A Nomenclatural and Taxonomic Revision of the Senecio squalidus Group (Asteraceae)
Abstract
1. Introduction
2. Materials and Methods
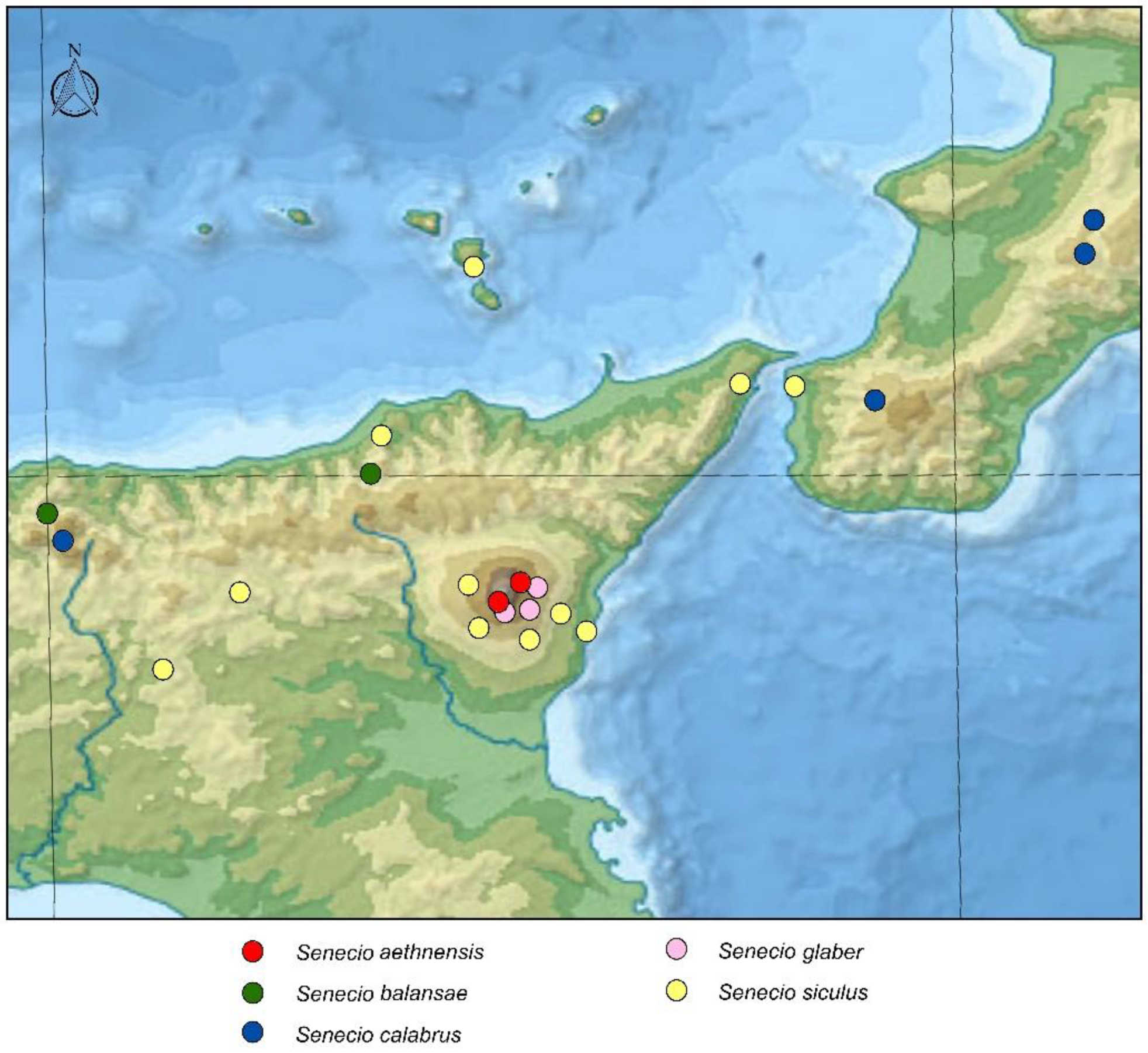
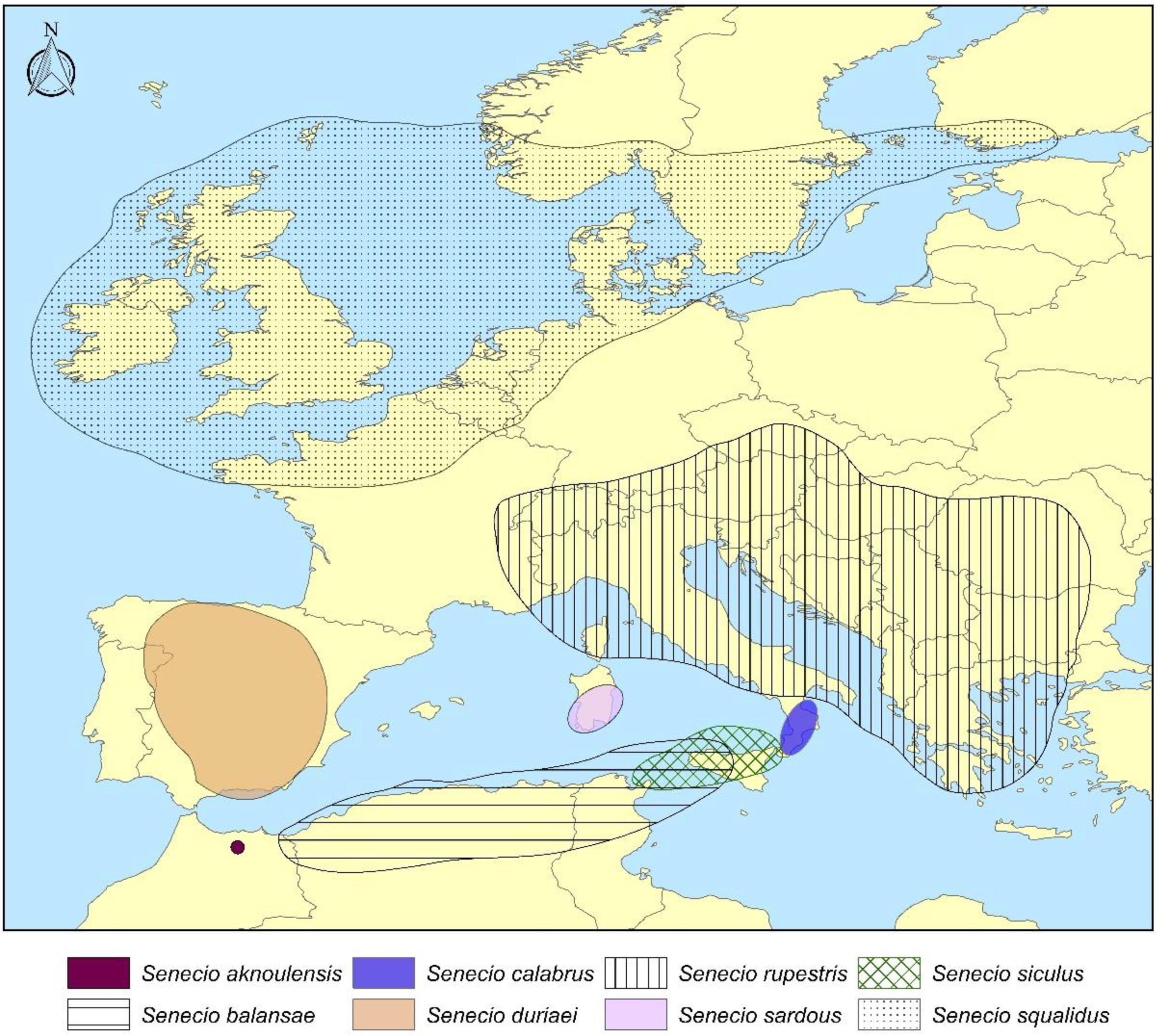
3. Results
Identification Key to the Species of Senecio Squalidus Group
| 1. | Basal leaves pinnatifid to pinnatipartite | 2 |
| 1. | Basal leaves entire to shallowly lobed | 7 |
| 2. | Leaf lobes narrow and long (length/width ratio > 3), with acute tips | 3 |
| 2. | Leaf lobes wide and short (length/width ratio ≤ 3), with rounded tips | 5 |
| 3. | Whole plant arachnoid, basal leaves widened at the apex | S. aknoulensis |
| 3. | Whole plant glabrous to sparsely arachnoid, basal leaves not or slightly widened at the apex | 4 |
| 4. | Leaves coarsely divided, lobes 3–5 mm wide or more | S. squalidus |
| 4. | Leaves finely divided, lobes 1–3 mm wide | S. siculus |
| 5. | Basal leaves lyrate, with apex at least twice as wide as the lateral lobes | S. balansae |
| 5. | Basal leaves not lyrate, with narrow apex, slightly wider than the lateral lobes | 6 |
| 6. | Plant glandular; leaves lobed, with crenate margin | S. duriaei |
| 6. | Plant eglandular; leaves deeply laciniate, with toothed margin | S. rupestris |
| 7. | Leaves thick and rigid | 8 |
| 7. | Leaves thin and soft | 9 |
| 8. | Leaves with short and acute lobes | S. ×glaber |
| 8. | Leaves with entire to dissected margins | S. aethnensis |
| 9. | Basal leaves not or slightly widened at the apex | S. calabrus |
| 9. | Basal leaves widened at the apex | S. sardous |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, J.C.M. The Mediterranean species of Senecio sections Senecio and Delphinifolius. Notes R. Bot. Gard. Edinb. 1979, 37, 387–428. [Google Scholar]
- Greuter, W. The Euro+Med treatment of Senecioneae and the minor Compositae tribes—Generic concepts and required new names, with an addendum to Cardueae (Notulae ad floram euro-mediterraneam pertinentes No. 8). Willdenowia 2003, 33, 245–250. [Google Scholar] [CrossRef]
- Greuter, W. Compositae (pro parte majore). In Compositae. Euro+Med PlantBase; Greuter, W., Raab-Straube von, E., Eds.; 2006; Available online: https://www.emplantbase.org/home.html (accessed on 8 August 2022).
