Co-Inoculation of Plant-Growth-Promoting Bacteria Modulates Physiological and Biochemical Responses of Perennial Ryegrass to Water Deficit
Abstract
1. Introduction
2. Results
2.1. Biomass Production under Water Deficit
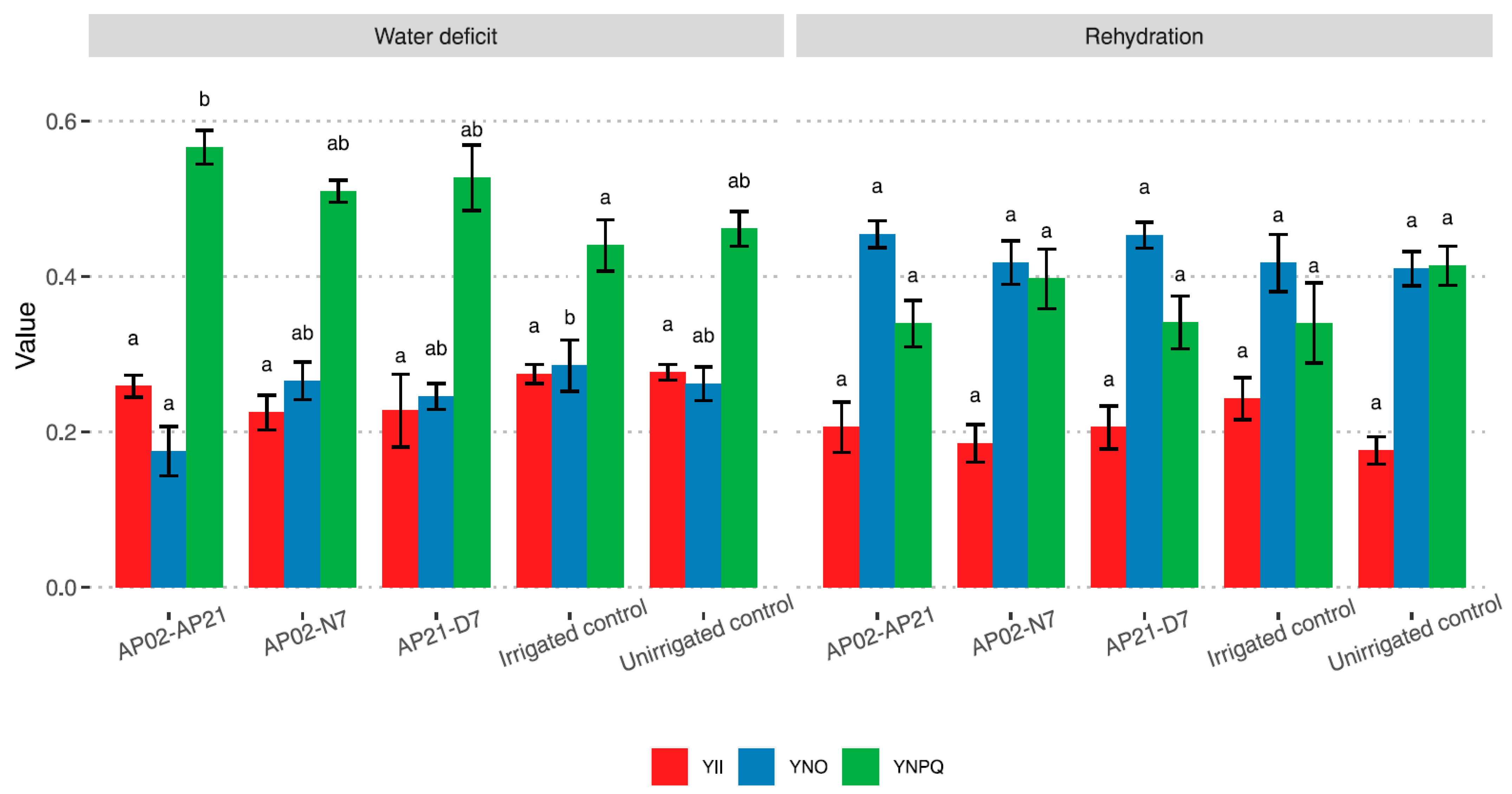
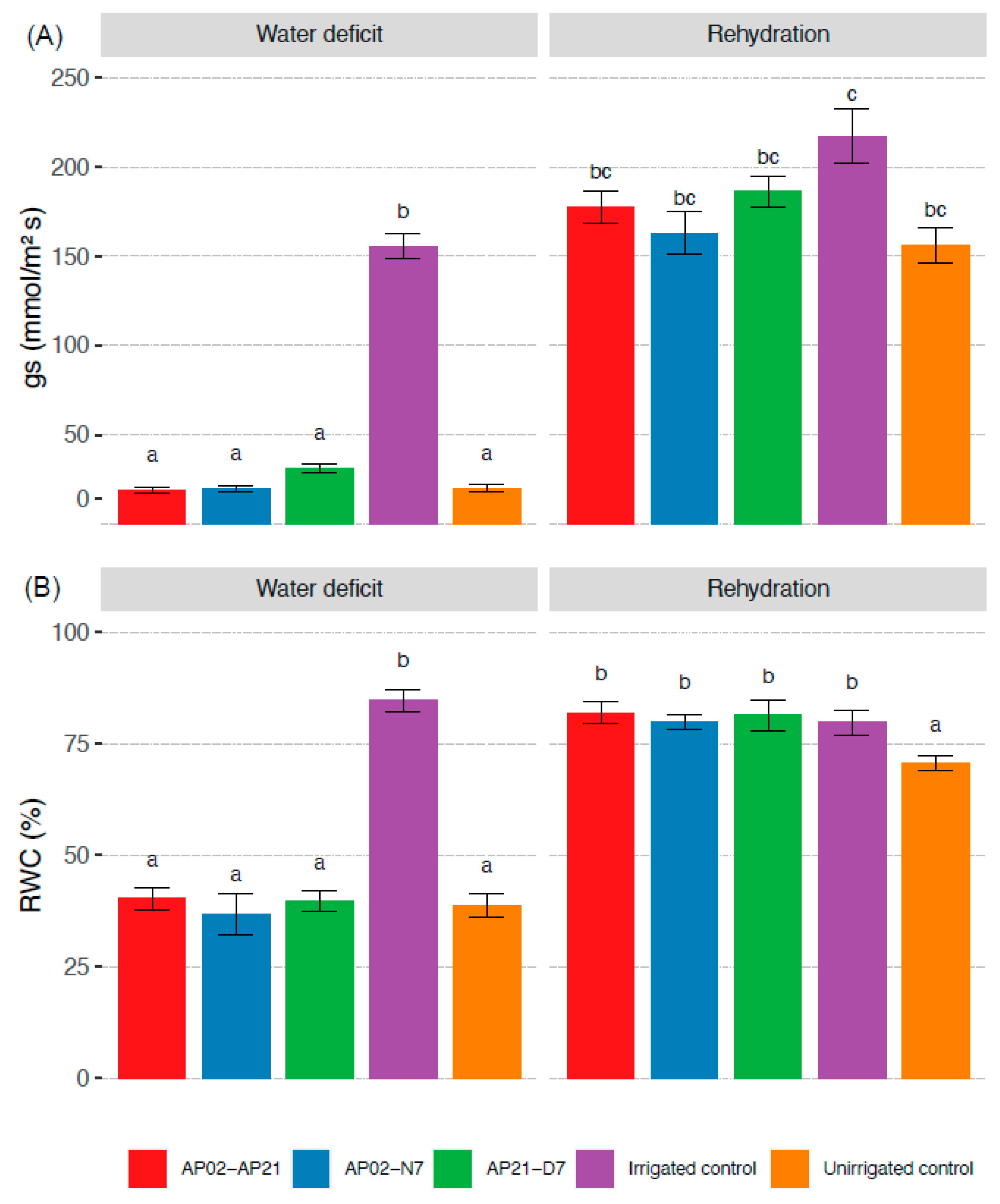
2.2. Plant Physiological Response under Water Deficit and after Rehydration
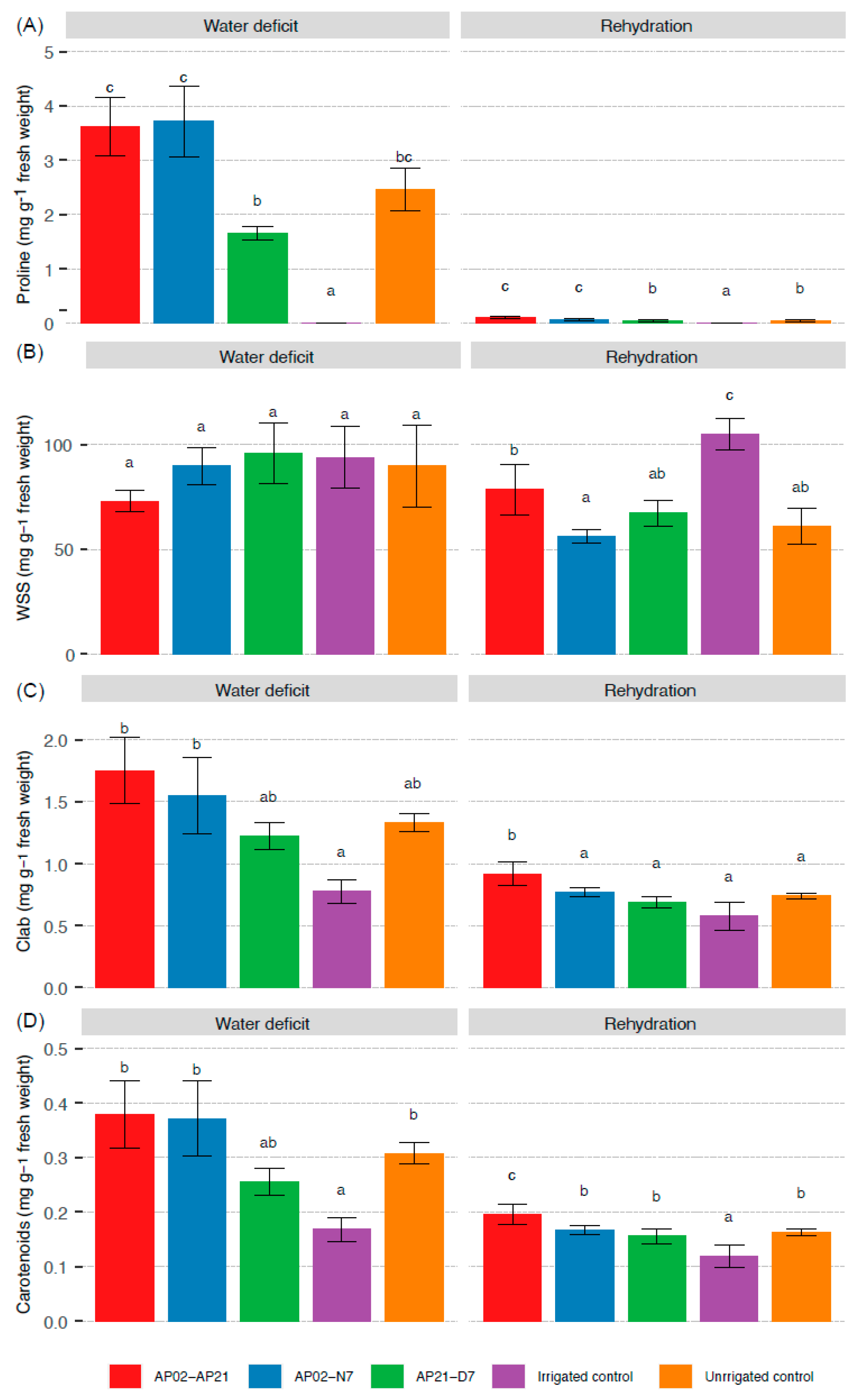
2.3. Plant Biochemical Response under Water Deficit and after Rehydration
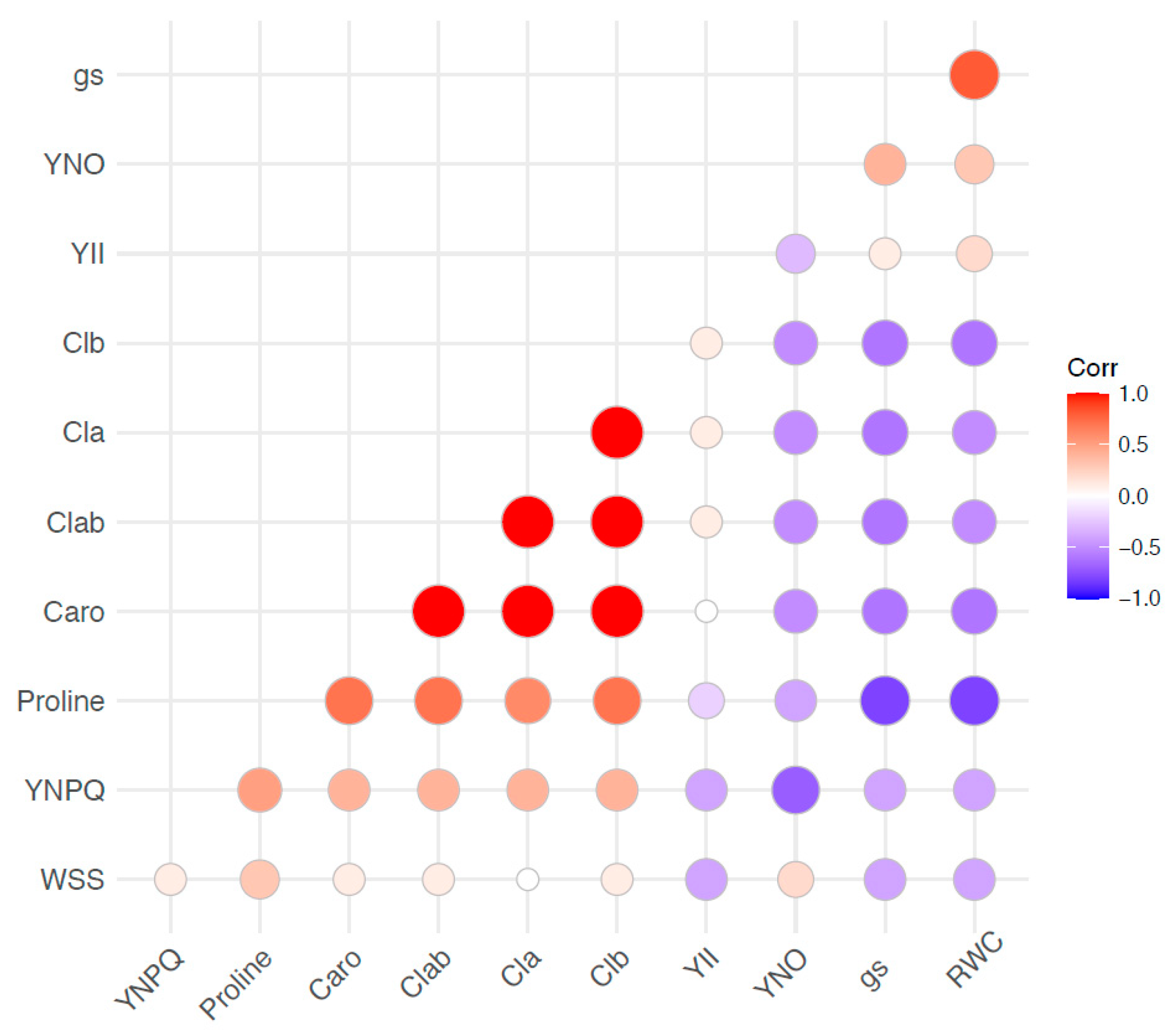
2.4. Correlation between the Physiological and Biochemical Responses of Perennial Ryegrass to Stress
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Consortia
4.2. Greenhouse Experiment
4.3. Determination of Physiological Parameters
4.4. Determination of Biochemical Parameters
4.5. Determination of Plant Development Parameters
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium, L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Foito, A.; Byrne, S.L.; Shepherd, T.; Stewart, D.; Barth, S. Transcriptional and metabolic profiles of Lolium perenne, L. genotypes in response to a PEG-induced water stress. Plant Biotechnol. J. 2009, 7, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Bothe, A.; Westermeier, P.; Wosnitza, A.; Willner, E.; Schum, A.; Dehmer, K.J.; Hartmann, S. Drought tolerance in perennial ryegrass (Lolium perenne, L.) as assessed by two contrasting phenotyping systems. J. Agron. Crop Sci. 2018, 204, 375–389. [Google Scholar] [CrossRef]
