6-Paradol Alleviates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Inhibiting AKT/mTOR Axis
Abstract
1. Introduction
1.1. Western Blot
1.2. Statistical Analysis
2. Results
2.1. Prostate Weights and Indices
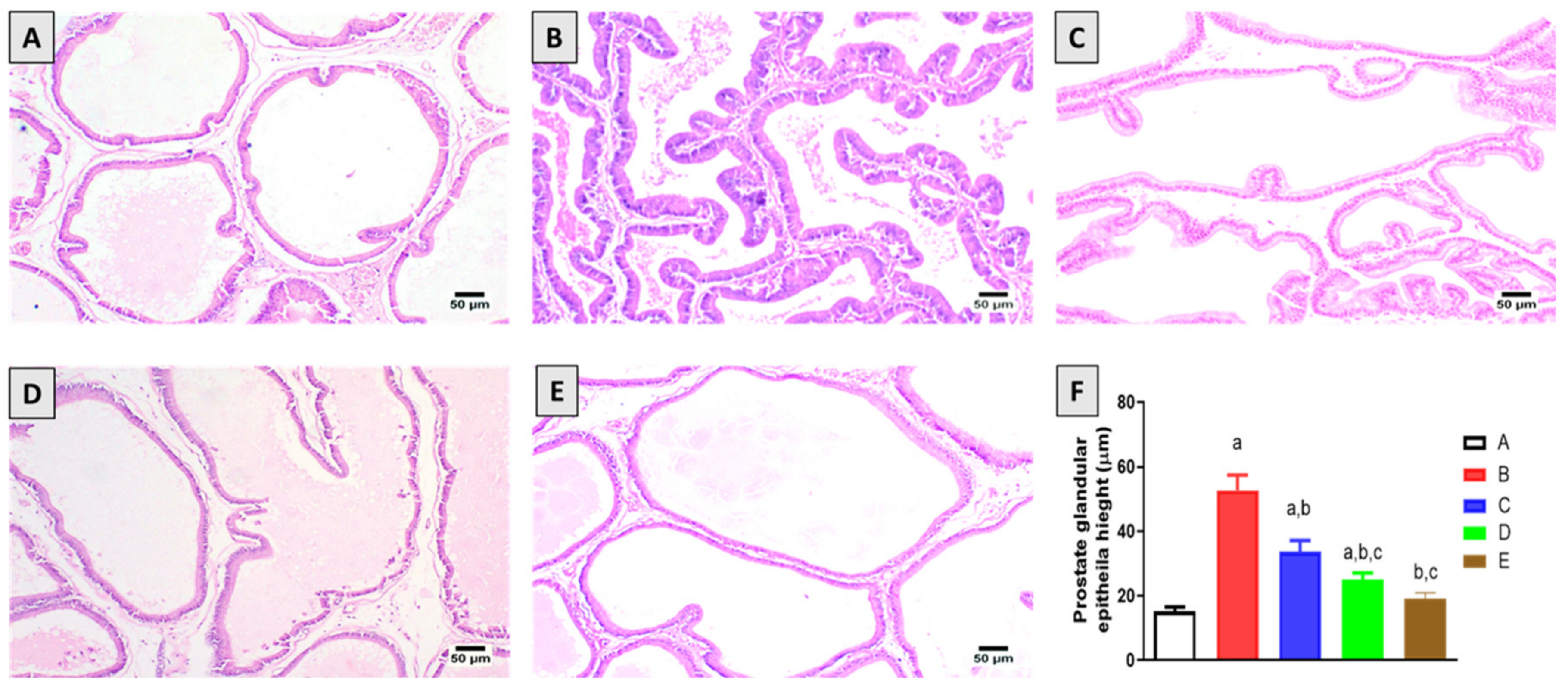
2.2. Histopathological Examination
2.3. Assessment of Cyclin-D1 Expression
2.4. Assessment of Bax and Bcl2 mRNA Expression
2.5. Assessment of Oxidative Stress Markers
2.6. Assessment of Inflammation Markers
2.7. Assessment of p-AKT and p-mTOR Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation of 6-PD
4.3. Animals
4.4. Experimental Design and Animal Treatment
4.5. Histological Examination
4.6. Assessment of Oxidative Stress Biomarkers
4.7. Immunohistochemical Staining
4.8. mRNA Expression of Bax and Bcl-2
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Baradhi, K.M. Benign Prostatic Hyperplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Abrams, P. New Words for Old: Lower Urinary Tract Symptoms for “Prostatism”. BMJ 1994, 308, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Benign Prostatic Hyperplasia: An Overview. Rev. Urol. 2005, 7 (Suppl. 9), S3–S14. [Google Scholar] [PubMed]
- Parsons, J.K. Modifiable Risk Factors for Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: New Approaches to Old Problems. J. Urol. 2007, 178, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.S. Pathology of Benign Prostatic Hyperplasia. Prostate Suppl. 2000, 9, 4–14. [Google Scholar] [CrossRef]
- Eleazu, C.; Eleazu, K.; Kalu, W. Management of Benign Prostatic Hyperplasia: Could Dietary Polyphenols Be an Alternative to Existing Therapies? Front. Pharmacol. 2017, 8, 234. [Google Scholar] [CrossRef]
