Digalloyl Glycoside: A Potential Inhibitor of Trypanosomal PFK from Euphorbia abyssinica J.F. Gmel
Abstract
:1. Introduction
2. Results
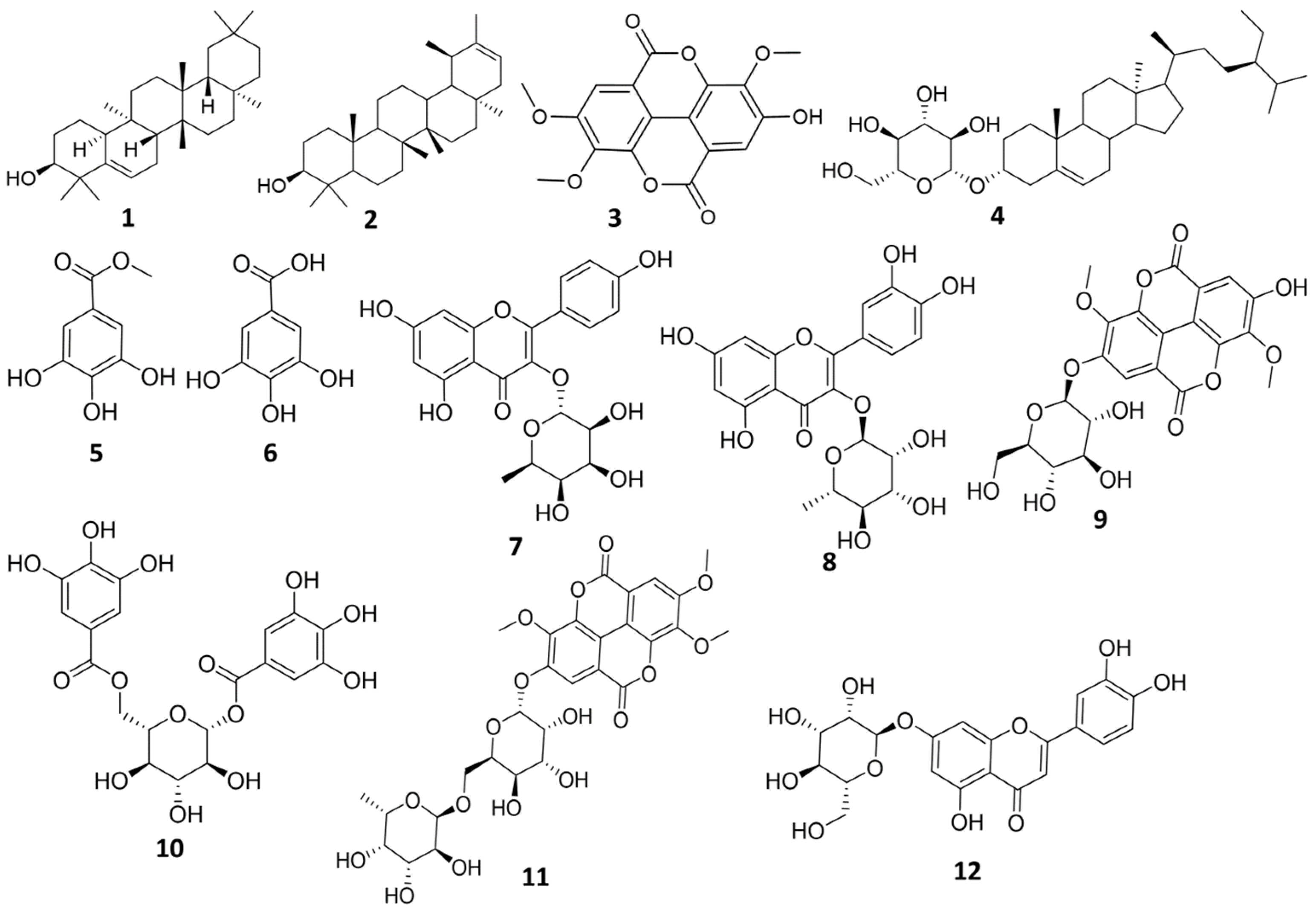
2.1. Investigation of Methylene Chloride Fraction of E. abyssinica J.F. Gmel
2.2. Investigation of Ethyl Acetate Fraction of E. abyssinica J.F. Gmel
2.3. Investigation of N-Butanol Fraction of E. abyssinica J.F. Gmel
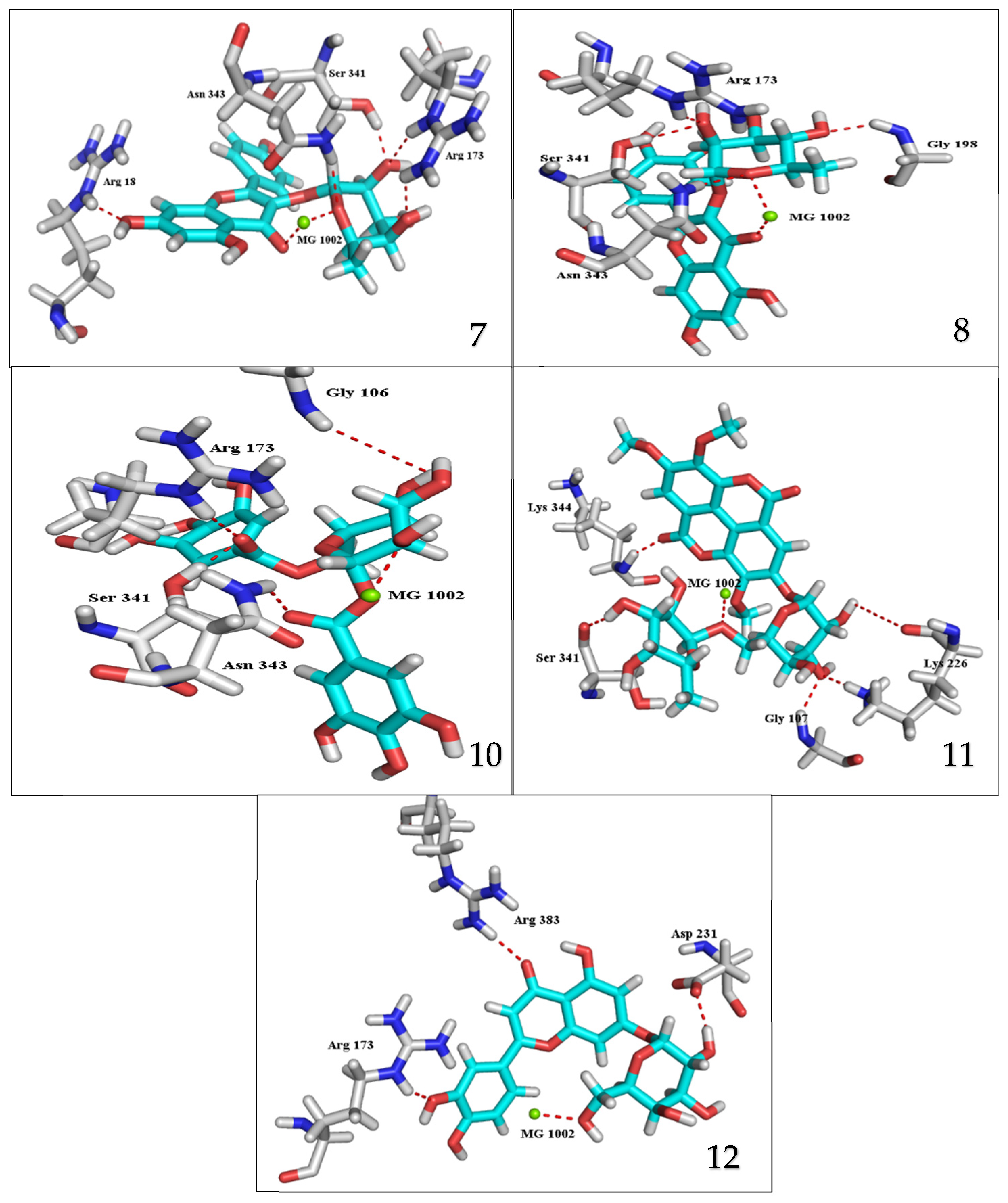
2.4. Docking Study for Anti-Trypanosomal Activity
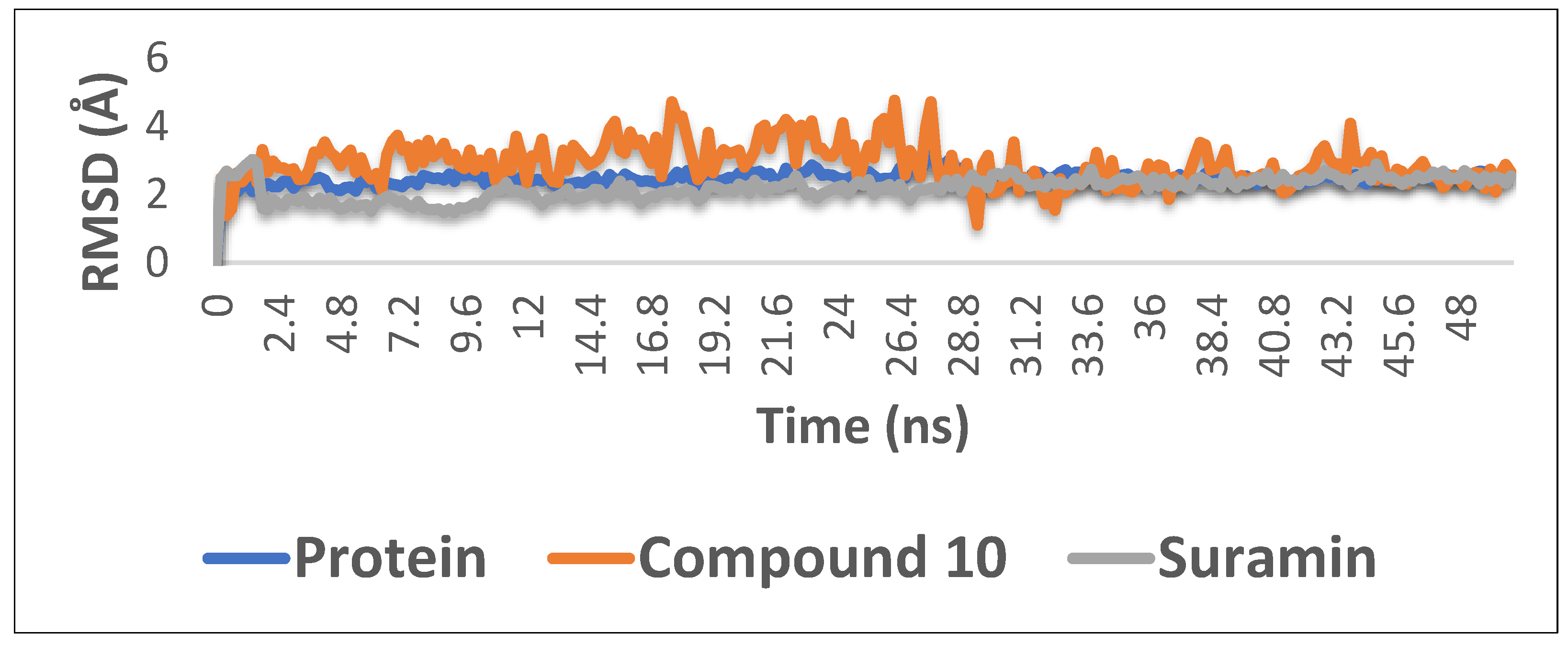
2.5. Molecular Dynamics Simulations
2.6. Prediction of the Pharmacokinetic Properties and Toxicological Properties Using ADMET
3. Discussion
3.1. Identification of the Isolated Compounds
3.2. Docking Study for Anti-Trypanosomal Activity
3.3. Molecular Dynamics Simulations
3.4. Prediction of the Pharmacokinetic Properties and Toxicological Properties Using ADMET
4. Materials and Methods
4.1. Plant Material
4.2. Chromatographic Materials and Apparatus
4.3. Extraction and Fractionation
4.4. Investigation of Methylene Chloride Fraction of E. abyssinica J.F. Gmel
4.5. Investigation of Ethyl Acetate Fraction of E. abyssinica J.F. Gmel
4.6. Investigation of N-Butanol Fraction of E. abyssinica J.F. Gmel
4.7. Docking Study for Anti-Trypanosomal Activity
4.8. Molecular Dynamics Simulations
4.9. Prediction of the Pharmacokinetic Properties and Toxicological Properties Using ADMET
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merritt, C.; Silva, L.E.; Tanner, A.L.; Stuart, K.; Pollastri, M.P. Kinases as druggable targets in trypanosomatid protozoan parasites. Chem. Rev. 2014, 114, 11280–11304. [Google Scholar] [CrossRef] [Green Version]