- Jonavičienė, K.; Statkevičiūtė, G.; Kemešytė, V.; Brazauskas, G. Genetic and phenotypic diversity for drought tolerance in perennial ryegrass (Lolium perenne, L.). Zemdirb.-Agric. 2014, 101, 411–418. [Google Scholar] [CrossRef][Green Version]
- Yates, S.; Jaškūnė, K.; Liebisch, F.; Nagelmüller, S.; Kirchgessner, N.; Kölliker, R.; Walter, A.; Brazauskas, G.; Studer, B. Phenotyping a dynamic trait: Leaf growth of perennial ryegrass under water limiting conditions. Front. Plant Sci. 2019, 10, 344. [Google Scholar] [CrossRef]
- Fariaszewska, A.; Aper, J.; van Huylenbroeck, J.; Baert, J.; de Riek, J.; Staniak, M.; Pecio, L. Mild Drought Stress-Induced Changes in Yield, Physiological Processes and Chemical Composition in Festuca, Lolium and Festulolium. J. Agron. Crop Sci. 2017, 203, 103–116. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, Y.; Pei, Z.; Liu, H.; Sun, S. Growth Response and Gene Expression to Deficit Irrigation and Recovery of Two Perennial Ryegrass Accessions Contrasting in Drought Tolerance. Hortscience 2016, 51, 921–926. [Google Scholar] [CrossRef]
- He, L.; Hatier, J.-H.B.; Matthew, C. Drought tolerance of two perennial ryegrass cultivars with and without AR37 endophyte. N. Z. J. Agric. Res. 2017, 60, 173–188. [Google Scholar] [CrossRef]
- He, L.; Hatier, J.H.; Card, S.D.; Matthew, C. Endophyte-infection reduces leaf dehydration of ryegrass and tall fescue plants under moderate water deficit. N. Z. Grassl. Assoc. 2013, 75, 151–156. [Google Scholar] [CrossRef]
