Mapping of Two Major QTLs Controlling Flowering Time in Brassica napus Using a High-Density Genetic Map
Abstract
1. Introduction
2. Results
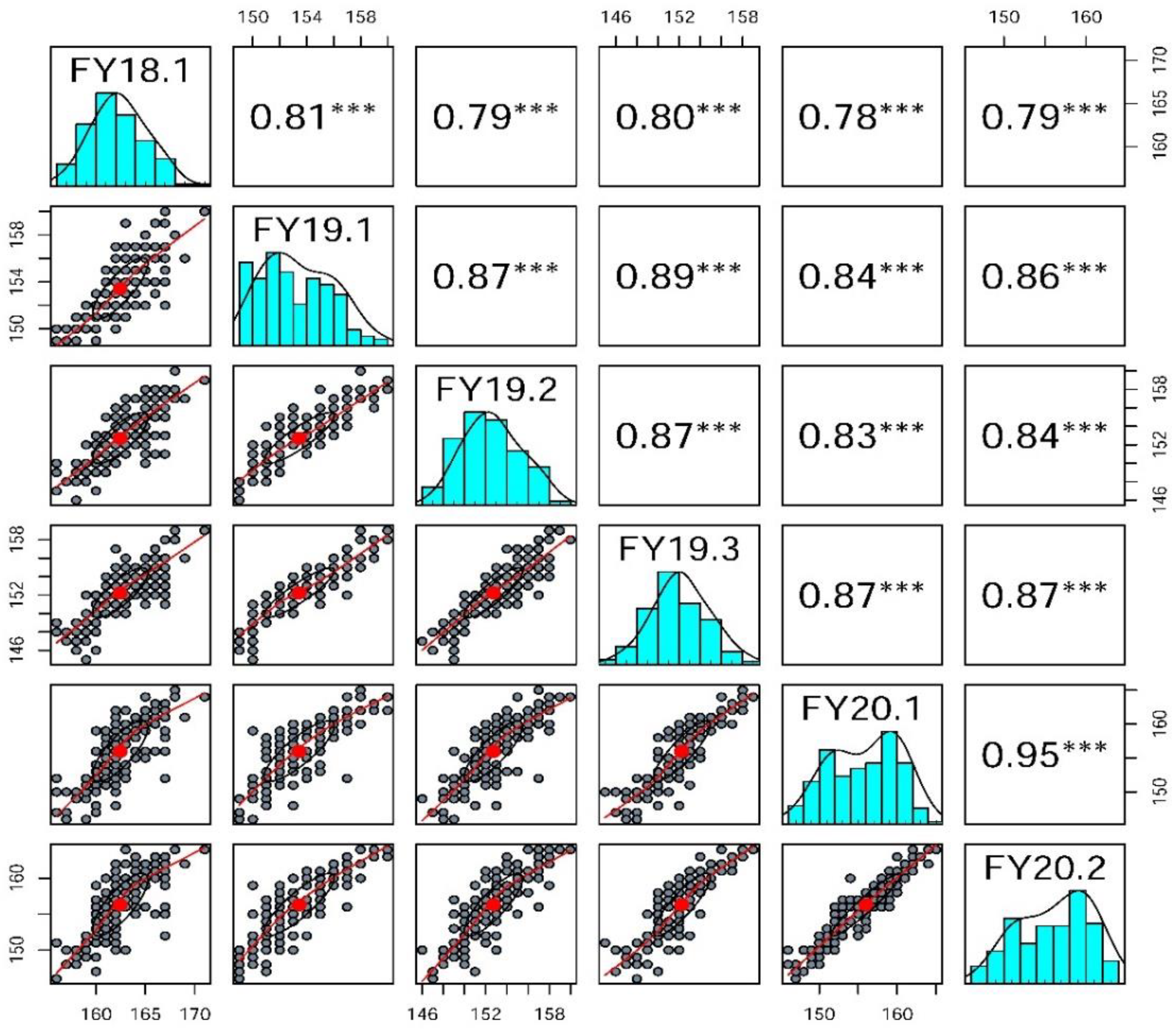
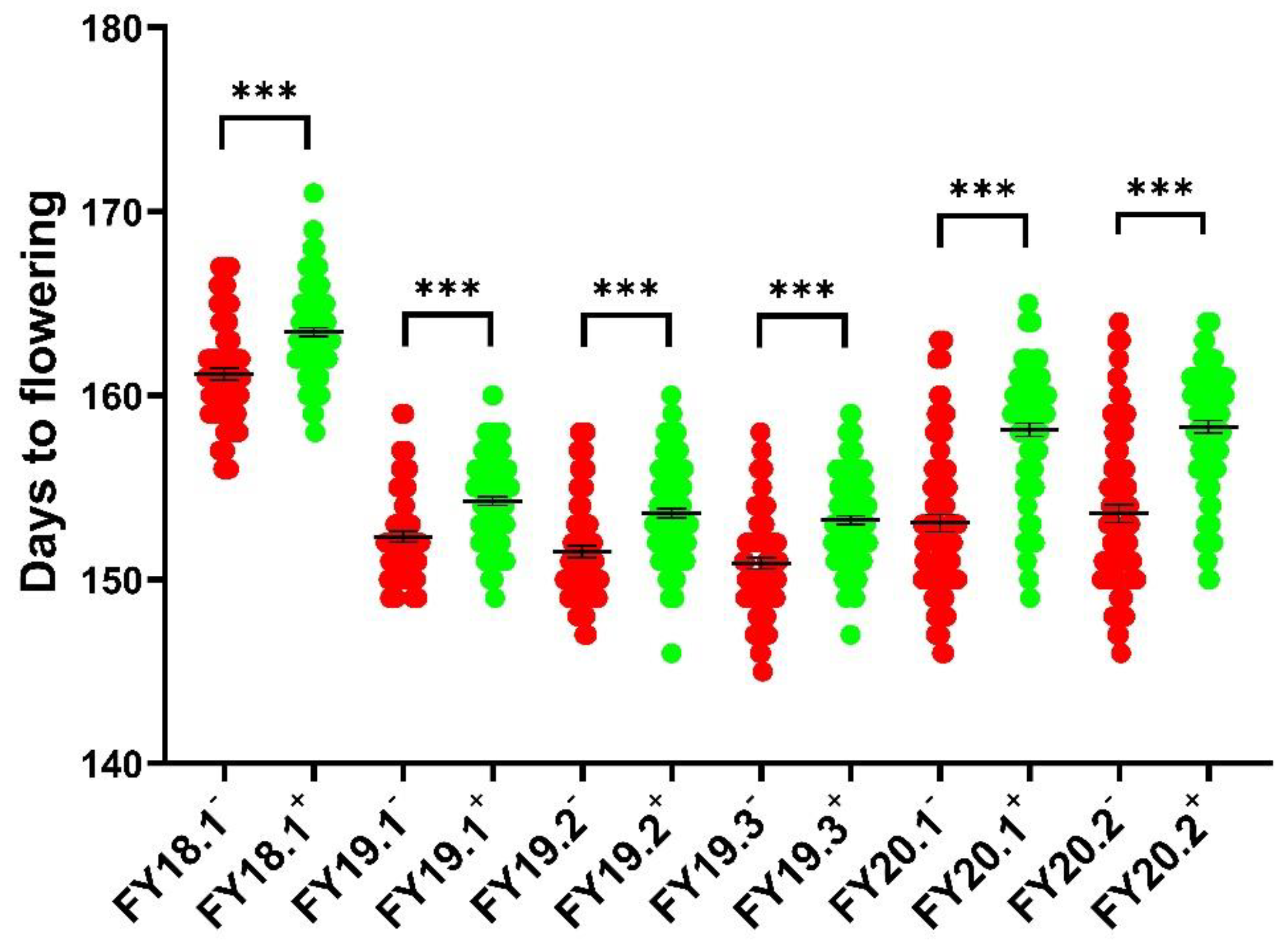
2.1. Phenotyping of Two Parents and the 158A-SGDH Population
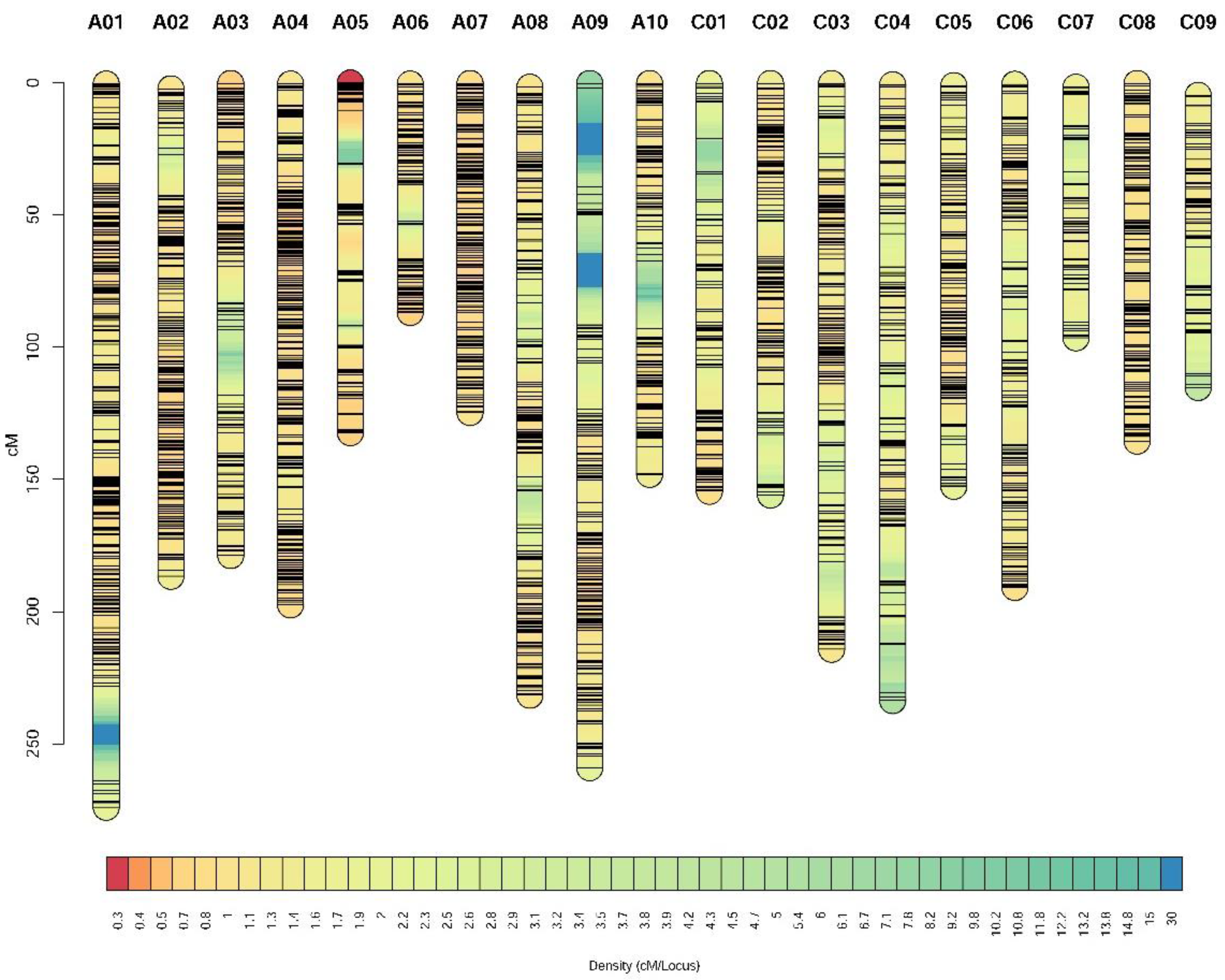
2.2. High-Density Genetic Linkage Map Construction
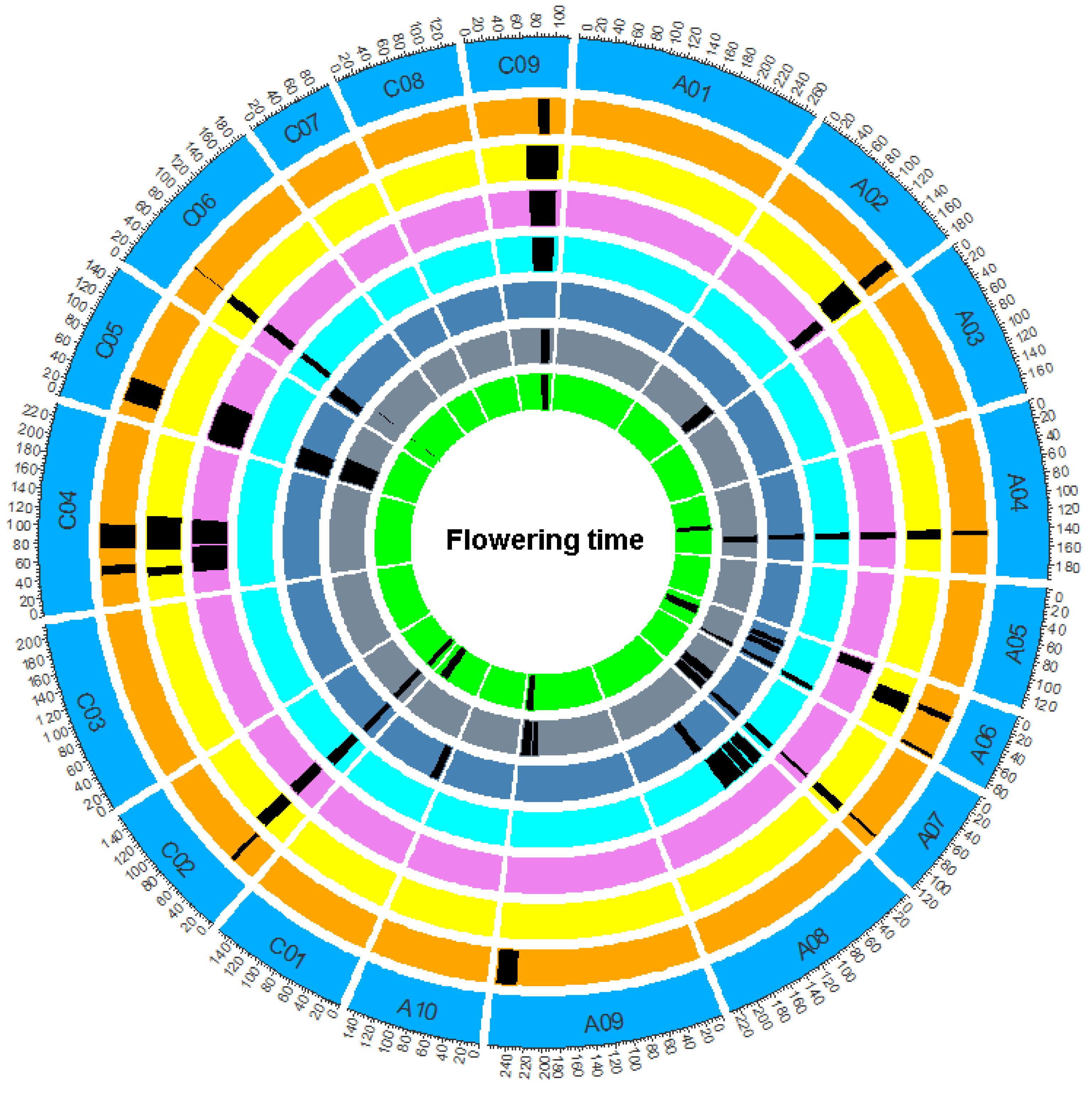
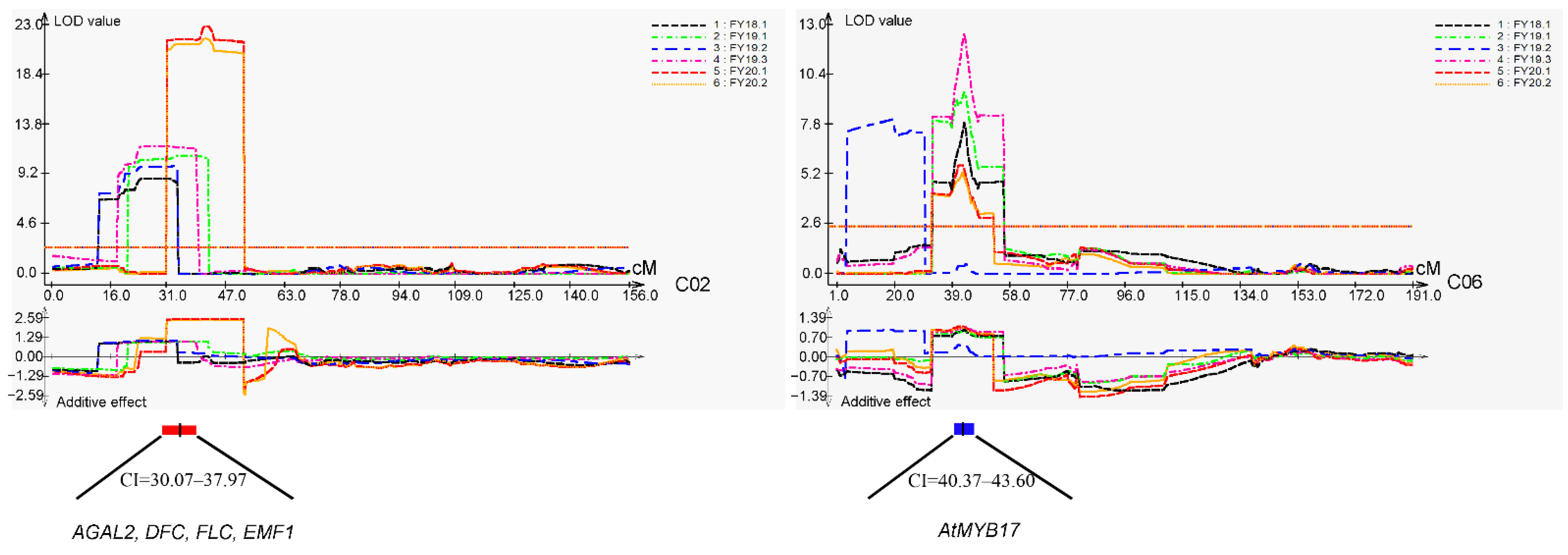
2.3. QTL Mapping for DTF
2.4. Candidate Gene Prediction of the Two Major Flowering time QTL Regions
2.5. InDel Markers Linked to Flowering Time in the Target Intervals
3. Discussion
3.1. Importance of Flowering Time in the Crops
3.2. Construction of the High-Density Genetic Map for the 158A-SGDH Population
3.3. Analysis of Two Major Flowering Time QTLs in the 158A-SGDH Population
3.4. Analysis of Candidate Genes Associated with the Two Major Flowering Time QTLs
4. Materials and Methods
4.1. Plant Materials
4.2. Field Experiment and Phenotypic Measurements
4.3. Genome Sequencing and Genotyping
4.4. Linkage Map Construction and QTL Mapping
4.5. Candidate Gene Analysis within Target Intervals
4.6. Development of InDel Markers in the Candidate Regions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shrestha, R.; Gomez-Ariza, J.; Brambilla, V.; Fornara, F. Molecular control of seasonal flowering in rice, arabidopsis and temperate cereals. Ann. Bot. 2014, 114, 1445–1458. [Google Scholar] [CrossRef]
- Bouché, F.; Lobet, G.; Tocquin, P.; Périlleux, C. FLOR-ID: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2016, 44, D1167–D1171. [Google Scholar] [CrossRef]
- Koornneef, M.; Alonso-Blanco, C.; Vreugdenhil, D. Naturally occurring genetic variation in Arabidopsis thaliana. Annu. Rev. Plant Biol. 2004, 55, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Blumel, M.; Dally, N.; Jung, C. Flowering time regulation in crops-what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015, 32, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, S. Regulation and Subfunctionalization of Flowering Time Genes in the Allotetraploid Oil Crop Brassica napus. Front. Plant Sci. 2020, 11, 605155. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Gomez, N.V.; Agosti, M.B.; Miralles, D.J. Global trends of rapeseed grain yield stability and rapeseed-to-wheat yield ratio in the last four decades. Eur. J. Agron. 2012, 37, 56–65. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Wu, X.M.; Bhat, S.R. History, evolution, and domestication of Brassica crops. Plant Breed. Rev. 2011, 35, 19–84. [Google Scholar]