- Ayyildiz, M.; Celiker, S.; Ozhelvaci, F.; Akten, E.D. Identification of Alternative Allosteric Sites in Glycolytic Enzymes for Potential Use as Species-Specific Drug Targets. Front. Mol. Biosci. 2020, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- McNae, I.W.; Kinkead, J.; Malik, D.; Yen, L.-H.; Walker, M.K.; Swain, C.; Webster, S.P.; Gray, N.; Fernandes, P.M.; Myburgh, E. Fast acting allosteric phosphofructokinase inhibitors block trypanosome glycolysis and cure acute African trypanosomiasis in mice. Nat. Commun. 2021, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, G.A. A review of animal phosphofructokinase isozymes with an emphasis on their physiological role. Mol. Cell. Biochem. 1983, 52, 75–91. [Google Scholar] [CrossRef] [PubMed]
- McNae, I.W.; Martinez-Oyanedel, J.; Keillor, J.W.; Michels, P.A.M.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D. The crystal structure of ATP-bound phosphofructokinase from Trypanosoma brucei reveals conformational transitions different from those of other phosphofructokinases. J. Mol. Biol. 2009, 385, 1519–1533. [Google Scholar] [CrossRef]
- Albert, M.-A.; Haanstra, J.R.; Hannaert, V.; Van Roy, J.; Opperdoes, F.R.; Bakker, B.M.; Michels, P.A.M. Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei. J. Biol. Chem. 2005, 280, 28306–28315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opperdoes, F.R. Compartmentation of carbohydrate metabolism in trypanosomes. Annu. Rev. Microbiol. 1987, 41, 127–151. [Google Scholar] [CrossRef]
- Martinez-Oyanedel, J.; McNae, I.W.; Nowicki, M.W.; Keillor, J.W.; Michels, P.A.M.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D. The first crystal structure of phosphofructokinase from a eukaryote: Trypanosoma brucei. J. Mol. Biol. 2007, 366, 1185–1198. [Google Scholar] [CrossRef]
- T Jacobs, R.; Nare, B.; A Phillips, M. State of the art in African trypanosome drug discovery. Curr. Top. Med. Chem. 2011, 11, 1255–1274. [Google Scholar] [CrossRef] [Green Version]
