1. Introduction
The practice of managing forests has been used throughout history worldwide for economic and ecological purposes [
1]. Black cherry (
Prunus serotina Ehrh.) is an important tree species that is managed for its ecological and economic value in the northeastern USA [
2]. The lumber is highly esteemed by woodworkers and carpenters, and the fruit is consumed by a wide diversity of wildlife [
3]. Black cherry is widely distributed throughout the eastern USA and can grow in a variety of different climatic conditions. However, the highest quality black cherry timber originates from the Allegheny Plateau region of the USA where the environment is cool, moist, and temperate [
3]. In particular, the Allegheny National Forest (ANF) located in northwestern Pennsylvania produces some of the highest-quality black cherry in the world. Over the last few decades, the land managers in the ANF have noticed a decline in natural regeneration. The cause of this decline is still unknown, though one hypothesis is a lack of adequate pollination and pollinators for the consequence of reduced fruit formation.
The management of timber species like black cherry requires the manipulation of a forest ecosystem using heavy machinery, which can have an impact on plant and animal communities including pollinators. Previous studies [
4,
5] showed that loss of tree habitat and soil erosion and compaction caused by silvicultural practices could have a lasting negative impact on some species of Lepidoptera (moths and butterflies). Other studies [
6,
7] suggest there is a positive response to open canopies or recently removed clear cuts by some solitary bee communities. However, when the dense herbaceous understory took over, the bee community’s richness and abundance were noticeably lowered [
7]. These earlier studies indicate that the application of silvicultural management could affect insect abundance and diversity.
Currently, foresters in the ANF utilize even-aged regeneration treatments to manage shade-intolerant species like black cherry [
8]. The preferred method for black cherry regeneration is the shelterwood seed tree cut (referred to as “seed-tree” hereafter) followed in succession by the shelterwood removal cut (referred to as “removal” hereafter). The seed-tree treatment is preferred where natural regeneration is desirable and when there is an opportunity on the landscape to increase management success [
8]. This sequence of management involves harvesting approximately one-third of the overstory or mature trees originally found in the unmanaged stand (
Figure 1a), leaving well-distributed trees that provide seeds for the next generation (
Figure 1b). The seed-tree treatment provides sufficient light to reach the understory and promotes seedling establishment and growth, without giving enough light to undesired weeds. The removal cut is conducted approximately 3–15 years after the seed-tree treatment [
8] (
Figure 1c). The removal-cut proceeds when seedlings have been established, and a stand is at full maturity and ready to have all merchantable tree species harvested, leaving only the residual wildlife reserve trees [
8]. This method is considered the best for the regeneration of Allegheny hardwoods [
9]. Therefore, if there is not a significant quantity of black cherry saplings growing in the stands at the time of removal treatment, the potential for regeneration failure is significant. This failure is likely to occur because the canopy opening created by the removal treatment allows a new cohort of fast-growing herbaceous species to develop and out-compete any regenerating young black cherry trees. Additionally, a lack of viable seeds produced by the parent trees in the seed-tree stands could also be the cause of the black cherry regeneration problem in the ANF.
Only a couple of previous studies on black cherry pollinators have been published. Surveys conducted in a non-forest stand [
10] and forest edge [
11] reported that Hymenoptera (bees and wasps) and Diptera (true flies) are the most prevalent insect groups visiting black cherry flowers. Our previous study conducted in unmanaged black cherry stands [
12] showed that the most abundant insects that visited black cherry canopies were insects belonging to the order Diptera followed by Coleoptera (beetles) and Hymenoptera. In addition, the study also found that insects, in particular dipterans, are attracted to the canopy of black cherry trees when flowers are present and thus likely contribute to pollination, and black cherry flowers emit a volatile blend that is similar to that of other pollinator-dependent
Prunus species. However, little is currently known about how black cherry pollinators are affected by the management of respective forest stands, which can be a significant factor affecting the regeneration of black cherry.