- Hou, J.N.; Long, Y.; Raman, H.; Zou, X.X.; Wang, J.; Dai, S.T.; Xiao, Q.Q.; Li, C.; Fan, L.J.; Liu, B.; et al. A Tourist-like MITE insertion in the upstream region of the BnFLC.A10 gene is associated with vernalization requirement in rapeseed (Brassica napus L.). BMC Plant Biol. 2012, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, W.; Li, D.; Chao, H.; Zhao, X.; Ta, N.; Li, Y.; Guan, Z.; Guo, L.; Zhang, L.; et al. Genetic dissection of the mechanism of flowering time based on an environmentally stable and specific QTL in Brassica napus. Plant Sci. 2018, 277, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Tadege, M.; Sheldon, C.C.; Helliwell, C.A.; Stoutjesdijk, P.; Dennis, E.S.; Peacock, W.J. Control of flowering time by FLC orthologues in Brassica napus. Plant J. 2001, 28, 545–553. [Google Scholar] [CrossRef]
- Zou, X.X.; Suppanz, I.; Raman, H.; Hou, J.N.; Wang, J.; Long, Y.; Jung, C.; Meng, J.L. Comparative Analysis of FLC Homologues in Brassicaceae Provides Insight into Their Role in the Evolution of Oilseed Rape. PLoS ONE 2012, 7, e45751. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Wan, M.; Guo, C.; Wang, B.; Li, H.; Li, G.; Tian, Y.; Ge, X.; King, G.J.; Liu, K.; et al. Transposon insertions within alleles of BnaFLC.A10 and BnaFLC.A2 are associated with seasonal crop type in rapeseed. J. Exp. Bot. 2020, 71, 4729–4741. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Shi, J.; Qiu, D.; Li, R.; Zhang, C.; Wang, J.; Hou, J.; Zhao, J.; Shi, L.; Park, B.S.; et al. Flowering time quantitative trait loci analysis of oilseed Brassica in multiple environments and genomewide alignment with Arabidopsis. Genetics 2007, 177, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, Y.; Wu, B.D.; Liu, J.; Jiang, C.C.; Shi, L.; Zhao, J.W.; King, G.J.; Meng, J.L. The evolution of Brassica napus FLOWERING LOCUS T paralogues in the context of inverted chromosomal duplication blocks. BMC Evol. Biol. 2009, 9. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Eckermann, P.; Coombes, N.; Manoli, S.; Zou, X.; Edwards, D.; Meng, J.; Prangnell, R.; Stiller, J.; et al. Genetic and physical mapping of flowering time loci in canola (Brassica napus L.). Theor. Appl. Genet. 2013, 126, 119–132. [Google Scholar] [CrossRef]