- Jarvis, C.E.; Turland, N. Typification of Linnaean specific and varietal names in the Compositae (Asteraceae). Taxon 1998, 47, 347–370. [Google Scholar] [CrossRef]
- Iamonico, D.; Managlia, A. Lectotypification of the Bertoloni’s names in the genus Senecio L. (Asteraceae). Plant Biosyst. 2013, 149, 48–53. [Google Scholar] [CrossRef]
- Arrigoni, P.V. Contributo alla conoscenza della flora della Sardegna: Nuove specie di Taraxacum e altri reperti. Parlatorea 2007, 9, 87–94. [Google Scholar]
- Ronsisvalle, G.A. Osservazioni biometriche su alcune specie di Senecio dell’Etna. Boll. Accad. Gioenia Catania 1968, 9, 332–345. [Google Scholar]
- Fici, S.; Lo Presti, R.M. Variation in the Senecio aethnensis group (Asteraceae) along an altitudinal gradient. Plant Biosyst. 2003, 137, 305–312. [Google Scholar] [CrossRef]
- Kent, D.H. Senecio squalidus L. in the British Isles. 1. Early records (to 1877). Proc. Bot. Soc. Br. Isles 1956, 2, 115–118. [Google Scholar]
- Crisp, P. Cytotaxonomic Studies in the Section Annui of Senecio. Ph.D. Dissertation, University of London, London, UK, 1972. [Google Scholar]
- Abbott, R.J.; James, J.K.; Irwin, J.A.; Comes, H.P. Hybrid origin of the Oxford ragwort, Senecio squalidus L. Watsonia 2000, 23, 123–138. [Google Scholar]
- Abbott, R.J.; Brennan, A.C.; James, J.K.; Forbes, D.F.; Hegarty, M.J.; Hiscock, S.J. Recent hybrid origin and invasion in the British Isles by a self-incompatible species, Oxford ragwort (Senecio squalidus L., Asteraceae). Biol. Invasions 2009, 11, 1145–1158. [Google Scholar] [CrossRef]
- James, J.K.; Abbott, R.J. Recent, allopatric, homoploid hybrid speciation: The origin of Senecio squalidus (Asteraceae) in the British Isles from a hybrid zone on Mount Etna, Sicily. Evolution 2005, 59, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.C.; Barker, D.; Hiscock, S.J.; Abbott, R.J. Molecular genetic and quantitative trait divergence associated with recent homoploid hybrid speciation: A study of Senecio squalidus (Asteraceae). Heredity 2012, 108, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.C.; Hiscock, S.J.; Abbott, R.J. Completing the hybridization triangle: The inheritance of genetic incompatibilities during homoploid hybrid speciation in ragworts (Senecio). AoB Plants 2019, 11, ply078. [Google Scholar] [CrossRef] [PubMed]
