Organic Amendments Effects on Nutrient Uptake, Secondary Metabolites, and Antioxidant Properties of Melastoma malabathricum L.
Abstract
:1. Introduction
2. Results
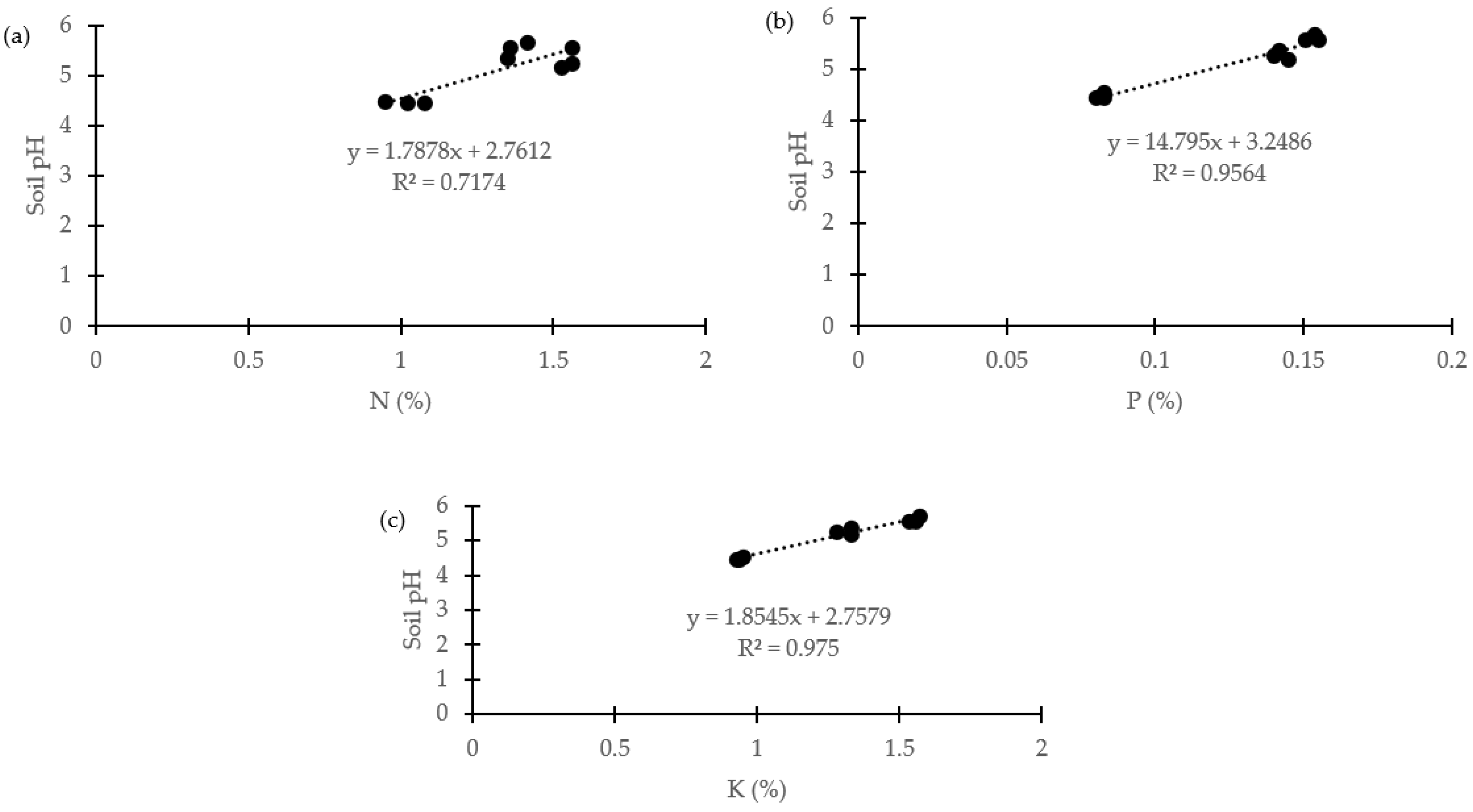
2.1. Soil Analysis and Mineral Content
2.2. Phytochemical Screening
2.3. Determination of Total Chlorophyll and Carotenoid Content
2.4. Determination of Total Anthocyanin, Flavonoid and Phenolic Contents
2.5. Antioxidant Potential of M. malabathricum L. methanolic Extracts
2.6. Significant Pearson’s Correlation between Measured Parameters
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Sample Preparation
4.2. Soil Analysis
4.3. Phytochemical Screening of Bioactive Compounds in M. malabathricum L.
4.4. Determination of Total Chlorophyll and Carotenoid Content
4.5. Measurement of Total Anthocyanin Content
MW (Molecular weight of cyanidin − 3 − glucoside) = 449.2 g/mol
DF = dilution factor
ε = 26, 900
4.6. Measurement of Total Phenolic Content
4.7. Measurement of Total Flavonoid Content
4.8. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Activity Assay
4.9. ABTS (2,2-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid)) Radical Scavenging Activity Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Liu, H.-T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef]
- Drinkwater, L.E.; Letourneau, D.K.; Workneh, F.; van Bruggen, A.H.C.; Shennan, C. Fundamental Differences between Conventional and Organic Tomato Agroecosystems in California. Ecol. Appl. 1995, 5, 1098–1112. [Google Scholar] [CrossRef]
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.H.; Monaco, F.D.; Antmann, E.; Chorath, P. Sustainable approaches for minimizing biosolids production and maximizing reuse options in sludge management: A review. J. Environ. Manag. 2015, 158, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Curea, C. Sustainable Societies and Municipal Solid Waste Management in Southeast Asia. In Sustainable Asia; World Scientific: Singapore, 2017; pp. 391–415. [Google Scholar]
- Dang, Q.; Wang, Y.; Xiong, S.; Yu, H.; Zhao, X.; Tan, W.; Cui, D.; Xi, B. Untangling the response of fungal community structure, composition and function in soil aggregate fractions to food waste compost addition. Sci. Total Environ. 2021, 769, 145248. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.; Ramadass, K.; Vinu, A.; Kirkham, M.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef] [PubMed]
- Van Fan, Y.; Lee, C.T.; Klemeš, J.J.; Chua, L.S.; Sarmidi, M.R.; Leow, C.W. Evaluation of Effective Microorganisms on home scale organic waste composting. J. Environ. Manag. 2018, 216, 41–48. [Google Scholar] [CrossRef]
- Hansen, V.; Hauggaard-Nielsen, H.; Petersen, C.T.; Mikkelsen, T.N.; Müller-Stöver, D. Effects of gasification biochar on plant-available water capacity and plant growth in two contrasting soil types. Soil Tillage Res. 2016, 161, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 789–813. [Google Scholar] [CrossRef] [Green Version]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Impact of Biochar Organic and Inorganic Carbon on Soil CO2 and N2O Emissions. J. Environ. Qual. 2017, 46, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herath, H.; Arbestain, M.C.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209–210, 188–197. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Sun, Y.; Wu, D.; Meng, J.; Chen, W. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. Catena 2019, 173, 481–493. [Google Scholar] [CrossRef]