- Brimacombe, K.R.; Walsh, M.J.; Liu, L.; Vásquez-Valdivieso, M.G.; Morgan, H.P.; McNae, I.; Fothergill-Gilmore, L.A.; Michels, P.A.M.; Auld, D.S.; Simeonov, A. Identification of ML251, a potent inhibitor of T. brucei and T. cruzi phosphofructokinase. ACS Med. Chem. Lett. 2014, 5, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Girdhar, S.; Girdhar, A.; Lather, V.; Pandita, D. Novel therapeutic targets for human African trypanosomiasis. Curr. Treat. Options Infect. Dis. 2017, 9, 200–209. [Google Scholar] [CrossRef]
- Luisi, G.; Carradori, S. New Compounds for the Management of Trypanosoma brucei Infection. In Topics in Medicinal Chemistry; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- EL-Fiky, F.; Asres, K.; Gibbons, S.; Hammoda, H.; Badr, J.; Umer, S. Phytochemical and Antimicrobial Investigation of Latex from Euphorbia abyssinica Gmel. Nat. Prod. Commun. 2008, 3, 1505–1508. [Google Scholar] [CrossRef] [Green Version]
- Dorsey, B.L.; Haevermans, T.; Aubriot, X.; Morawetz, J.J.; Riina, R.; Steinmann, V.W.; Berry, P.E. Phylogenetics, morphological evolution, and classification of Euphorbia subgenus Euphorbia. Taxon 2013, 62, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, J.A.; Sanz-Cervera, J.F.; Ropero, F.J.; Checa, J.; Fraga, B.M. Ingenane and lathyrane diterpenes from the latex of Euphorbia acrurensis. Phytochemistry 1998, 49, 1095–1099. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Mohammed, R.; Tawfike, A.F.; Lithy, N.M.; AbouZid, S.F.; Amin, M.N.; Abdelmohsen, U.R.; Amin, E. Cytotoxic activity and metabolic profiling of fifteen Euphorbia Species. Metabolites 2021, 11, 15. [Google Scholar] [CrossRef]
- Muluye, A.B.; Desta, A.G.; Abate, S.K.; Dano, G.T. Anti-malarial activity of the root extract of Euphorbia abyssinica (Euphorbiaceae) against Plasmodium berghei infection in mice. Malar. J. 2019, 18, 261. [Google Scholar] [CrossRef] [Green Version]
- Boniface, P.K.; Elizabeth, F.I. Flavonoid-derived privileged scaffolds in anti-trypanosoma brucei drug discovery. Curr. Drug Targets 2019, 20, 1295–1314. [Google Scholar] [CrossRef]