To assist land managers in sustaining or even increasing black cherry populations, a further understanding of how different silvicultural systems affect the pollinator community is needed. Therefore, we conducted a 2-year field experiment to determine whether silvicultural treatments significantly affect the insect community in black cherry stands. Specifically, our study used pan traps installed in the canopy and on the grounds to capture insects visiting black cherry during flowering to determine if two silvicultural treatments (i.e., seed-tree and removal treatments) increase or decrease the species abundance, richness, and diversity (see details in
Section 4) of insects compared to unmanaged black cherry trees (i.e., control). We hypothesized that the two silvicultural treatments would reduce the abundance, richness, and diversity of insect species visiting black cherry flowers. We further hypothesized that insects utilizing and pollinating black cherry flowers would be found in the stands with the highest concentration of black cherry (i.e., seed-tree treatment).
3. Discussion
Black cherry is an economically and environmentally important species to the Allegheny Plateau region of the USA, and silvicultural management of the species is necessary for timber production. The goal of this study was to examine if there was a potential deficiency in the insect communities in black cherry stands during the flowering period and if forest silvicultural treatments influence the species diversity and richness of the insects associated with black cherry. Our results showed that the major insect order was Diptera regardless of stand type and trap position, comprising ~73% of 17,375 insects captured in this study (
Table 1). Many insects collected in this study are known to feed on pollen or are associated with flowers. Our previous study [
12] via electron microscopy analysis demonstrated that insects collected in the canopy traps, representing 12 different families in three major orders (Coleoptera, Diptera, and Hymenoptera), carried pollen grains of black cherry on their bodies, legs, and antennae. These insects included
Antocha sp. (Diptera: Limoniidae),