- Nevado, B.; Harris, S.A.; Beaumont, M.A.; Hiscock, S.J. Rapid homoploid hybrid speciation in British gardens: The origin of Oxford ragwort (Senecio squalidus). Mol. Ecol. 2020, 29, 4221–4233. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.L.Y.; Hiscock, S.J.; Filatov, D.A. The role of interspecific hybridisation in adaptation and speciation: Insights from studies in Senecio. Front. Plant Sci. 2022, 13, 907363. [Google Scholar] [CrossRef]
- Peruzzi, L.; Bedini, G.; Andreucci, A. Homoploid hybrid speciation in Doronicum L. (Asteraceae)? Morphological, karyological and molecular evidences. Plant Biosyst. 2011, 146, 867–877. [Google Scholar] [CrossRef]
- Von Hagen, K.B.; Seidler, G.; Welk, E. New evidence for a postglacial homoploid hybrid origin of the widespread Central European Scabiosa columbaria L. s. str. (Dipsacaceae). Plant Syst. Evol. 2008, 274, 179–191. [Google Scholar] [CrossRef]
- Soltis, D.E.; Avrodiev, E.V.; Meyers, S.C.; Severns, P.M.; Zhang, L.; Gitzendanner, M.A.; Ayers, T.; Chester, M.; Soltis, P.S. Additional origins of Ownbey’s Tragopogon mirus. Bot. J. Linn. Soc. 2012, 169, 297–311. [Google Scholar] [CrossRef]
- Banfi, E.; Galasso, G. Speciazione alloctona, opportunità imprevista della bioglobalizzazione. Inf. Bot. Ital. 2015, 47, 338–341. [Google Scholar]
- Viciani, D.; Vidali, M.; Gigante, D.; Bolpagni, R.; Villani, M.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; Angiolini, C.; et al. A first checklist of the alien-dominated vegetation in Italy. Plant Sociol. 2020, 57, 29–54. [Google Scholar] [CrossRef]
- Linnaeus, C. Species Plantarum, Exhibentes Plantas Rite Cognitas, ad Genera Relatas, cum Differentiis Specificis, Nominibus Trivialibus, Locis Natalibus, Ecundum Systema Sexuale Digestas; Impensis Laurentii Salvii: Holmiae, Sweden, 1753; Volume 2. [Google Scholar] [CrossRef]
- Walker, R. Flora of Oxfordshire and Its Contiguous Counties; Slatter: Oxford, UK, 1833. [Google Scholar]