- Doan, T.T.; Henry-Des-Tureaux, T.; Rumpel, C.; Janeau, J.-L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef]
- Liew, R.K.; Nam, W.L.; Chong, M.Y.; Phang, X.Y.; Su, M.H.; Yek, P.N.Y.; Ma, N.L.; Cheng, C.K.; Chong, C.T.; Lam, S.S. Oil palm waste: An abundant and promising feedstock for microwave pyrolysis conversion into good quality biochar with potential multi-applications. Process. Saf. Environ. Prot. 2018, 115, 57–69. [Google Scholar] [CrossRef]
- Abdullah, R.; Osman, N.; Yusoff, S.; Yusof, H.M.; Halim, N.A.; Rosli, N.M. Effects of Palm Kernel Biochar and Food Waste Compost on the Growth of Palm Lily (Cordyline Fruticosa), Coleus (Coleus Sp.), And Boat Lily (Rhoeo Discolor). Appl. Ecol. Environ. Res. 2021, 19, 205–218. [Google Scholar] [CrossRef]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in Southeast Asia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef]
- Guo, X.; Han, W.; Zhang, G.; Yang, Y.; Wei, Z.; He, Q.; Wu, Q. Effect of inorganic and organic amendments on maize biomass, heavy metals uptake and their availability in calcareous and acidic washed soil. Environ. Technol. Innov. 2020, 19, 101038. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How Do Crop Plants Tolerate Acid Soils? Mechanisms of Aluminum Tolerance and Phosphorous Efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Y.; Chen, Q.; Li, Y.; Guo, D.; Nie, X.; Peng, X. Assessment of heavy metal pollution and the effect on bacterial community in acidic and neutral soils. Ecol. Indic. 2020, 117, 106626. [Google Scholar] [CrossRef]
- Cifu, M.; Xiaonan, L.; Zhihong, C.; Zhengyi, H.; Wanzhu, M. Long-term effects of lime application on soil acidity and crop yields on a red soil in Central Zhejiang. Plant Soil 2004, 265, 101–109. [Google Scholar] [CrossRef]
- Ishikawa, S.; Wagatsuma, T.; Sasaki, R.; Ofei-Manu, P. Comparison of the amount of citric and malic acids in Al media of seven plant species and two cultivars each in five plant species. Soil Sci. Plant Nutr. 2000, 46, 751–758. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.; Verstraten, J.M. Mobility of Fe(II), Fe(III) and Al in acidic forest soils mediated by dissolved organic matter: Influence of solution pH and metal/organic carbon ratios. Geoderma 2003, 113, 323–340. [Google Scholar] [CrossRef]
- Kinraide, T.B. Toxicity factors in acidic forest soils: Attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Eur. J. Soil Sci. 2003, 54, 323–333. [Google Scholar] [CrossRef]
- Wong, W. Melastoma malabathricum: Too Beautiful to Be Called a Weed; Green Culture: Singapore, 2008; pp. 1–7. [Google Scholar]
- Joffry, S.M.; Yob, N.J.; Rofiee, M.S.; Affandi, M.M.R.M.M.; Suhaili, Z.; Othman, F.; Akim, A.M.; Desa, M.N.M.; Zakaria, Z.A. Melastoma malabathricum (L.) Smith Ethnomedicinal Uses, Chemical Constituents, and Pharmacological Properties: A Review. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Dévéhat, F.L.-L.; Bakhtiar, A.; Bézivin, C.; Amoros, M.; Boustie, J. Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia 2002, 73, 400–405. [Google Scholar] [CrossRef]