Atalantycha bilineata (Coleoptera: Cantharidae),
Camponotus pennsylvanicus (Hymenoptera: Formicidae),
Trichopion sp. (Coleoptera: Curculionidae) and
Drosophila sp. (Diptera: Drosophilidae).
Our study also revealed that the canopy traps captured significantly more insects (~60% of all insects captured in this study) than the ground traps (
Table 1,
Figure 3). In line with our previous study [
12] two specific insect orders (i.e., Lepidoptera and Thysanoptera) were found to be significantly more abundant in the canopy of black cherry, indicating that these insects might be utilizing flowers and thus contributing to the pollination of black cherry. Likewise, the number of Diptera captured in the canopy of black cherry trees was higher than that on the ground (
Table 1), however, in contrast to our previous study [
12], the difference in Diptera captured in the two trap positions was not significant (
p = 0.123) in the current study (
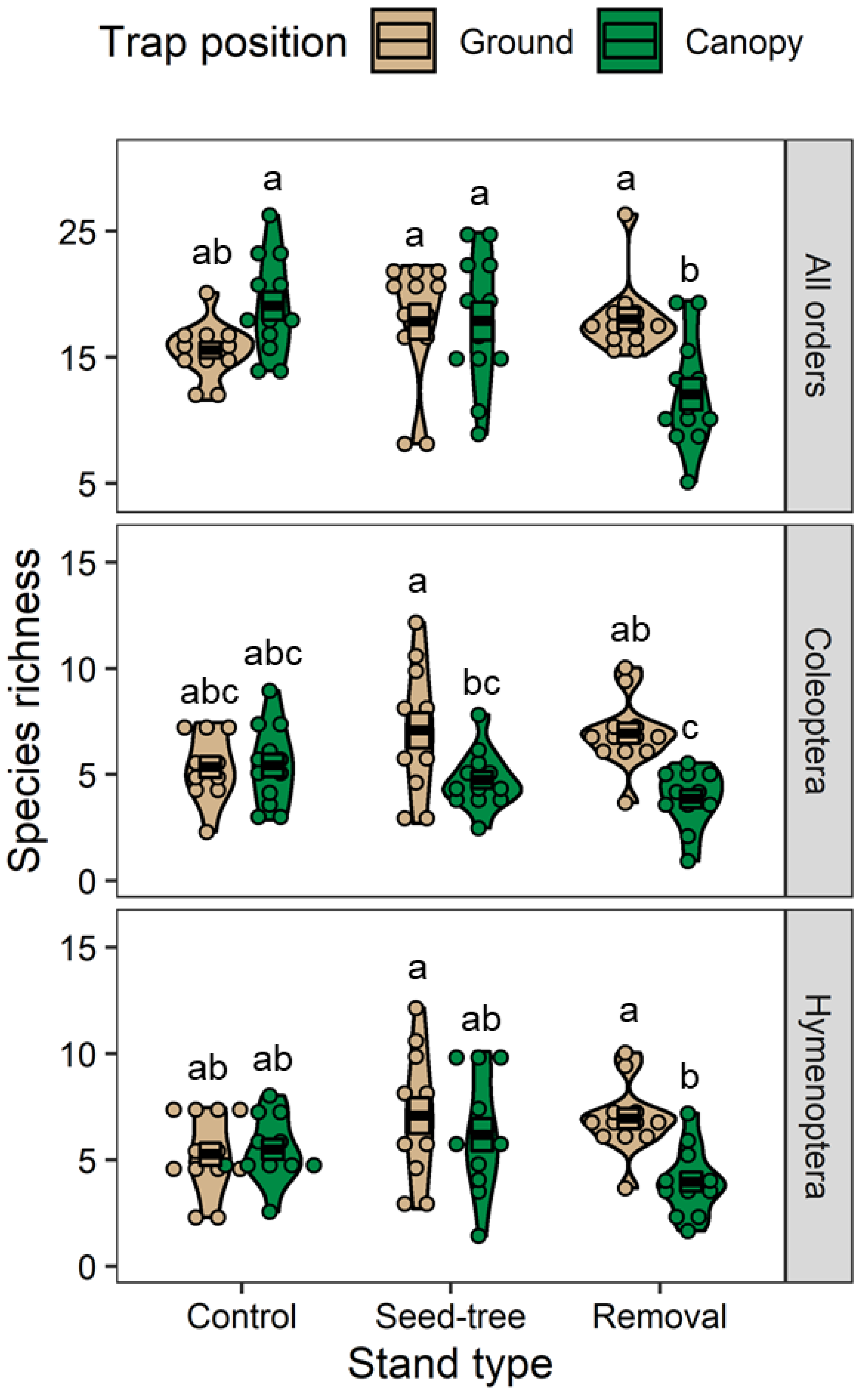
Figure 3). Remarkably, the species diversity of all the insect species and that of Diptera in the removal treatment were significantly higher in the ground traps than in canopy traps (
Figure 5), suggesting that the resources (i.e., nectar and pollen) in the removal stands were less abundant in the canopy than on the ground. The lack of contiguous canopy cover for the insect to move within might also contribute to the decreased number of insects found in the canopies in removal stands. Although many dipteran insects are predators (such as syrphid larvae) or associated with pasture or livestock, we did not find such dipteran species in our study because it was conducted inside the forest distant from field crops and livestock farms. In addition, honeydew produced by sap-sucking insects such as aphids can attract other insects including Coleoptera and Diptera. As the flowering period of black cherry is quite early in spring, we did not observe a high number of aphids at the time of black cherry flowering. Additionally, we did not find or recognize any sooty mold growing on lower leaves which might have been caused by a high abundance of honeydew-producing insects.