- Jabalia, N.; Kumar, A.; Kumar, V.; Rani, R. In Silico Approach in Drug Design and Drug Discovery: An Update. In Innovations and Implementations of Computer Aided Drug Discovery Strategies in Rational Drug Design; Springer: Singapore, 2021; pp. 245–271. [Google Scholar]
- Kanapeckaitė, A.; Beaurivage, C.; Jančorienė, L.; Mažeikienė, A. In silico drug discovery for a complex immunotherapeutic target-human c-Rel protein. Biophys. Chem. 2021, 276, 106593. [Google Scholar] [CrossRef]
- Silakari, O.; Singh, P.K. Concepts and Experimental Protocols of Modelling and Informatics in Drug Design; Academic Press: Cambridge, MA, USA, 2020; ISBN 0128205466. [Google Scholar]
- El-Hawary, S.S.; Lithy, N.M.; Amin, E.; AbouZid, S.F.; Mohammed, R. Anti-trypanosomal activity and DNA fingerprinting of fifteen Euphorbia species using ISSR and SCoT markers. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 54. [Google Scholar] [CrossRef]
- Onawole, A.T.; Kolapo, T.U.; Sulaiman, K.O.; Adegoke, R.O. Structure based virtual screening of the Ebola virus trimeric glycoprotein using consensus scoring. Comput. Biol. Chem. 2018, 72, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Yamamoto, K.; Tamura, T.; Kimura, Y.; Iida, T.; Nambara, T.; Chang, F.C. Triterpenoid Ketones from Lingnania chungii MoClure: Arborinone, friedelin and glutinone. Chem. Pharm. Bull. 1992, 40, 789–791. [Google Scholar] [CrossRef] [Green Version]
- Olea, R.S.G.; Torres, L.M.B.; Roque, L.C.; Roque, N.F. 13C NMR spectroscopic data for glutinol and derivatives. Magn. Reson. Chem. 1994, 32, 378–379. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Chaiang, Y.-M. Five new taraxastane-type triterpenes from the aerial roots of Ficus microcarpa. Chem. Pharm. Bull. 1999, 47, 498–500. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, W.F.; McLean, S.; Poplawski, J.; Enriquez, R.G.; Escobar, L.I.; Leon, I. Total assignment of 13C and 1H spectra of three isomeric triterpenol derivatives by 2D NMR: An investigation of the potential utility of 1H chemical shifts in structural investigations of complex natural products. Tetrahedron 1986, 42, 3419–3428. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, T.J.; Peng, S.L.; Liu, Z.R.; Ding, L.S. Two new triterpenoids from the roots of Sanguisorba officinalis L. J. Integr. Plant Biol. 2005, 47, 251–256. [Google Scholar] [CrossRef]
- Vo, T.K.; Ta, Q.T.H.; Chu, Q.T.; Nguyen, T.T.; Vo, V.G. Anti-Hepatocellular-Cancer Activity Exerted by β-Sitosterol and β-Sitosterol-Glucoside from Indigofera zollingeriana Miq. Molecules 2020, 25, 3021. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Heredia, N.; Camacho-Corona, M.D.R.; García, S. Isolation, characterization and mode of antimicrobial action against Vibrio cholerae of methyl gallate isolated from Acacia farnesiana. J. Appl. Microbiol. 2013, 115, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, E.; García, A.; Avalos-Alanís, F.G.; Rivas-Galindo, V.M.; Delgadillo-Puga, C.; Camacho-Corona, M.D.R. Nuclear magnetic resonance spectroscopy data of isolated compounds from Acacia farnesiana (L) Willd fruits and two esterified derivatives. Data Br. 2019, 22, 255–268. [Google Scholar] [CrossRef]
- Farag, M.A.; Al-Mahdy, D.A.; Salah El Dine, R.; Fahmy, S.; Yassin, A.; Porzel, A.; Brandt, W. Structure Activity Relationships of Antimicrobial Gallic Acid Derivatives from Pomegranate and Acacia Fruit Extracts against Potato Bacterial Wilt Pathogen. Chem. Biodivers. 2015, 12, 955–962. [Google Scholar] [CrossRef]
- Lee, S.Y.; So, Y.-J.; Shin, M.S.; Cho, J.Y.; Lee, J. Antibacterial effects of afzelin isolated from Cornus macrophylla on Pseudomonas aeruginosa, a leading cause of illness in immunocompromised individuals. Molecules 2014, 19, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Nono, R.N.; Barboni, L.; Teponno, R.B.