- Zakaria, Z.; Nor, R.R.M.; Kumar, G.H.; Ghani, Z.A.; Sulaiman, M.; Devi, G.R.; Jais, A.M.; Somchit, N.; Fatimah, C. Antinociceptive, anti-inflammatory and antipyretic properties of Melastoma malabathricum leaves aqueous extract in experimental animals. Can. J. Physiol. Pharmacol. 2006, 84, 1291–1299. [Google Scholar] [CrossRef]
- Anuar, N.; Adnan, A.F.M.; Saat, N.; Aziz, N.; Taha, R.M. Optimization of Extraction Parameters by Using Response Surface Methodology, Purification, and Identification of Anthocyanin Pigments in Melastoma malabathricum Fruit. Sci. World J. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.K.; Kumar, A. Evaluation of Total Phenol, Flavonoid and in vitro Antioxidant Activity of Methanolic Extract of Leaves of Melastoma malabathricum Linn. Asian J. Chem. 2011, 23, 434–438. [Google Scholar]
- Watanabe, T.; Jansen, S.; Osaki, M. Al–Fe interactions and growth enhancement in Melastoma malabathricum and Miscanthus sinensis dominating acid sulphate soils. Plant Cell Environ. 2006, 29, 2124–2132. [Google Scholar] [CrossRef]
- Maejima, E.; Osaki, M.; Wagatsuma, T.; Watanabe, T. Contribution of constitutive characteristics of lipids and phenolics in roots of tree species in Myrtales to aluminum tolerance. Physiol. Plant. 2016, 160, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimee, H.; Normaniza, O. Physiological Responses of Melastoma malabathricum at Different Slope Orientations. J. Trop. Plant Physiol. 2014, 6, 10–22. [Google Scholar]
- Saifuddin, M.; Osman, N.; Idris, R.M.; Halim, A. The effects of pre-aluminum treatment on morphology and physiology of potential acidic slope plants. Kuwait J. Sci. 2016, 43, 199–220. [Google Scholar]
- Serrano, E. Banana Soil Acidification in the Caribbean Coast of Costa Rica and its Relationship with Increased Aluminium Concentrations. In Proceedings of the Banana Root System: Towards a Better Understanding for its Productive Management: Proceedings of an International Symposium/Sistema Radical del Banano: Hacia un Mejor Conocimiento para su Manejo Productivo: Memorias de un Simposio Internacional; Inibap: San Jose, Costa Rica, 2005; p. 144. [Google Scholar]
- Osaki, M.; Watanabe, T.; Tadano, T. Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci. Plant Nutr. 1997, 43, 551–563. [Google Scholar] [CrossRef]
- Watanabe, T.; Osaki, M.; Yoshihara, T.; Tadano, T. Distribution and chemical speciation of aluminum in the Al accumulator plant, Melastoma malabathricum L. Plant Soil 1998, 201, 165–173. [Google Scholar] [CrossRef]
- Ch’Ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving Phosphorus Availability, Nutrient Uptake and Dry Matter Production of Zea Mays L. on a Tropical Acid Soil Using Poultry Manure Biochar and Pineapple Leaves Compost. Exp. Agric. 2016, 52, 447–465. [Google Scholar] [CrossRef]
- Wong, M.T.F.; Nortcliff, S.; Swift, R.S. Method for determining the acid ameliorating capacity of plant residue compost, urban waste compost, farmyard manure, and peat applied to tropical soils. Commun. Soil Sci. Plant Anal. 1998, 29, 2927–2937. [Google Scholar] [CrossRef]