A mature black cherry in the ANF can grow to heights of up to 35 m, which is a significant distance for a small flower-visiting insect living on the ground to fly upward to find a source of pollen and nectar in the canopy. Therefore, we believe that most of the insects we found in the canopy during flowering were attracted to the flowers and voluntarily flew up to the canopy. In addition, significantly higher overall insect abundance was observed in the seed-tree stands, suggesting that the seed-tree treatment created a resource-abundant environment that attracted these species to the canopy of the remaining flowering black cherry trees. The resource concentration hypothesis predicts that if there is a resource abundance it will support higher loads of plant-specific species [
13]. Conversely, resource dilution will not support plant-specific insect species and thus their number will be reduced [
14]. In our study, the seed-tree stands had a higher concentration of resources for black cherry-specific flower visitors, which could explain why the flower-visiting insects including Dipteran were more abundant where black cherry trees were in higher concentrations. This can be seen in the control stand where black cherry comprises <3% of the total tree species. Conversely, in the seed-tree stands where we observed a higher percentage of black cherry (~30%), we also observed a higher population of insects. Furthermore, when the resources are removed in the next step of the treatments (i.e., removal treatment), we observed a drop in the insect population back to low black cherry concentration conditions.
The seed-tree treatment removes approximately one-third of the competing overstory and understory tree species in a forest stand (
Figure 1), which lowers the tree diversity in the stand in favor of mature black cherry in the overstory. Some insects in the Diptera and Lepidoptera orders that indeed are associated with and use black cherry flowers, were concentrated in the canopy of these remaining seed-tree stands (
Table 2). Many lepidopterans we found in this study are polyphagous and can feed on the vegetation of many different tree and plant species as larvae. In contrast, the significantly lower number of dipterans and lepidopterans found in the removal stands could be explained by the substantial loss of overstory canopy structure caused by the clearcutting or removal of most black cherry trees and in consequence the disturbance of habitats. This treatment is designed to drastically lower the number of trees per ha and tree diversity in a stand by harvesting all merchantable tree species. This creates large openings that decrease humidity, increase sunlight to the understory, and raise windspeeds in the stand. These conditions, along with the temporary reduction in the diversity of floral resources, are less favorable for flower-visiting insects. We conducted ground surveys of the understory herbaceous vegetation during the peak flowering period of black cherry. From the understory vegetation survey, we found 39 plant species in the control, 22 species in the seed-tree cut, and 18 species in the removal treatments (
Table S1).
Our study revealed that seed-tree treatments increased insect species abundance in two major insect orders (i.e., Diptera and Hymenoptera) and species richness was significantly affected by trap position in two insect orders (i.e., Coleoptera and Hymenoptera). The only order to show an interaction of stand type and trap position was Lepidoptera (data not shown). From our observations, we have seen that lepidopterans prefer diverse vegetation and inhabit the canopies of trees. These results indicate that the captures of lepidopterans depend on silvicultural treatments and the location of insect traps. If the stand has more diverse overstory vegetation higher numbers of lepidopterans are expected to be captured in the canopies.
A total of 544 morphospecies in the five major insect orders (i.e., Diptera, Coleoptera, Lepidoptera, Hymenoptera, and Thysanoptera) were found in this study, and the seed-tree treatment significantly affected the species abundance, richness, and diversity. In the seed-tree and control stands, lepidopterans were more abundant in the canopy, suggesting that lepidopterans prefer a stand with more diverse and abundant overstory; these insects are likely using the canopy to feed as larvae and as a source of nectar as adults. The lepidopteran species most frequently found in the canopy was
M. canadaria. This is a polyphagous insect that is known to feed on the leaves of nearly every deciduous tree species found in the control and seed-tree stands as a larva [
15]. The polyphagous adults have also been recorded to carry black cherry pollen on their bodies which indicates they use black cherry by feeding on nectar and likely contribute to pollination [
16]. Notably, our study showed the scarcity of hymenopterans found inside the forest, compared to a previous study conducted on the forest edge [
11] that reported a high number of hymenopterans. Major hymenopteran species found in our study was gall-inducing wasps which are known to be associated with oak, but their ecological importance to black cherry pollination is not known.
The main species of thrips captured in the canopy was identified as
Frankliniella occidentalis (Thysanoptera: Thripidae), western flower thrips.