- Li, F.; Deng, J.; Nzabanita, C.; Li, Y.; Duan, T. Growth and physiological responses of perennial ryegrass to an AMF and an Epichloë endophyte under different soil water contents. Symbiosis 2019, 79, 151–161. [Google Scholar] [CrossRef]
- He, A.; Niu, S.; Yang, D.; Ren, W.; Zhao, L.; Sun, Y.; Meng, L.; Zhao, Q.; Paré, P.W.; Zhang, J. Two PGPR strains from the rhizosphere of Haloxylon ammodendron promoted growth and enhanced drought tolerance of ryegrass. Plant Physiol. Biochem. 2021, 161, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Su, A.Y.; Niu, S.Q.; Liu, Y.Z.; He, A.L.; Zhao, Q.; Paré, P.W.; Li, M.-F.; Han, Q.-Q.; Khan, S.A.; Zhang, J.-L. Synergistic effects of bacillus amyloliquefaciens (GB03) and water retaining agent on drought tolerance of perennial ryegrass. Int. J. Mol. Sci. 2017, 18, 2651. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; George, R.; Das, G.; Paramithiotis, S.; Shin, H. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-Plant-Microbe Interactions in Stressed Agriculture Management: A Review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Shiva, S.; Prasad, K.; Vardharajula, S.; Shrivastava, M.; Skz, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Mohammadipanah, F.; Zamanzadeh, M. Bacterial Mechanisms Promoting the Tolerance to Drought Stress in Plants. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H.B., Ed.; Springer: Singapore, 2019; pp. 185–224. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Vaishnav, A.; Varma, A.; Tuteja, N.; Choudhary, D.K. Characterization of Bacterial Volatiles and Their Impact on Plant Health Under Abiotic Stress. In Volatiles and Food Security: Role of Volatiles in Agro-Ecosystems; Choudhary, D.K., Agarwal, P., Tuteja, N., Sharma, A.K., Varma, A., Eds.; Springer: Singapore, 2017; pp. 25–43. [Google Scholar] [CrossRef]