- Hue, N.V.; Craddock, G.R.; Adams, F. Effect of Organic Acids on Aluminum Toxicity in Subsoils. Soil Sci. Soc. Am. J. 1986, 50, 28–34. [Google Scholar] [CrossRef]
- Ch’Ng, H.Y.; Haruna, A.O.; Majid, N.M.N.A.; Jalloh, M.B. Improving soil phosphorus availability and yield of Zea mays L. using biochar and compost derived from agro-industrial wastes. Ital. J. Agron. 2019, 14, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Hwang, T.; Neculita, C.M. In Situ Immobilization of Heavy Metals in Severely Weathered Tailings Amended with Food Waste-Based Compost and Zeolite. Water Air Soil Pollut. 2013, 224, 1–9. [Google Scholar] [CrossRef]
- Jahnke, R.A.; Craven, D.B. Quantifying the role of heterotrophic bacteria in the carbon cycle: A need for respiration rate measurements1. Limnol. Oceanogr. 1995, 40, 436–441. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Paulin, B.; O’Malley, P. Compost Production and Use in Horticulture. 2008. Available online: https://researchlibrary.agric.wa.gov.au/cgi/viewcontent.cgi?article=1197&context=bulletins (accessed on 1 November 2021).
- Sierra, J.; Fontaine, S.; Desfontaines, L. Factors controlling N mineralization, nitrification, and nitrogen losses in an Oxisol amended with sewage sludge. Soil Res. 2001, 39, 519–534. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Engelhard, M. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Mkhabela, M.; Warman, P. The influence of municipal solid waste compost on yield, soil phosphorus availability and uptake by two vegetable crops grown in a Pugwash sandy loam soil in Nova Scotia. Agric. Ecosyst. Environ. 2005, 106, 57–67. [Google Scholar] [CrossRef]
- Iyamuremye, F.; Dick, R. Organic Amendments and Phosphorus Sorption by Soils. Adv. Agron. 1996, 139–185. [Google Scholar]
- Ajeng, A.A.; Abdullah, R.; Kadir, W.R.A.; Suki, N.I.A.; Sa’adah, N. Growth of Maize and Residual Nutrients in Soil Treated with Palm Kernel Shell Biochar. Growth 2021, 52, 2979–2994. [Google Scholar]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Piccolo, A.; Pietramellara, G.; Mbagwu, J. Use of humic substances as soil conditioners to increase aggregate stability. Geoderma 1997, 75, 267–277. [Google Scholar] [CrossRef]
- Whalen, J.K.; Willms, W.D.; Dormaar, J.F. Soil Carbon, Nitrogen and Phosphorus in Modified Rangeland Communities. J. Range Manag. 2003, 56, 665. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Ochoa, M.V.; Hinojosa, M.B.; Muñoz, B.G. Improved soil quality after 16 years of olive mill pomace application in olive oil groves. Agron. Sustain. Dev. 2012, 32, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Smider, B.; Singh, B. Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Berek, A.K.; Hue, N.V. Characterization of Biochars and Their Use as an Amendment to Acid Soils. Soil Sci. 2016, 181, 412–426. [Google Scholar] [CrossRef]