- Thomas, D.; Chughtai, B.; Kini, M.; Te, A. Emerging Drugs for the Treatment of Benign Prostatic Hyperplasia. Expert Opin. Emerg. Drugs 2017, 22, 201–212. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, M.J.; Sun, H.Y.; Lee, B.; Li, S.; Khandwala, Y.; Del Giudice, F.; Chung, B.I. Efficacy and Safety of 5 Alpha-Reductase Inhibitor Monotherapy in Patients with Benign Prostatic Hyperplasia: A Meta-Analysis. PLoS ONE 2018, 13, e0203479. [Google Scholar] [CrossRef]
- Bapir, R.; Bhatti, K.H.; Eliwa, A.; García-Perdomo, H.A.; Gherabi, N.; Hennessey, D.; Magri, V.; Mourmouris, P.; Ouattara, A.; Perletti, G.; et al. Effect of Alpha-Adrenoceptor Antagonists on Sexual Function. A Systematic Review and Meta-Analysis. Arch. Ital. Urol. Androl. 2022, 94, 252–263. [Google Scholar] [CrossRef]
- Yu, Z.-J.; Yan, H.-L.; Xu, F.-H.; Chao, H.-C.; Deng, L.-H.; Xu, X.-D.; Huang, J.-B.; Zeng, T. Efficacy and Side Effects of Drugs Commonly Used for the Treatment of Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia. Front. Pharmacol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Wohlmuth, H. Phytochemistry and Pharmacology of Plants from the Ginger Family, Zingiberaceae. Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2008. [Google Scholar]
- Karna, P.; Chagani, S.; Gundala, S.R.; Rida, P.C.G.; Asif, G.; Sharma, V.; Gupta, M.V.; Aneja, R. Benefits of Whole Ginger Extract in Prostate Cancer. Br. J. Nutr. 2012, 107, 473–484. [Google Scholar] [CrossRef]
- Liu, C.-M.; Kao, C.-L.; Tseng, Y.-T.; Lo, Y.-C.; Chen, C.-Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 2017, 22, 1477. [Google Scholar] [CrossRef] [PubMed]
- Eid, B.G.; Mosli, H.; Hasan, A.; El-Bassossy, H.M. Ginger Ingredients Alleviate Diabetic Prostatic Complications: Effect on Oxidative Stress and Fibrosis. Available online: https://www.hindawi.com/journals/ecam/2017/6090269/ (accessed on 4 June 2020).