- Chen, L.; Dong, F.; Cai, J.; Xin, Q.; Fang, C.; Liu, L.; Wan, L.; Yang, G.; Hong, D. A 2.833-kb Insertion in BnFLC.A2 and Its Homeologous Exchange with BnFLC.C2 during Breeding Selection Generated Early-Flowering Rapeseed. Mol. Plant 2018, 11, 222–225. [Google Scholar] [CrossRef]
- Tudor, E.H.; Jones, D.M.; He, Z.; Bancroft, I.; Trick, M.; Wells, R.; Irwin, J.A.; Dean, C. QTL-seq identifies BnaFT.A02 and BnaFLC.A02 as candidates for variation in vernalization requirement and response in winter oilseed rape (Brassica napus). Plant Biotechnol. J. 2020, 18, 2466–2481. [Google Scholar] [CrossRef]
- Song, J.; Li, B.; Cui, Y.; Zhuo, C.; Gu, Y.; Hu, K.; Wen, J.; Yi, B.; Shen, J.; Ma, C.; et al. QTL Mapping and Diurnal Transcriptome Analysis Identify Candidate Genes Regulating Brassica napus Flowering Time. Int. J. Mol. Sci. 2021, 22, 7559. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, B.; Ma, N.; Liu, X.; Qin, M.; Zhang, Y.; Wang, K.; Guo, N.; Zuo, K.; Liu, X.; et al. Quantitative Trait Locus Mapping and Identification of Candidate Genes Controlling Flowering Time in Brassica napus L. Front. Plant Sci. 2020, 11, 626205. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, Z.; Wang, P.; Song, Y.; Ahmad, A.; Dong, F.; Hong, D.; Yang, G. Heterosis Derived From Nonadditive Effects of the BnFLC Homologs Coordinates Early Flowering and High Yield in Rapeseed (Brassica napus L.). Front. Plant Sci. 2021, 12, 798371. [Google Scholar] [CrossRef] [PubMed]
- Raman, H.; Raman, R.; Coombes, N.; Song, J.; Prangnell, R.; Bandaranayake, C.; Tahira, R.; Sundaramoorthi, V.; Killian, A.; Meng, J.; et al. Genome-wide association analyses reveal complex genetic architecture underlying natural variation for flowering time in canola. Plant Cell Environ. 2016, 39, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, K.; Zhang, Z.; Guan, C.; Chen, S.; Hua, W.; Li, J.; Wen, J.; Yi, B.; Shen, J.; et al. Genome-wide association study reveals the genetic architecture of flowering time in rapeseed (Brassica napus L.). DNA Res. 2016, 23, 43–52. [Google Scholar] [CrossRef]