- Hass, A.; Gonzalez, J.M.; Lima, I.M.; Godwin, H.W.; Halvorson, J.J.; Boyer, D.G. Chicken Manure Biochar as Liming and Nutrient Source for Acid Appalachian Soil. J. Environ. Qual. 2012, 41, 1096–1106. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.; Silva, L.; Six, J.; Parikh, S.J. Use of Chemical and Physical Characteristics to Investigate Trends in Biochar Feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Berek, A.K.; Hue, N.V.; Radovich, T.J.K.; Ahmad, A.A. Biochars Improve Nutrient Phyto-Availability of Hawai’i’s Highly Weathered Soils. Agronomy 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of Nutrient-Enriched Biochar as a Soil Amendment during Maize Growth: Exploring Practical Alternatives to Recycle Agricultural Residuals and to Reduce Chemical Fertilizer Demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef] [Green Version]
- Sigua, G.; Novak, J.; Watts, D.; Johnson, M.; Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Ruttens, A.; Mench, M.; Colpaert, J.; Boisson, J.; Carleer, R.; Vangronsveld, J. Phytostabilization of a metal contaminated sandy soil. I: Influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environ. Pollut. 2006, 144, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Till. Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Roy, R.; Romi, I.J.; Hasan, M.; Bhuiyan, M.K.H.; Khan, M.M.H. Phytochemical screening, antioxidant and antibacterial activity of some medicinal plants grown in Sylhet region. IOSR J. Pharm. Biol. Sci. 2019, 14, 26–37. [Google Scholar]
- Danladi, S.; Wan-Azemin, A.; Sani, Y.N.; Mohd, K.S.; Us, M.; Mansor, S.M.; Dharmaraj, S. Phytochemical screening, antioxidant potential and cytotoxic activity of Melastoma malabathricum Linn. from different locations. Int. J. Pharm. Pharm. Sci. 2015, 7, 408–413. [Google Scholar]
- Danladi, S.; Wan-Azemin, A.; Sani, Y.N.; Mohd, K.S.; Us, M.R.; Mansor, S.M.; Dharmaraj, S. Phytochemical Screening, Total Phenolic and Total Flavonoid Content, and Antioxidant Activity of Different Parts of Melastoma malabathricum. J. Teknol. 2015, 77, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Sari, N.M.; Kuspradini, H.; Amirta, R.; Kusuma, I.W. Antioxidant activity of an invasive plant, Melastoma malabathricum and its potential as herbal tea product. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 144, p. 12029. [Google Scholar]
- Neagoe, A.; Ebenå, G.; Carlsson, E. The effect of soil amendments on plant performance in an area affected by acid mine drainage. Geochemistry 2005, 65, 115–129. [Google Scholar] [CrossRef]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Marôco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Yusof, Z.; Ramasamy, S.; Mahmood, N.Z.; Yaacob, J.S. Vermicompost Supplementation Improves the Stability of Bioactive Anthocyanin and Phenolic Compounds in Clinacanthus nutans Lindau. Molecules 2018, 23, 1345. [Google Scholar] [CrossRef] [Green Version]
- Namdeo, A. Plant cell elicitation for production of secondary metabolites: A review. Pharm. Rev. 2007, 1, 69–79. [Google Scholar]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and Biotic Elicitors–Role in Secondary Metabolites Production through In Vitro Culture of Medicinal Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2016; pp. 247–277. [Google Scholar]