- Jochum, M.; McWilliams, K.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.-K. Bioprospecting Plant Growth-Promoting Rhizobacteria That Mitigate Drought Stress in Grasses. Front. Microbiol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Moreno-Galván, A.E.; Cortés-Patiño, S.; Romero-Perdomo, F.; Uribe-Vélez, D.; Bashan, Y.; Bonilla, R.R. Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl. Soil Ecol. 2020, 147, 103367. [Google Scholar] [CrossRef]
- Cortés-Patiño, S.; Vargas, C.; Álvarez-Flórez, F.; Bonilla, R.; Estrada-Bonilla, G. Potential of herbaspirillum and azospirillum consortium to promote growth of perennial ryegrass under water deficit. Microorganisms 2021, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Res 2016, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kościelniak, J.; Filek, W.; Biesaga-Kościelniak, J. The effect of drought stress on chlorophyll fluorescence in Lolium-Festuca hybrids. Acta Physiol. Plant 2006, 28, 149–158. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Pardo-Díaz, S.; Romero-Perdomo, F.; Mendoza-Labrador, J.; Delgadillo-Duran, D.; Castro-Rincon, E.; Silva, A.M.M.; Rojas-Tapias, D.F.; Cardoso, E.J.B.N.; Estrada-Bonilla, G.A. Endophytic PGPB Improves Plant Growth and Quality, and Modulates the Bacterial Community of an Intercropping System. Front. Sustain. Food Syst. 2021, 5, 354. [Google Scholar] [CrossRef]