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Lupidi, G.; Tapondjou, A.L. Antimicrobial, antioxidant, anti-inflammatory activities and phytoconstituents of extracts from the roots of Dissotis thollonii Cogn. (Melastomataceae). S. Afr. J. Bot. 2014, 93, 19–26. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Tannins and related compounds. X. Rhubarb (2): Isolation and structures of a glycerol gallate, gallic acid glucoside gallates, galloylglucoses and isolindleyin. Chem. Pharm. Bull. 1983, 31, 1652–1658. [Google Scholar] [CrossRef] [Green Version]
- Li, C.W.; Dong, H.J.; Cui, C. Bin The synthesis and antitumor activity of twelve galloyl glucosides. Molecules 2015, 20, 2034–2060. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.J.; Yeh, C.H.; Yang, L.M.; Liu, P.C.; Hsu, F.L. Phenolic compounds from Formosan Euphorbia tirucalli. J. Chin. Chem. Soc. 2001, 48, 105–108. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.-C.; Ho, C.-T. Antioxidative phenolic compounds from sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Uchida, H.; Yamashita, H.; Anai, T.; Muranaka, T.; Ohyama, K. Agrobacterium-Mediated Transformation of Euphorbia tirucalli Callus. Biosci. Biotechnol. Biochem. 2010, 74, 851–853. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.Q.; Ahmed, Z.; Malik, A. Further triterpenes from the stem bark of Euphorbia tirucalli. Planta Med. 1987, 53, 577. [Google Scholar] [CrossRef]
- Jia, H.Y.; Liao, Z.X.; Liu, F.Y.; Wu, L.; Xu, C.; Zuo, B. A new phenylpropanoid from the roots of Euphorbia nematocypha. Nat. Prod. Res. 2015, 29, 650–655. [Google Scholar] [CrossRef]
- Wang, L.; Yu, M.-M.; Chi, Y.-Q.; Ouyang, W.-B.; Zang, Z.; Zhao, Y. Chemical constituents of Euphorbia dracunculoides. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi= China J. Chin. Mater. Med. 2014, 39, 3969–3973. [Google Scholar]
- Le, D.T.K.; Bui, H.X.; Nguyen, T.T.A.; Pham, T.N.K.; Duong, H.T. Chemical constituents of Euphorbia tirucalli L. Sci. Technol. Dev. J. Sci. 2018, 2, 76–82. [Google Scholar] [CrossRef]