- Chan, L.K.; Koay, S.S.; Boey, P.L.; Bhatt, A. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biol. Res. 2010, 43, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Lakhdar, A.; Falleh, H.; Ouni, Y.; Oueslati, S.; Debez, A.; Ksouri, R.; Abdelly, C. Municipal solid waste compost application improves productivity, polyphenol content, and antioxidant capacity of Mesembryanthemum edule. J. Hazard. Mater. 2011, 191, 373–379. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Chakrabarti, K.; Chakraborty, A.; Nayak, D.; Tripathy, S.; Powell, M. Municipal waste compost as an alternative to cattle manure for supplying potassium to lowland rice. Chemosphere 2007, 66, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Singvilay, O.; Shin, W.; Kim, E.; Chung, J.; Sa, T. Effects of Long-Term Compost and Fertilizer Application on Soil Phosphorus Status Under Paddy Cropping System. Commun. Soil Sci. Plant Anal. 2004, 35, 1635–1644. [Google Scholar] [CrossRef]
- Ashokhan, S.; Ramasamy, S.; Karsani, S.A.; Othman, R.; Yaacob, J.S. Analysis of bioactive pigments in coloured callus of Azadirachta indica for possible use as functional natural colourants. Pigment Resin Technol. 2019, 48, 9–19. [Google Scholar] [CrossRef]
- Shariff, A.H.M.; Miller, H. Site fertility and its influence on the stocking of dipterocarp species in the tropical rain forest of peninsular Malaysia. J. Trop. For. Sci. 1992, Mar 1. 189–201. [Google Scholar]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Leelamanie, D.A.L.; Liyanage, T.D.P.; Rajarathna, I.M.L.V. A comparison of weight loss and c analysis methods in determining organic matter content in Sri Lankan soils. Trop. Agric. Res. Ext. 2015, 18, 117. [Google Scholar] [CrossRef]
- Bremmer, J.; Mulvaney, C. Nutrient Total. Methods Soil Anal. 1982, 595–642. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publication: Oxford, UK, 1974; p. 565. [Google Scholar]
- Solihah, M.; Rosli, W.W.; Nurhanan, A. Phytochemicals screening and total phenolic content of Malaysian Zea mays hair extracts. Int. Food Res. J. 2012, 19, 1533–1538. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent Author links open overlay panel. Met. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]


| Treatments | Total N | Available P | Exchangeable Cation | |||
|---|---|---|---|---|---|---|
| K | Ca | Mg | Na | |||
| % | mg/kg | meq/100g | ||||
| T1 | 3.33 × 10−2 ± 0.003 b | 2.500 ± 0.500 c | 15.3 × 10−2 ± 0.013 c | 1.287 ± 0.090 b | 28.7 × 10−2 ± 0.027 a | 3.03 × 10−2 ± 0.000 a |
| T2 | 11.5 × 10−2 ± 0.005 a | 79.500 ± 0.500 b | 107 × 10−2 ± 0.130 b | 1.370 ± 0.100 b | 21.3 × 10−2 ± 0.015 a | 3.5 × 10−2 ± 0.005 a |
| T3 | 12.3 × 10−2 ± 0.009 a | 190.500 ± 1.500 a | 170 × 10−2 ± 0.151 a | 2.280 ± 0.142 a | 27.7 × 10−2 ± 0.020 a | 4.0 × 10−2 ± 0.006 a |
| Treatments | Micronutrient | |||||
| Zn | Fe | Mn | Cu | Cd | Al | |
| mg/kg | ||||||
| T1 | 2.653 ± 0.018 a | 191.270 ± 5.047 a | 2.653 ± 0.034 a | 31.090 ± 0.517 a | 79.3 × 10−2 ± 0.054 a | 2.1 × 10−2 ± 0.001 a |
| T2 | 1.927 ± 0.070 c | 77.227 ± 1.998 c | 1.783 ± 0.107 c | 4.273 ± 0.069 b | 72.3 × 10−2 ± 0.049 a | 2.0 × 10−2 ± 0.000 a |
| T3 | 2.140 ± 0.047 b | 133.747 ± 2.139 b | 2.340 ± 0.050 b | 4.613 ± 0.178 b | 67.0 × 10−2 ± 0.072 a | 1.9 × 10−2 ± 0.000 a |
| Phytochemical Screening | Leaves | Stem | Roots | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| Alkaloids I | - | - | - | - | - | - | - | - | - |
| Alkaloids II | + | + | + | - | - | - | - | - | - |
| Alkaloids III | - | - | - | - | - | - | - | - | - |