- Lu, K.; Wei, L.; Li, X.; Wang, Y.; Wu, J.; Liu, M.; Zhang, C.; Chen, Z.; Xiao, Z.; Jian, H.; et al. Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat. Commun. 2019, 10, 1154. [Google Scholar] [CrossRef]
- Wu, D.; Liang, Z.; Yan, T.; Xu, Y.; Xuan, L.; Tang, J.; Zhou, G.; Lohwasser, U.; Hua, S.; Wang, H.; et al. Whole-Genome Resequencing of a Worldwide Collection of Rapeseed Accessions Reveals the Genetic Basis of Ecotype Divergence. Mol. Plant 2019, 12, 30–43. [Google Scholar] [CrossRef]
- Helal, M.; Gill, R.A.; Tang, M.; Yang, L.; Hu, M.; Yang, L.; Xie, M.; Zhao, C.; Cheng, X.; Zhang, Y.; et al. SNP- and Haplotype-Based GWAS of Flowering-Related Traits in Brassica napus. Plants 2021, 10, 2475. [Google Scholar] [CrossRef]
- Huang, L.; Min, Y.; Schiessl, S.; Xiong, X.; Jan, H.U.; He, X.; Qian, W.; Guan, C.; Snowdon, R.J.; Hua, W.; et al. Integrative analysis of GWAS and transcriptome to reveal novel loci regulation flowering time in semi-winter rapeseed. Plant Sci. 2021, 310, 110980. [Google Scholar] [CrossRef]
- Vollrath, P.; Chawla, H.S.; Schiessl, S.V.; Gabur, I.; Lee, H.; Snowdon, R.J.; Obermeier, C. A novel deletion in FLOWERING LOCUS T modulates flowering time in winter oilseed rape. Theor. Appl. Genet. 2021, 134, 1217–1231. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Q.; Qian, Q.; Zhao, Q.; Wang, L.; Wang, A.; Guan, J.; Fan, D.; Weng, Q.; Huang, T.; et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009, 19, 1068–1076. [Google Scholar] [CrossRef]
- Ke, L.; Lei, W.; Yang, W.; Wang, J.; Gao, J.; Cheng, J.; Sun, Y.; Fan, Z.; Yu, D. Genome-wide identification of cold responsive transcription factors in Brassica napus L. BMC Plant Biol. 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Muller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Kondra, Z. A genetic study of growth characters and yield characters of oilseed rape. Euphytica 1978, 27, 177–183. [Google Scholar] [CrossRef]
- Amiri-Oghan, H.; Fotokian, M.; Javidfar, F.; Alizadeh, B. Genetic analysis of grain yield, days to flowering and maturity in oilseed rape (Brassica napus L.) using diallel crosses. Int. J. Plant Prod. 2009, 3, 19–26. [Google Scholar]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef]