- Yoshida, T.; Yokoyama, K.; Namba, O.; Okuda, T. Tannins and Related Polyphenols of Euphorbiaceous Plants. VII. Tirucallins A, B and Euphorbin F, Monomeric and Dimeric Ellagitannins from Euphorbia tirucalli L. Chem. Pharm. Bull. 1991, 39, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.S.; Li, Z.L.; Wei, J.G.; Yang, Y.P.; Li, X.L. Chemical constituents of Euphorbia royleana. Chin. Tradit. Herb. Drugs 2013, 44, 2039–2043. [Google Scholar]
- Tian, Y.; Sun, L.-M.; Liu, X.-Q.; Li, B.; Wang, Q.; Dong, J.-X. Anti-HBV active flavone glucosides from Euphorbia humifusa Willd. Fitoterapia 2010, 81, 799–802. [Google Scholar] [CrossRef]
- Santos, R.T.D.; Hiramoto, L.L.; Lago, J.H.G.; Sartorelli, P.; Tempone, A.G.; Pinto, E.G. Anti-trypanosomal activity of 1, 2, 3, 4, 6-penta-O-galloyl-β-D-glucose isolated from Plectranthus barbatus Andrews (Lamiaceae). Quim. Nova 2012, 35, 2229–2332. [Google Scholar] [CrossRef] [Green Version]
- Amisigo, C.M.; Antwi, C.A.; Adjimani, J.P.; Gwira, T.M. In vitro anti-trypanosomal effects of selected phenolic acids on Trypanosoma brucei. PLoS ONE 2019, 14, e0216078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanrewaju Timothy, O.; Odumosu Patricia, O.; Eyong Kenneth, O. Anti-trypanosomal evaluation of Ximenia americana root bark and chromatographic-mass spectrometric profile. GSC Biol. Pharm. Sci. 2019, 7, 108–117. [Google Scholar] [CrossRef]
- Guterres, H.; Im, W. Improving protein-ligand docking results with high-throughput molecular dynamics simulations. J. Chem. Inf. Model. 2020, 60, 2189–2198. [Google Scholar] [CrossRef]
- Hecker, E. Cocarcinogenic Principles from the Seed Oil of Croton tiglium and from Other Euphorbiaceae. Cancer Res. 1968, 28, 2338–2349. [Google Scholar] [PubMed]
- Markham, K.R. Techniques of Flavonoid Identification; Academic press: Cambridge, MA, USA, 1982; ISBN 0124726801. [Google Scholar]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [Green Version]