| Flavonoids I Flavonoid II | ++ | ++ | ++ | + | + | + | + | ++ | ++ |
| +++ | +++ | +++ | + | + | ++ | + | ++ | ++ | |
| Phenol | ++ | ++ | ++ | + | + | + | + | + | + |
| Phlobatannins | - | - | - | - | - | - | - | - | - |
| Saponins | - | - | - | - | - | - | - | - | - |
| Tannins | ++ | ++ | ++ | - | - | - | + | + | + |
| Ca (µg g−1 DW) | Cb (µg g−1 DW) | Ca + Cb (µg g−1 DW) | Ca/Cb Ratio | C(x+c) (µg g−1 DW) | Ca + Cb/C(x+c) Ratio | |
|---|---|---|---|---|---|---|
| T1 | 182.623 ± 14.475 b | 90.628 ± 5.298 b | 273.252 ± 19.720 c | 2.010 ± 0.049 b | 149.212 ± 12.748 b | 1.836 ±0.028 a |
| T2 | 261.837 ± 6.519 a | 95.008 ± 2.069 b | 356.845 ± 8.576 b | 2.756 ± 0.011 a | 224.698 ± 5.66 a | 1.588 ± 0.006 b |
| T3 | 289.441 ± 8.881 a | 144.237 ± 4.585 a | 433.678 ± 13.224 a | 2.007 ± 0.025 b | 237.733 ± 7.224 a | 1.824 ± 0.014 a |
| TAC (mg/g DW) | TPC (mg GAE/g DE) | TFC (mg QE/g DE) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Stem | Roots | Leaves | Stem | Roots | Leaves | Stem | Roots | |
| T1 | 10.2 × 10−2 ± 0.036 b | 1.5 × 10−2 ± 0.005 a | 2.0 × 10−2 ± 0.006 a | 9505.160 ± 182.057 a | 2174.517 ± 27.789 b | 3606.996 ± 9.396 b | 1088.224 ± 31.536 b | 183.353 ± 1.264 b | 175.845 ± 4.395 c |
| T2 | 26.5 × 10−2 ± 0.038 a | 6.1 × 10−2 ± 0.024 a | 6.5 × 10−2 ± 0.034 a | 9933.322 ± 30.217 a | 2267.808 ± 58.939 b | 5419.291 ± 36.121 a | 1524.796 ± 38.125 a | 183.572 ± 0.922 b | 643.268 ± 5.946 a |
| T3 | 36.1 × 10−2 ± 0.034 a | 3.7 × 10−2 ± 0.015 a | 8.9 × 10−2 ± 0.020 a | 9857.329 ± 49.172 a | 4930.956 ± 16.025 a | 5396.671 ± 8.200 a | 1464.902 ± 16.032 a | 209.984 ± 0.572 a | 232.944 ± 2.511 b |
| ABTS | DPPH | |||||
|---|---|---|---|---|---|---|
| IC50 (mg/mL) | IC50 (mg/mL) | |||||
| Leaves | Stem | Roots | Leaves | Stem | Roots | |
| T1 | 46.4 × 10−2 ± 0.057 a | 450.7 × 10−2 ± 0.733 a | 133.5 × 10−2 ± 0.036 a | 22.3 × 10−2 ± 0.008 a | 376.9 × 10−2 ± 0.014 a | 299.9 × 10−2 ± 0.008 a |
| T2 | 29.3 × 10−2 ± 0.027 b | 16.5 × 10−2 ± 0.002 b | 102.3 × 10−2 ± 0.061 b | 16.3 × 10−2 ± 0.002 b | 73.4 × 10−2 ± 0.039 c | 76.3 × 10−2 ± 0.035 b |
| T3 | 27.9 × 10−2 ± 0.020 b | 19.7 × 10−2 ± 0.003 b | 14.8 × 10−2 ± 0.000 c | 13.1 × 10−2 ± 0.001 c | 276.6 × 10−2 ± 0.182 b | 49.1 × 10−2 ± 0.032 c |
| pH | N | P | K | Ca | Mg | Na | Zn | Fe | Mn | Cu | Cd | Ca | Cb | Ca + Cb | Car | TAC | TPC | TFC | DPPH | ABTS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | ||||||||||||||||||||
| N | 0.929 ** | 1 | |||||||||||||||||||
| P | 0.938 ** | 0.887 * | 1 | ||||||||||||||||||
| K | 0.944 ** | 0.953 ** | 0.950 * | 1 | |||||||||||||||||
| Ca | 0.787 * | 0.802 * | 0.943 * | 0.903 ** | 1 | ||||||||||||||||
| Mg | −0.253 | −0.40 | 0.115 | −0.176 | 0.307 | 1 | |||||||||||||||
| Na | 0.530 | 0.675 | 0.516 | 0.762 * | 0.434 | −0.249 | 1 | ||||||||||||||
| Zn | −0.802 ** | −0.862 ** | −0.628 | −0.707 * | −0.459 | 0.490 | −0.297 | 1 | |||||||||||||
| Fe | −0.666 | −0.814 * | −0.405 | −0.614 | −0.177 | 0.731 * | −0.353 | 0.939 ** | 1 | ||||||||||||
| Mn | −0.515 | −0.655 | −0.233 | −0.427 | −0.115 | 0.575 | −0.144 | 0.914 ** | 0.935 ** | 1 | |||||||||||
| Cu | −0.940 ** | −0.975 ** | −0.805 | −0.907 ** | −0.619 | 0.446 | −0.549 | 0.938 ** | 0.868 ** | 0.751 * | 1 | ||||||||||