- Curá, J.A.; Franz, D.R.; Filosofía, J.E.; Balestrasse, K.B.; Burgueño, L.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria Increases the Tolerance of Maize to Drought Stress. Microorganisms 2017, 5, 41. [Google Scholar] [CrossRef]
- Alves, G.C.; Videira, S.S.; Urquiaga, S.; Reis, V.M. Differential plant growth promotion and nitrogen fixation in two genotypes of maize by several Herbaspirillum inoculants. Plant Soil 2014, 387, 307–321. [Google Scholar] [CrossRef]
- Rossi, M.; Borromeo, I.; Capo, C.; Glick, B.R.; Del Gallo, M.; Pietrini, F.; Forni, C. PGPB improve photosynthetic activity and tolerance to oxidative stress in brassica napus grown on Salinized soils. Appl. Sci. 2021, 11, 11442. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, J.; Wang, X.; Jiang, Y. Phenotypic and Genotypic Diversity for Drought Tolerance among and within Perennial Ryegrass Accessions. Hortscience 2015, 50, 1148–1154. [Google Scholar] [CrossRef]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa, L. Plant Soil 2019, 451, 57–73. [Google Scholar] [CrossRef]
- Brilli, F.; Pollastri, S.; Raio, A.; Baraldi, R.; Neri, L.; Bartolini, P.; Podda, A.; Loreto, F.; Maserti, B.E.; Balestrini, R. Root colonization by Pseudomonas chlororaphis primes tomato (Lycopersicum esculentum) plants for enhanced tolerance to water stress. J. Plant Physiol. 2019, 232, 82–93. [Google Scholar] [CrossRef]