- Vázquez, F.M.; García, D.; Márquez, F. (2851) Proposal to reject the name Senecio nebrodensis (Asteraceae). Taxon 2021, 70, 1370. [Google Scholar] [CrossRef]
- Fiori, A. Senecio. In Flora Analitica d’Italia; Fiori, A., Béguinot, A., Paoletti, G., Eds.; Tipografia del Seminario: Padova, Italy, 1903; Volume 3, pp. 208–221. [Google Scholar]
- Fiori, A. Senecio. In Nuova Flora Analitica d’ltalia; Tipografia Ricci: Firenze, Italy, 1927; Volume 2, pp. 587–602. [Google Scholar]
- Bernardo, L.; Passalacqua, N.G.; Peruzzi, L. Notulae: 1738–1749—Notulae alla checklist della flora vascolare italiana 10 (1682–1750). Inf. Bot. Ital. 2010, 42, 529–532. [Google Scholar]
- Peruzzi, L.; Domina, G.; Bartolucci, F.; Galasso, G.; Peccenini, S.; Raimondo, F.M.; Albano, A.; Alessandrini, A.; Banfi, E.; Barberis, G.; et al. An inventory of the names of vascular plants endemic to Italy, their loci classici and types. Phytotaxa 2015, 196, 1–217. [Google Scholar] [CrossRef]
- Domina, G.; Giusso del Galdo, G.; Gargano, D.; Labra, M.; Peccenini, S.; Peruzzi, L.; Raimondo, F.M. The Italian Loci Classici census. Taxon 2012, 61, 1351–1354. [Google Scholar] [CrossRef]
- Peruzzi, L.; Galasso, G.; Domina, G.; Bartolucci, F.; Santangelo, A.; Alessandrini, A.; Astuti, G.; D’Antraccoli, M.; Roma-Marzio, F.; Ardenghi, N.M.G.; et al. An inventory of the names of native, non-endemic vascular plants described from Italy, their loci classici and types. Phytotaxa 2019, 410, 1–215. [Google Scholar] [CrossRef]
- Brundu, G.; Peruzzi, L.; Domina, G.; Bartolucci, F.; Galasso, G.; Peccenini, S.; Raimondo, F.M.; Albano, A.; Alessandrini, A.; Banfi, E.; et al. At the intersection of cultural and natural heritage: Distribution and conservation of the type localities of the Italian endemic vascular plants. Biol. Conserv. 2017, 214, 109–118. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 4 June 2022).
- Stafleu, F.A.; Cowan, R.S. Taxonomic Literature: A Selective Guide to Botanical Publications and Collections with Dates, Commentaries and Types, 2nd ed.; Bohn, Scheltema & Holkema: Utrecht, The Netherlands, 1983; Volume 4. [Google Scholar]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile; Koeltz Botanical Books: Glashütten, Germany, 2019; p. 159. [Google Scholar] [CrossRef]