| Cd | −0.547 | −0.489 | −0.952 ** | −0.490 | −0.351 | 0.415 | −0.335 | 0.326 | 0.322 | 0.197 | 0.460 | 1 | |||||||||
| Ca | 0.960 ** | 0.902 ** | 0.891 * | 0.899 ** | 0.771 * | −0.343 | 0.494 | −0.794 * | −0.691 * | −0.556 | −0.912 ** | −0.643 | 1 | ||||||||
| Cb | 0.778 * | 0.649 | 0.905 * | 0.805 * | 0.935 ** | 0.167 | 0.460 | −0.290 | −0.084 | 0.072 | −0.533 | −0.577 | 0.763 * | 1 | |||||||
| Ca + Cb | 0.950 ** | 0.851 ** | 0.968 ** | 0.904 ** | 0.862 ** | −0.177 | 0.502 | −0.657 | −0.510 | −0.360 | −0.827 ** | −0.657 | 0.972 ** | 0.894 ** | 1 | ||||||
| Car | 0.945 ** | 0.914 ** | 0.829 * | 0.883 ** | 0.720 * | −0.412 | 0.486 | −0.843 ** | −0.762 * | −0.639 | −0.934 ** | −0.622 | 0.994 ** | 0.687 * | 0.940 ** | 1 | |||||
| TAC | 0.869 ** | 0.879 ** | 0.912 * | 0.903 ** | 0.798 * | −0.107 | 0.548 | −0.717 * | −0.573 | −0.424 | −0.843 ** | −0.215 | 0.803 ** | 0.669 * | 0.801 ** | 0.792 * | 1 | ||||
| TPC | 0.675 * | 0.767 * | 0.548 | 0.616 | 0.340 | −0.60 | 0.350 | −0.748 * | −0.733 * | −0.670 * | −0.739 * | −0.490 | 0.702 * | 0.360 | 0.618 | 0.727 * | 0.442 | 1 | |||
| TFC | 0.883 ** | 0.939 ** | 0.657 | 0.848 ** | 0.576 | −0.524 | 0.514 | −0.936 ** | −0.902 ** | −0.816 ** | −0.967 ** | −0.533 | 0.921 ** | 0.469 | 0.810 ** | 0.956 ** | 0.739 * | 0.749 * | 1 | ||
| DPPH | −0.979 ** | −0.948 ** | −0.943 ** | −0.970 ** | −0.817 * | 0.240 | −0.622 | 0.759 * | 0.628 | 0.486 | 0.915 ** | 0.562 | −0.954 ** | −0.802 ** | −0.954 ** | −0.935 ** | −0.840 ** | −0.734 * | −0.867 ** | 1 | |
| ABTS | −0.822 ** | −0.785 * | −0.583 | −0.779 * | −0.628 | 0.398 | −0.464 | 0.745 * | 0.713 * | 0.585 | 0.836 ** | 0.582 | −0.909 ** | −0.575 | −0.840 ** | −0.925 ** | −0.743 * | −0.460 | −0.896 ** | 0.780 * | 1 |
| Properties | Soil | PK Biochar | FW Compost |
|---|---|---|---|
| pH | 3.90 | 8.61 | 6.60 |
| EC (dS/M) | 0.10 | 3.67 | 2.84 |
| Texture | Sandy loam | - | - |
| Total OC (%) | 3.97 | 43.41 | 14.34 |
| N (%) | 0.06 | 0.5 | 2.39 |
| Available P (mg/kg) | 0.29 | 0.15 | 2.82 |
| K (meq/100 g) | 0.11 | 0.74 | 0.21 |
| Ca (meq/100 g) | 1.20 | 2.27 | 0.76 |
| Mg (meq/100 g) | 0.26 | 0.25 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusli, L.S.; Abdullah, R.; Yaacob, J.S.; Osman, N. Organic Amendments Effects on Nutrient Uptake, Secondary Metabolites, and Antioxidant Properties of Melastoma malabathricum L. Plants 2022, 11, 153. https://doi.org/10.3390/plants11020153
Rusli LS, Abdullah R, Yaacob JS, Osman N. Organic Amendments Effects on Nutrient Uptake, Secondary Metabolites, and Antioxidant Properties of Melastoma malabathricum L. Plants. 2022; 11(2):153. https://doi.org/10.3390/plants11020153
Chicago/Turabian StyleRusli, Lili Syahani, Rosazlin Abdullah, Jamilah Syafawati Yaacob, and Normaniza Osman. 2022. "Organic Amendments Effects on Nutrient Uptake, Secondary Metabolites, and Antioxidant Properties of Melastoma malabathricum L." Plants 11, no. 2: 153. https://doi.org/10.3390/plants11020153
APA StyleRusli, L. S., Abdullah, R., Yaacob, J. S., & Osman, N. (2022). Organic Amendments Effects on Nutrient Uptake, Secondary Metabolites, and Antioxidant Properties of Melastoma malabathricum L. Plants, 11(2), 153. https://doi.org/10.3390/plants11020153