F. occidentalis is known as a flower dweller and adults live amongst the foliage, fruits, and flowers of a wide variety of plant species [
17]. The larvae typically drop to the ground when they are ready to pupate but can remain on the plant if the floral structure is complex enough [
16]. Since the 1970s
F. occidentalis has spread from its native range in western North America to become a major pest of horticultural and agricultural crops worldwide [
18]. It is believed that this species arrived in the area through infested plants being transported to greenhouses in Pennsylvania [
18]. These are important pests economically due to their feeding habits on flowers (florivore) and their ability to transmit viruses [
19]. Our previous study [
12] found that some of the volatile organic compounds (VOCs) emitted by the flowers of black cherry match VOCs that have been demonstrated to be attractive to
F. occidentalis [
20].
Prunus necrotic ringspot virus (PNRSV), and prune dwarf virus (PDV) can be transmitted through pollen found on the bodies of thrips and can cause up to 50% yield loss in sweet cherry and 100% yield loss in peach [
21,
22]. Although these viruses have not been confirmed in black cherry, there is the potential for a negative impact on black cherry regeneration.
Based on our findings, a potential reason for the decline in the natural regeneration of black cherry observed by land managers in the ANF could be a lack of adequate pollination of the flowers. One might expect that the increased abundance of all insects upon the seed-tree treatment (
Table 1,
Figure 2) and in particular of Diptera, which we had found previously to carry black cherry pollen [
14], would result in an improved pollination rate. However, pollination in black cherry, like in other
Prunus species, is controlled by a self-incompatibility mechanism [
23,
24] which is genetically regulated by the multi-allelic S-locus. Thus, for successful pollination black cherry requires the transfer of pollen from flowers of a tree with one set of S-locus alleles to the flowers of another tree carrying a distinct set of S-locus alleles to produce viable seeds. In contrast, when the transferred pollen and the pistil of the receiving flower express the same S-locus allele the pollen is rejected which results in a failure to fertilize the flower as well as to form seeds and a fruit. The gradual reduction in the number of black cherry trees in the seed-tree and removal treatments concomitantly increases the distance between individual black cherry trees. Many insects visiting flowering black cherry trees are relatively small in size and therefore have a weak ability to fly suggesting that an increased distance between trees after these silvicultural treatments might actually reduce the rate of required cross-pollination. Moreover, the removal of trees in forest stands likely also leads to a reduced representation of diverse S-locus alleles in the black cherry population, which in consequence might increase the chance of pollen rejection due to the flower pistil expressing the same S-allele. Further studies will be required to verify if the silvicultural treatments currently used in the ANF not only alter the insect assemblage associated with black cherry but also create a potential genetic bottleneck in the tree population which could contribute to the decline in natural regeneration.
In this study, we examined how the insect community visiting black cherry trees would be influenced by silvicultural management. We hypothesized that the two silvicultural treatments (i.e., shelterwood seed-tree and removal treatments) would reduce the abundance, richness, and diversity of insect species visiting black cherry flowers. The results of our experiment showed that the seed-tree treatment increases the overall abundance of insects visiting black cherry during the flowering period, and the species richness and diversity of insects captured in the canopy of black cherry were lower in removal treatments, indicating that silvicultural treatments affected insect community significantly. In addition, our study showed that Diptera was the most abundant insect order, and we listed 10 major insect species visiting flowers of black cherry in the seed-tree and removal treatments, suggesting they are key players in the pollination of black cherry. Furthermore, our previous study [
12] showed a significant increase in dipteran species when black cherry is flowering. The current study revealed that the concentration of black cherry in a managed forest stand also has an effect on dipteran abundance, further providing evidence that dipterans are attracted by the flowers of black cherry and are potentially significant pollinators.
Although our study reported various aspects of insect community responses to the silvicultural treatments in black cherry stands, future studies are needed to investigate if the structural changes in the canopy resulting from silvicultural treatments can impact the movement of pollinator insects between trees, which is required to overcome self-incompatibility issues in black cherry pollination. In addition, further studies should investigate whether the major insects we captured are efficiently cross-pollinating black cherry trees. As the silvicultural practices presented in this manuscript are successive interventions, a long-term study including both practices would provide a more comprehensive understanding of their effect on pollinator insects.