- Krieger, U.; Lippman, Z.B.; Zamir, D. The flowering gene SINGLE FLOWER TRUSS drives heterosis for yield in tomato. Nat. Genet. 2010, 42, 459–463. [Google Scholar] [CrossRef]
- Endo-Higashi, N.; Izawa, T. Flowering time genes Heading date 1 and Early heading date 1 together control panicle development in rice. Plant Cell Physiol. 2011, 52, 1083–1094. [Google Scholar] [CrossRef]
- Park, S.J.; Jiang, K.; Tal, L.; Yichie, Y.; Gar, O.; Zamir, D.; Eshed, Y.; Lippman, Z.B. Optimization of crop productivity in tomato using induced mutations in the florigen pathway. Nat. Genet. 2014, 46, 1337–1342. [Google Scholar] [CrossRef]
- Guo, Y.; Hans, H.; Christian, J.; Molina, C. Mutations in single FT- and TFL1-paralogs of rapeseed (Brassica napus L.) and their impact on flowering time and yield components. Front. Plant Sci. 2014, 5, 282. [Google Scholar] [CrossRef]
- Jian, H.; Zhang, A.; Ma, J.; Wang, T.; Yang, B.; Shuang, L.S.; Liu, M.; Li, J.; Xu, X.; Paterson, A.H.; et al. Joint QTL mapping and transcriptome sequencing analysis reveal candidate flowering time genes in Brassica napus L. BMC Genom. 2019, 20, 21. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and phylogeny of the Brassicaceae (Cruciferae): An overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Chrost, B.; Kolukisaoglu, U.; Schulz, B.; Krupinska, K. An alpha-galactosidase with an essential function during leaf development. Planta 2007, 225, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Swiezewski, S.; Crevillen, P.; Liu, F.; Ecker, J.R.; Jerzmanowski, A.; Dean, C. Small RNA-mediated chromatin silencing directed to the 3’ region of the Arabidopsis gene encoding the developmental regulator, FLC. Proc. Natl. Acad. Sci. USA 2007, 104, 3633–3638. [Google Scholar] [CrossRef] [PubMed]
- Michaels, S.D.; Amasino, R.M. Flowering Locus C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, C.C.; Burn, J.E.; Perez, P.P.; Metzger, J.; Edwards, J.A.; Peacock, W.J.; Dennis, E.S. The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 1999, 11, 445–458. [Google Scholar] [CrossRef]
- Helliwell, C.A.; Wood, C.C.; Robertson, M.; Peacock, W.J.; Dennis, E.S. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006, 46, 183–192. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Yuan, W.; Schmitz, R.J.; He, Y. Photoperiodic control of the floral transition through a distinct polycomb repressive complex. Dev. Cell 2014, 28, 727–736. [Google Scholar] [CrossRef]