- Ibn Tattou, M.; Senecio, L. Flore Pratique du Maroc; Fennane, M., Ibn Tattou, M., El Oualidi, J., Eds.; Institut Scientifique de Rabat: Rabat, Morocco, 2014; Volume 3, pp. 261–268. [Google Scholar]
- Calvo, J.; Aedo, C. Senecio. In Flora Iberica; Benedí, C., Buira, A., Rico, E., Crespo, M.B., Quintanar, A., Aedo, C., Eds.; CSIC: Madrid, Spain, 2019; Volume 16, pp. 1506–1562. ISBN 9788400104603. [Google Scholar]
- Pastena, C.; Anselmo, A.; Zimmardi, M.C. (Eds.) Francesco Cupani, Panphyton Siculum; Regione Siciliana, Assessorato dei Beni Culturali ed Ambientali e Della Pubblica Istruzione, Dipartimento dei Beni Culturali ed Ambientali e Dell’educazione permanente, Biblioteca Centrale Della Regione Siciliana: Palermo, Italy, 2003. [Google Scholar]
- Mineo, C.; Domina, G.; Mazzola, P.; Raimondo, F.M. Studi su antichi erbari siciliani. Inf. Bot. Ital. 2005, 37, 444–445. [Google Scholar]
- Chrtek, J.; Skočdopolová, B. Waldstein’s collection in the herbarium of the National Museum in Prague. Sborn. Nár. Mus. Praze Řada B Přír. Vědy 1982, 38, 201–238. [Google Scholar]
- Boccone, P. Icones & Descriptiones Rariorum Plantarum Siciliae, Melitae, Galliae, & Italiae; E. Theatro Sheldoniano prostand apud Robertum Scott Bibliopolam: London, UK, 1674. [Google Scholar]
- Hind, N.; King, C. 1012 Senecio squalidus Compositae. Curtis’s Bot. Mag. 2022, 39, 113–134. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 8 August 2022).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Harris, S.A. Introduction of Oxford ragwort, Senecio squalidus L. (Asteraceae), to the United Kingdom. Watsonia 2002, 24, 31–43. [Google Scholar] [CrossRef]
- Schluter, D. The Ecology of Adaptive Radiation; Oxford University Press Inc.: New York, NY, USA, 2000; ISBN 0198505221. [Google Scholar]
- Gavrilets, S.; Losos, J.B. Adaptive radiation: Contrasting theory with data. Science 2009, 323, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, B.; Lledó, M.D.; Vargas, P. Adaptive radiation in Mediterranean Cistus (Cistaceae). PLoS ONE 2009, 4, e6362. [Google Scholar] [CrossRef] [PubMed]