- Wilson, S. Intraspecific Differences in the Response of Perennial Ryegrass (Lolium perenne, L.) to Drought. Doctoral Dissertation, Lincoln University, Lincoln, New Zealand, 2016. [Google Scholar]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant 2015, 153, 79–90. [Google Scholar] [CrossRef]
- Neiverth, A.; Delai, S.; Garcia, D.M.; Saatkamp, K.; de Souza, E.M.; Pedrosa, F.D.O.; Guimarães, V.F.; dos Santos, M.F.; Vendruscolo, E.C.G.; da Costa, A.C.T. Performance of different wheat genotypes inoculated with the plant growth promoting bacterium Herbaspirillum seropedicae. Eur. J. Soil Biol. 2014, 64, 1–5. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Shameer, S.; Prasad, T.N.V.K.V. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Rodriguez Caceres, E.A. Improved medium for isolation of Azospirillum spp. Appl. Environ. Microbiol. 1982, 44, 990–991. [Google Scholar] [CrossRef] [PubMed]
- Nihorimbere, V.; Cawoy, H.; Seyer, A.; Brunelle, A.; Thonart, P.; Ongena, M. Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol. Ecol. 2012, 79, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Soil Science Division Staff. Soil Survey Manual Soil Science Division Staff Agriculture Handbook No. 18; Government Printing Office: Washington, DC, USA, 2017. [Google Scholar]
- Sade, N.; Galkin, E.; Moshelion, M. Measuring Arabidopsis, Tomato and Barley Leaf Relative Water Content (RWC). Bio. Protoc. 2015, 5, e1451. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kolde, R. Package ‘Pheatmap’. 2018. Available online: https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf (accessed on 11 May 2022).