- Searle, I.; He, Y.H.; Turck, F.; Vincent, C.; Fornara, F.; Krober, S.; Amasino, R.A.; Coupland, G. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Gene Dev. 2006, 20, 898–912. [Google Scholar] [CrossRef]
- Simon, R.; Igeno, M.I.; Coupland, G. Activation of floral meristem identity genes in Arabidopsis. Nature 1996, 384, 59–62. [Google Scholar] [CrossRef]
- Wagner, D.; Sablowski, R.W.; Meyerowitz, E.M. Transcriptional activation of APETALA1 by LEAFY. Science 1999, 285, 582–584. [Google Scholar] [CrossRef]
- Sessions, A.; Yanofsky, M.F.; Weigel, D. Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science 2000, 289, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, G.; Qu, L.J.; Gu, H. Characterization of Arabidopsis MYB transcription factor gene AtMYB17 and its possible regulation by LEAFY and AGL15. J. Genet. Genom. 2009, 36, 99–107. [Google Scholar] [CrossRef]
- Pastore, J.J.; Limpuangthip, A.; Yamaguchi, N.; Wu, M.F.; Sang, Y.; Han, S.K.; Malaspina, L.; Chavdaroff, N.; Yamaguchi, A.; Wagner, D. LATE MERISTEM IDENTITY2 acts together with LEAFY to activate APETALA1. Development 2011, 138, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Merk, H.L.; Yarnes, S.C.; Van Deynze, A.; Tong, N.; Menda, N.; Mueller, L.A.; Mutschler, M.A.; Loewen, S.A.; Myers, J.R.; Francis, D.M. Trait Diversity and Potential for Selection Indices Based on Variation Among Regionally Adapted Processing Tomato Germplasm. J. Amer. Soc. Hortic. Sci. 2012, 137, 427–437. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull 1987, 19, 11–15. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The high-quality genome of Brassica napus cultivar ’ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef]
- Song, J.M.; Guan, Z.; Hu, J.; Guo, C.; Yang, Z.; Wang, S.; Liu, D.; Wang, B.; Lu, S.; Zhou, R.; et al. Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nat. Plants 2020, 6, 34–45. [Google Scholar] [CrossRef]
- Wu, Y.; Bhat, P.R.; Close, T.J.; Lonardi, S. Efficient and accurate construction of genetic linkage maps from the minimum spanning tree of a graph. PLoS Genet. 2008, 4, e1000212. [Google Scholar] [CrossRef]