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Fajrin, F.A.; Nugroho, A.E.; Nurrochmad, A.; Susilowati, R. Ginger Extract and Its Compound, 6-Shogaol, Attenuates Painful Diabetic Neuropathy in Mice via Reducing TRPV1 and NMDAR2B Expressions in the Spinal Cord. J. Ethnopharmacol. 2020, 249, 112396. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.Y.; Jung, Y.J.; Surh, Y.J.; Lee, S.S.; Park, K.K. Antioxidative and Antitumor Promoting Effects of [6]-Paradol and Its Homologs. Mutat. Res. 2001, 496, 199–206. [Google Scholar] [CrossRef]
- Ilic, N.M.; Dey, M.; Poulev, A.A.; Logendra, S.; Kuhn, P.E.; Raskin, I. Anti-Inflammatory Activity of Grains of Paradise (Aframomum Melegueta Schum) Extract. J. Agric. Food Chem. 2014, 62, 10452–10457. [Google Scholar] [CrossRef]
- El Dine, R.S.; Elfaky, M.A.; Asfour, H.; El Halawany, A.M. Anti-Adhesive Activity of Aframomum Melegueta Major Phenolics on Lower Respiratory Tract Pathogens. Nat. Prod. Res. 2021, 35, 539–547. [Google Scholar] [CrossRef]
- Gaire, B.P.; Kwon, O.W.; Park, S.H.; Chun, K.-H.; Kim, S.Y.; Shin, D.Y.; Choi, J.W. Neuroprotective Effect of 6-Paradol in Focal Cerebral Ischemia Involves the Attenuation of Neuroinflammatory Responses in Activated Microglia. PLoS ONE 2015, 10, e0120203. [Google Scholar] [CrossRef]
- Sapkota, A.; Park, S.J.; Choi, J.W. Neuroprotective Effects of 6-Shogaol and Its Metabolite, 6-Paradol, in a Mouse Model of Multiple Sclerosis. Biomol. Ther. (Seoul) 2019, 27, 152–159. [Google Scholar] [CrossRef]
- Hattori, H.; Mori, T.; Shibata, T.; Kita, M.; Mitsunaga, T. 6-Paradol Acts as a Potential Anti-Obesity Vanilloid from Grains of Paradise. Mol. Nutr. Food Res. 2021, 65, e2100185. [Google Scholar] [CrossRef]
- Rafeeq, M.; Murad, H.A.S.; Abdallah, H.M.; El-Halawany, A.M. Protective Effect of 6-Paradol in Acetic Acid-Induced Ulcerative Colitis in Rats. BMC Complement. Med. Ther. 2021, 21, 28. [Google Scholar] [CrossRef]
- Suresh, K.; Manoharan, S.; Vijayaanand, M.A.; Sugunadevi, G. Chemopreventive and Antioxidant Efficacy of (6)-Paradol in 7,12-Dimethylbenz(a)Anthracene Induced Hamster Buccal Pouch Carcinogenesis. Pharmacol. Rep. 2010, 62, 1178–1185. [Google Scholar] [CrossRef]
- Lee, E.; Surh, Y.J. Induction of Apoptosis in HL-60 Cells by Pungent Vanilloids, [6]-Gingerol and [6]-Paradol. Cancer Lett. 1998, 134, 163–168. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Chen, P.; He, Z.; Xu, J.; Chen, Y.; Liu, X.; Jiang, J. [6]-Paradol Suppresses Proliferation and Metastases of Pancreatic Cancer by Decreasing EGFR and Inactivating PI3K/AKT Signaling. Cancer Cell Int. 2021, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, W.H.; Hassan, M.; Abdou, R.M.; El-Dine, R.S.; Aboushousha, T.; El-Tanbouly, N.D.; El-Sayed, A.M. 6-Paradol Alleviates Diclofenac-Induced Acute Kidney Injury via Autophagy Enhancement-Mediated by AMPK/AKT/MTOR and NLRP3 Inflammasome Pathways. Environ. Toxicol. Pharmacol. 2022, 91, 103817. [Google Scholar] [CrossRef]
- Shukla, Y.; Prasad, S.; Tripathi, C.; Singh, M.; George, J.; Kalra, N. In Vitro and in Vivo Modulation of Testosterone Mediated Alterations in Apoptosis Related Proteins by [6]-Gingerol. Mol. Nutr. Food Res. 2007, 51, 1492–1502. [Google Scholar] [CrossRef]
- Donnell, R.F. Benign Prostate Hyperplasia: A Review of the Year’s Progress from Bench to Clinic. Curr. Opin. Urol. 2011, 21, 22–26. [Google Scholar] [CrossRef]
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef]
- Pawlicki, B.; Zieliński, H.; Dabrowski, M. Role of apoptosis and chronic prostatitis in the pathogenesis of benign prostatic hyperplasia. Pol. Merkur. Lekarski 2004, 17, 307–310. [Google Scholar]