- Zickler, D. Senecio (suite). Caryosyst. Cytogén. 1968, 2, 5–8. [Google Scholar]
- Larsen, K.; Laegaard, S. Chromosome studies of the Sicilian flora. Bot. Tidsskr. 1971, 66, 249–268. [Google Scholar]
- Raimondo, F.M.; Garbari, F. Numeri cromosomici per la flora italiana: 199–207. Inf. Bot. Ital. 1975, 7, 366–377. [Google Scholar]
- De Santis, C.; Pavone, P.; Zizza, A. Numeri cromosomici per la flora italiana: 232–237. Inf. Bot. Ital. 1976, 8, 74–81. [Google Scholar]
- Rossitto, M.; Ottonello, D.; Fici, S. Numeri cromosomici per la flora italiana: 993–1002. Inf. Bot. Ital. 1983, 15, 188–194. [Google Scholar]
- Napoli, M.; Zizza, A. Números cromosomáticos de plantas occidentales, 270–279. An. Jard. Bot. Madrid 1985, 40, 451–455. [Google Scholar]
- Kadereit, J.W. Chromosome number reports: LXXXVI. Taxon 1985, 34, 159. [Google Scholar] [CrossRef]
- Löve, A.; Kjellqvist, E. Cytotaxonomy of Spanish plants. IV. Dicotyledons: Caesalpiniaceae—Asteraceae. Lagascalia 1974, 4, 153–211. [Google Scholar]
- Gallego, M.J. Números cromosómicos para la flora española. 290–294. Lagascalia 1983, 12, 131–132. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, G.; Domina, G.; Bartolucci, F.; Galasso, G.; Peruzzi, L. A Nomenclatural and Taxonomic Revision of the Senecio squalidus Group (Asteraceae). Plants 2022, 11, 2597. https://doi.org/10.3390/plants11192597
Barone G, Domina G, Bartolucci F, Galasso G, Peruzzi L. A Nomenclatural and Taxonomic Revision of the Senecio squalidus Group (Asteraceae). Plants. 2022; 11(19):2597. https://doi.org/10.3390/plants11192597
Chicago/Turabian StyleBarone, Giulio, Gianniantonio Domina, Fabrizio Bartolucci, Gabriele Galasso, and Lorenzo Peruzzi. 2022. "A Nomenclatural and Taxonomic Revision of the Senecio squalidus Group (Asteraceae)" Plants 11, no. 19: 2597. https://doi.org/10.3390/plants11192597
APA StyleBarone, G., Domina, G., Bartolucci, F., Galasso, G., & Peruzzi, L. (2022). A Nomenclatural and Taxonomic Revision of the Senecio squalidus Group (Asteraceae). Plants, 11(19), 2597. https://doi.org/10.3390/plants11192597