- Kosambi, D.D. The estimation of map distances from recombination values. Ann. Eugen. 1943, 12, 172–175. [Google Scholar] [CrossRef]
- Zeng, Z.B. Precision mapping of quantitative trait loci. Genetics 1994, 136, 1457–1468. [Google Scholar] [CrossRef]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef]
- Arcade, A.; Labourdette, A.; Falque, M.; Mangin, B.; Chardon, F.; Charcosset, A.; Joets, J. BioMercator: Integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics 2004, 20, 2324–2326. [Google Scholar] [CrossRef]
- Maccaferri, M.; Sanguineti, M.C.; Corneti, S.; Ortega, J.L.; Salem, M.B.; Bort, J.; DeAmbrogio, E.; del Moral, L.F.; Demontis, A.; El-Ahmed, A.; et al. Quantitative trait loci for grain yield and adaptation of durum wheat (Triticum durum Desf.) across a wide range of water availability. Genetics 2008, 178, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, L.; Wei, L.; Yu, P.; Wang, J.; Zhao, H.; Zhang, Y.; Zhang, S.; Yang, Z.; Chen, G.; et al. BnTIR: An online transcriptome platform for exploring RNA-seq libraries for oil crop Brassica napus. Plant Biotechnol. J. 2021, 19, 1895–1897. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, C.; Wei, L.; Wang, S.; Yin, F.; Liu, D.; Guo, L.; Zhou, Y.; Yang, Q.Y. BnVIR: Bridging the genotype-phenotype gap to accelerate mining of candidate variations underlying agronomic traits in Brassica napus. Mol. Plant 2022, 15, 779–782. [Google Scholar] [CrossRef] [PubMed]

| Environment | Parents | 158A-SGDH Lines | ||||
|---|---|---|---|---|---|---|
| 158A a | SGDH284 a | DH Lines Range | Mean ± SEM | Skewness | Kurtosis | |
| FY18.1 | 159.33 ± 0.88 | 166.67 ± 0.67 *** | 156–171 | 162.44 ± 0.21 | 0.14 | −0.17 |
| FY19.1 | 149 ± 0.00 | 157 ± 0.58 *** | 149–160 | 153.44 ± 0.20 | 0.30 | −0.77 |
| FY19.2 | 149 ± 0.00 | 157 ± 0.58 *** | 146–160 | 152.73 ± 0.21 | 0.18 | −0.46 |
| FY19.3 | 149 ± 0.00 | 157 ± 0.58 *** | 145–159 | 152.25 ± 0.20 | 0.04 | −0.04 |
| FY20.1 | 149.33 ± 0.88 | 162.33 ± 0.33 *** | 146–165 | 155.99 ± 0.33 | −0.21 | −0.97 |
| FY20.2 | 149.33 ± 0.88 | 162.33 ± 0.33 *** | 146–164 | 156.31 ± 0.33 | −0.27 | −0.92 |
| QTL | Chr | Environment | CI (cM) | Peak | LOD | PVE (%) a | Add b |
|---|---|---|---|---|---|---|---|
| cqDTF-A02 | A02 | FY19.3/20.1/20.2 | 160.25–175.34 | 167.8 | 2.51–4.11 | 2.77–4.24 | −0.56~−0.98 |