- Silva, J.; Silva, C.M.; Cruz, F. Current Medical Treatment of Lower Urinary Tract Symptoms/BPH: Do We Have a Standard? Curr. Opin. Urol. 2014, 24, 21–28. [Google Scholar] [CrossRef]
- Traish, A.M.; Hassani, J.; Guay, A.T.; Zitzmann, M.; Hansen, M.L. Adverse Side Effects of 5α-Reductase Inhibitors Therapy: Persistent Diminished Libido and Erectile Dysfunction and Depression in a Subset of Patients. J. Sex. Med. 2011, 8, 872–884. [Google Scholar] [CrossRef]
- Nickel, J.C.; Sander, S.; Moon, T.D. A Meta-Analysis of the Vascular-Related Safety Profile and Efficacy of Alpha-Adrenergic Blockers for Symptoms Related to Benign Prostatic Hyperplasia. Int. J. Clin. Pract. 2008, 62, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.; Kathiresan, S.; Muthusamy, R.; Kathiresan, S. Protective Effects of [6]-Paradol on Histological Lesions and Immunohistochemical Gene Expression in DMBA Induced Hamster Buccal Pouch Carcinogenesis. Asian Pac. J. Cancer Prev. 2013, 14, 3123–3129. [Google Scholar] [CrossRef]
- Vital, P.; Castro, P.; Ittmann, M. Oxidative Stress Promotes Benign Prostatic Hyperplasia. Prostate 2016, 76, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.E.; Esmat, A.; Hassona, M.D.H.; Tadros, M.G.; Abdel-Naim, A.B.; Guns, E.S.T. The Effect of Pomegranate Fruit Extract on Testosterone-Induced BPH in Rats. Prostate 2015, 75, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Y.; Li, L. The Anti-Hyperplasia, Anti-Oxidative and Anti-Inflammatory Properties of Qing Ye Dan and Swertiamarin in Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Toxicol. Lett. 2017, 265, 9–16. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Neamatallah, T.; Eid, B.G.; Esmat, A.; Alamoudi, A.J.; Abd El-Aziz, G.S.; Ashour, O.M. 2-Methoxyestradiol Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats through Inhibition of HIF-1α/TGF-β/Smad2 Axis. Available online: https://www.hindawi.com/journals/omcl/2018/4389484/ (accessed on 4 June 2020).
- Karunasagara, S.; Hong, G.-L.; Jung, D.-Y.; Kim, K.-H.; Cho, K.; Jung, J.-Y. Protective Effects of Combination of Stauntonia Hexaphylla and Cornus Officinalis on Testosterone-Induced Benign Prostatic Hyperplasia through Inhibition of 5α- Reductase Type 2 and Induced Cell Apoptosis. PLoS ONE 2020, 15, e0236879. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from Dried Ginger Inhibits Growth of Prostate Cancer Cells Both in Vitro and in Vivo through Inhibition of STAT3 and NF-ΚB Signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef]
- Kim, M.O.; Lee, M.-H.; Oi, N.; Kim, S.-H.; Bae, K.B.; Huang, Z.; Kim, D.J.; Reddy, K.; Lee, S.-Y.; Park, S.J.; et al. [6]-Shogaol Inhibits Growth and Induces Apoptosis of Non-Small Cell Lung Cancer Cells by Directly Regulating Akt1/2. Carcinogenesis 2014, 35, 683–691. [Google Scholar] [CrossRef]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The Role of Chronic Prostatic Inflammation in the Pathogenesis and Progression of Benign Prostatic Hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef]
- Robert, G.; Descazeaud, A.; Nicolaïew, N.; Terry, S.; Sirab, N.; Vacherot, F.; Maillé, P.; Allory, Y.; de la Taille, A. Inflammation in Benign Prostatic Hyperplasia: A 282 Patients’ Immunohistochemical Analysis. Prostate 2009, 69, 1774–1780. [Google Scholar] [CrossRef]