- Bertinetto, C.; Engel, J.; Jansen, J. ANOVA simultaneous component analysis: A tutorial review. Anal. Chim. Acta X 2020, 6, 100061. [Google Scholar] [CrossRef]
- Knudson, C. Monte Carlo Likelihood Approximation for Generalized Linear Mixed Models. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2016. [Google Scholar]
- Dorscheidt, T. MetStaT: Statistical Metabolomics Tools. 2013. Available online: https://CRAN.R-project.org/package=MetStaT (accessed on 2 June 2022).
| Treatment | Leaf Weight (g plant−1) | Root Weight (g plant−1) | Leaf Length (cm plant−1) | Root Length (cm plant−1) | Leaf Number (plant−1) | Tiller Number (plant−1) | Root/Shoot |
|---|---|---|---|---|---|---|---|
| AP02–AP21 | 2.66 ± 0.15 b | 1.38 ± 0.04 a | 26.80 ± 0.84 a | 19.02 ± 0.85 a | 106.50 ± 7.24 a | 35.00 ± 2.38 a | 0.53 ± 0.38 a |
| AP02–N7 | 2.32 ± 0.16 ab | 1.60 ± 0.06 a | 28.44 ± 0.65 a | 20.50 ± 1.01 ab | 84.80 ± 8.02 a | 31.40 ± 4.09 a | 0.75 ± 0.02 b |
| AP21–D7 | 1.97 ± 0.08 a | 1.44 ± 0.04 a | 29.52 ± 0.81 a | 23.35 ± 0.54 abc | 81.83 ± 5.24 a | 26.67 ± 2.53 a | 0.74 ± 0.04 b |
| Irrigated control | 2.45 ± 0.12 ab | 1.98 ± 0.12 b | 27.17 ± 2.22 a | 26.17 ± 1.65 c | 81.83 ± 7.34 a | 29.33 ± 3.62 a | 0.81 ± 0.04 b |
| Unirrigated control | 2.21 ± 0.06 ab | 1.62 ± 0.04 a | 29.20 ± 0.57 a | 24.55 ± 1.03 bc | 81.83 ± 5.64 a | 26.83 ± 1.53 a | 0.74 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Patiño, S.; Vargas, C.D.; Alvarez-Flórez, F.; Estrada-Bonilla, G. Co-Inoculation of Plant-Growth-Promoting Bacteria Modulates Physiological and Biochemical Responses of Perennial Ryegrass to Water Deficit. Plants 2022, 11, 2543. https://doi.org/10.3390/plants11192543
Cortés-Patiño S, Vargas CD, Alvarez-Flórez F, Estrada-Bonilla G. Co-Inoculation of Plant-Growth-Promoting Bacteria Modulates Physiological and Biochemical Responses of Perennial Ryegrass to Water Deficit. Plants. 2022; 11(19):2543. https://doi.org/10.3390/plants11192543
Chicago/Turabian StyleCortés-Patiño, Sandra, Christian D. Vargas, Fagua Alvarez-Flórez, and German Estrada-Bonilla. 2022. "Co-Inoculation of Plant-Growth-Promoting Bacteria Modulates Physiological and Biochemical Responses of Perennial Ryegrass to Water Deficit" Plants 11, no. 19: 2543. https://doi.org/10.3390/plants11192543
APA StyleCortés-Patiño, S., Vargas, C. D., Alvarez-Flórez, F., & Estrada-Bonilla, G. (2022). Co-Inoculation of Plant-Growth-Promoting Bacteria Modulates Physiological and Biochemical Responses of Perennial Ryegrass to Water Deficit. Plants, 11(19), 2543. https://doi.org/10.3390/plants11192543