| cqDTF-A04 | A04 | FY19.1/19.2/ 19.3/20.1/20.2 | 140.64–147.53 | 144.08 | 4.45–5.60 | 5.16–7.38 | 0.63~1.16 |
| cqDTF-A06-1 | A06 | FY18.1/19.2/ 20.1 | 28.11–38.09 | 33.1 | 3.49–5.91 | 4.53–7.63 | 0.60~1.16 |
| cqDTF-A06-2 | A06 | FY19.1/ 19.2/19.3 | 81.03–86.89 | 84.43 | 2.74–6.70 | 3.61–8.28 | 0.54~0.80 |
| cqDTF-A07 | A07 | FY19.1/19.2/19.3/ 20.1/20.2 | 106.28–111.41 | 108.85 | 3.93–8.23 | 4.07–9.57 | 0.55~1.37 |
| cqDTF-A09 | A09 | FY18.1/19.3 | 230.58–258.24 | 233.95 | 2.8–3.03 | 3.02–3.93 | 0.48~0.57 |
| cqDTF-C02 | C02 | FY18.1/19.1/19.2/ 19.3/20.1/20.2 | 30.07–37.97 | 34.02 | 8.86–22.92 | 13.54–32.04 | 1.06~2.59 |
| cqDTF-C04-1 | C04 | FY20.1/20.2 | 39.16–53.01 | 46.09 | 2.7–2.94 | 2.70–3.17 | 1.06~1.11 |
| cqDTF-C04-2 | C04 | FY20.1/20.2 | 72.88–104.75 | 88.82 | 2.97–3.03 | 3.1–3.28 | 1.11~1.12 |
| cqDTF-C05 | C05 | FY19.2/19.3//20.2 | 17.1–49.34 | 33.22 | 2.53–2.85 | 2.74–3.74 | 0.46~0.73 |
| cqDTF-C06 | C06 | FY18.1/19.1/ 19.3/20.1/20.2 | 40.37–43.60 | 41.98 | 5.31–12.6 | 6.07–16.13 | 0.93~1.12 |
| cqDTF-C09 | C09 | FY18.1/19.1/ 19.3/20.1/20.2 | 78.83–94.84 | 86.84 | 3.04–4.47 | 3.13–6.45 | 0.58~0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Lei, W.; He, W.; Wang, Y.; Tian, J.; Gong, J.; Hao, B.; Cheng, X.; Shu, Y.; Fan, Z. Mapping of Two Major QTLs Controlling Flowering Time in Brassica napus Using a High-Density Genetic Map. Plants 2022, 11, 2635. https://doi.org/10.3390/plants11192635
Chen L, Lei W, He W, Wang Y, Tian J, Gong J, Hao B, Cheng X, Shu Y, Fan Z. Mapping of Two Major QTLs Controlling Flowering Time in Brassica napus Using a High-Density Genetic Map. Plants. 2022; 11(19):2635. https://doi.org/10.3390/plants11192635
Chicago/Turabian StyleChen, Lei, Weixia Lei, Wangfei He, Yifan Wang, Jie Tian, Jihui Gong, Bing Hao, Xinxin Cheng, Yingjie Shu, and Zhixiong Fan. 2022. "Mapping of Two Major QTLs Controlling Flowering Time in Brassica napus Using a High-Density Genetic Map" Plants 11, no. 19: 2635. https://doi.org/10.3390/plants11192635
APA StyleChen, L., Lei, W., He, W., Wang, Y., Tian, J., Gong, J., Hao, B., Cheng, X., Shu, Y., & Fan, Z. (2022). Mapping of Two Major QTLs Controlling Flowering Time in Brassica napus Using a High-Density Genetic Map. Plants, 11(19), 2635. https://doi.org/10.3390/plants11192635