- Sayed, R.H.; Saad, M.A.; El-Sahar, A.E. Dapoxetine Attenuates Testosterone-Induced Prostatic Hyperplasia in Rats by the Regulation of Inflammatory and Apoptotic Proteins. Toxicol. Appl. Pharmacol. 2016, 311, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Oxidative Stress, Chromatin Remodeling and Gene Transcription in Inflammation and Chronic Lung Diseases. J. Biochem. Mol. Biol. 2003, 36, 95–109. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Nandeesha, H.; Dorairajan, L.N.; Nachiappa Ganesh, R. Over Expression of PI3K-AkT Reduces Apoptosis and Increases Prostate Size in Benign Prostatic Hyperplasia. Aging Male 2020, 23, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, Y.-H.; Peng, Y.-G.; Hu, S.; Lu, Q.; Yang, L.-Y. Effect of PI3K/AKT inhibitor on benign prostate hyperplasia and its mechanism: An experimental study. Zhonghua Nan Ke Xue 2010, 16, 1068–1075. [Google Scholar]
- Caggia, S.; Libra, M.; Malaponte, G.; Cardile, V. Modulation of YY1 and P53 Expression by Transforming Growth Factor-Β3 in Prostate Cell Lines. Cytokine 2011, 56, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-F.; Yu, D.-J.; Jiang, C.-Y.; Wang, X.-J.; Zhu, Y.-P.; Zhao, R.-Z.; Lv, Z.; Sun, X.-W. TRAF6 Regulates Proliferation of Stromal Cells in the Transition and Peripheral Zones of Benign Prostatic Hyperplasia via Akt/MTOR Signaling. Prostate 2018, 78, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; El-Halawany, A.M.; Darwish, K.M.; Algandaby, M.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Koshak, A.E.; Elhady, S.S.; Fadil, S.A.; Alqarni, A.A.; et al. Bio-Guided Isolation of SARS-CoV-2 Main Protease Inhibitors from Medicinal Plants: In Vitro Assay and Molecular Dynamics. Plants 2022, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Parameter | Control | Testosterone (T, 3.0 mg/kg) | T + 6-PD (2.5 mg/kg) | T + 6-PD (5.0 mg/kg) | T + Finasteride (0.5 mg/kg) |
|---|---|---|---|---|---|
| Final body weight (g) | 265 ± 33.1 | 291 ± 37.3 | 271 ± 35.2 | 262 ± 30.4 | 256 ± 32.3 |
| Prostate weight (mg) | 311 ± 26.6 | 830 a ± 55.1 | 530 a,b ± 46.2 | 433 a,b,c ± 47.1 | 370 b,c ± 43.2 |
| Prostate index × 103 | 1.2 ± 0.14 | 2.9 a ± 0.30 | 1.98 b ± 0.28 | 1.67 b ± 0.18 | 1.45 b ± 0.18 |
| Target Gene | Primer Sequence | Gene Bank Accession Number |
|---|---|---|
| Bax | Forward: 5’-CCTGAGCTGACCTTGGAGCA-3’ Reverse: 5’-GGTGGTTGCCCTTTTCTACT-3’ | U32098.1 |
| Bcl-2 | Forward: 5’-TGATAACCGGGAGATCGTGA-3’ Reverse: 5’-AAAGCACATCCAATAAAAAGC-3’ | NM_016993.1 |
| β-actin | Forward: 5’-5′TCCGTCGCCGGTCCACACCC-3’ Reverse: 5’-TCACCAACTGGGACGATATG-3’ | NM_031144.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binmahfouz, L.S.; Almukadi, H.; Alamoudi, A.J.; El-Halawany, A.M.; Abdallah, H.M.; Algandaby, M.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Alghamdi, F.A.; Al-Shaeri, M.; et al. 6-Paradol Alleviates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Inhibiting AKT/mTOR Axis. Plants 2022, 11, 2602. https://doi.org/10.3390/plants11192602
Binmahfouz LS, Almukadi H, Alamoudi AJ, El-Halawany AM, Abdallah HM, Algandaby MM, Mohamed GA, Ibrahim SRM, Alghamdi FA, Al-Shaeri M, et al. 6-Paradol Alleviates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Inhibiting AKT/mTOR Axis. Plants. 2022; 11(19):2602. https://doi.org/10.3390/plants11192602
Chicago/Turabian StyleBinmahfouz, Lenah S., Haifa Almukadi, Abdulmohsin J. Alamoudi, Ali M. El-Halawany, Hossam M. Abdallah, Mardi M. Algandaby, Gamal A. Mohamed, Sabrin R. M. Ibrahim, Faraj A. Alghamdi, Majed Al-Shaeri, and et al. 2022. "6-Paradol Alleviates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Inhibiting AKT/mTOR Axis" Plants 11, no. 19: 2602. https://doi.org/10.3390/plants11192602
APA StyleBinmahfouz, L. S., Almukadi, H., Alamoudi, A. J., El-Halawany, A. M., Abdallah, H. M., Algandaby, M. M., Mohamed, G. A., Ibrahim, S. R. M., Alghamdi, F. A., Al-Shaeri, M., & Abdel-Naim, A. B. (2022). 6-Paradol Alleviates Testosterone-Induced Benign Prostatic Hyperplasia in Rats by Inhibiting AKT/mTOR Axis. Plants, 11(19), 2602. https://doi.org/10.3390/plants11192602