- MacKerell Jr, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Jiang, W.; Lee, H.S.; Roux, B.; Im, W. CHARMM-GUI Ligand Binder for absolute binding free energy calculations and its application. J. Chem. Inf. Model. 2013, 53, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
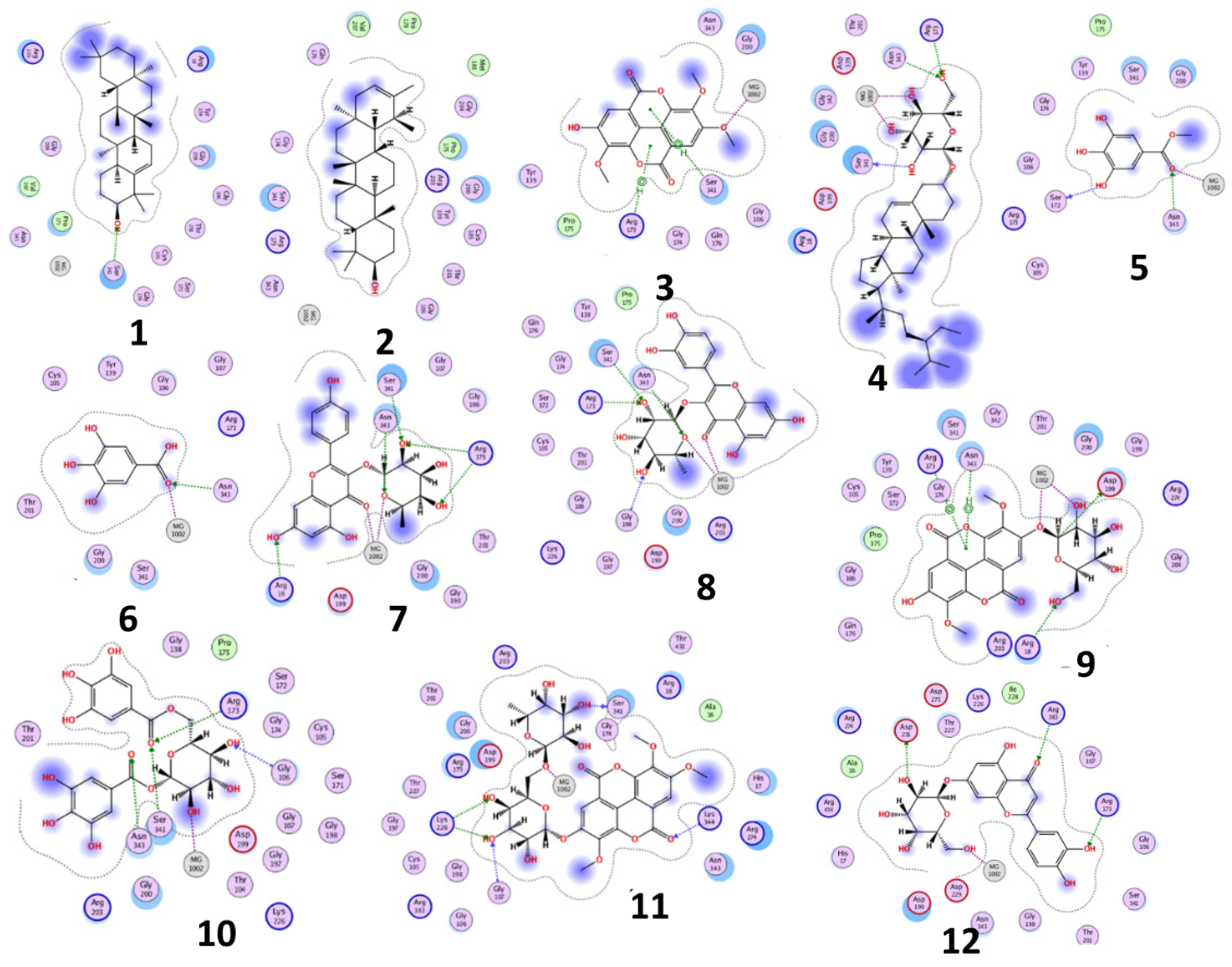


| Compound | Phosphofructokinase Enzyme (PDB ID:3F5M) | ||||
|---|---|---|---|---|---|
| Binding Free Energy (Kcal/mol) | Binding Affinity Constant (ki = nM) | Distance (in Å) from Main Residue | Interaction | ||
| Glut-5-en-3-β-ol (1) | −10.5283 | 19.6 | 2.05 | Ser341 | H-acceptor |
| ψ-Taraxasterol (2) | −5.5902 | 80830 | - | - | - |
| 3,3′,4-O-Trimethylellagic acid (3) | −9.7990 | 67.07 | 3.66 2.48 1.02 | Arg173 Ser341 MG1002 | pi-H pi-H Metal |
| β-Sitosterol glucoside (4) | −13.9894 | 0.057 | 1.96 1.74 1.48 1.13 1.12 | Arg173 Ser341 Asn343 MG1002 MG1002 | H-acceptor H-donor H-acceptor Metal Metal |
| Methyl gallate (5) | −13.0253 | 0.29 | 1.99 1.91 1.13 | Asn343 Ser172 MG1002 | H-acceptor H-donor Metal |
| Gallic acid (6) | −11.3316 | 5.06 | 2.10 1.10 | Asn343 MG1002 | H-acceptor Metal |
| Kaempferol-3-O-α-L-rhamnoside (7) (Afzelin) | −21.3948 | 2.22 × 10−7 | 2.35 1.97 1.75 1.94 2.01 1.05 1.02 | Arg173 Arg173 Ser341 Asn343 Arg18 MG1002 MG1002 | H-acceptor H-acceptor H-acceptor H-acceptor H-acceptor Metal Metal |
| Quercetin-3-O-α-L-rhamnopyrnosyl (8) (Quercitrin) | −20.3334 | 1.3 × 10−6 | 1.93 2.11 1.88 1.91 1.33 1.08 | Arg173 Ser341 Asn343 Gly198 MG1002 MG1002 | H-acceptor H-acceptor H-acceptor H-acceptor Metal Metal |
| 3,3′-Dimethylellagic acid-4′-O-β-d-glucopyranoside (9) | −12.8799 | 0.37 | 3.94 3.61 2.14 2.84 1.98 2.13 | Arg173 Asn343 Asp199 Arg18 MG1002 MG1002 | pi-cation pi-H H-donor H-acceptor Metal Metal |
| 1,6-di-O-galloyl-d-glucose (10) | −23.0767 | 1.3 × 10−8 | 2.42 1.58 1.84 2.11 1.22 | Arg173 Ser341 Asn343 Gly106 MG1002 | H-acceptor H-acceptor H-acceptor H-acceptor Metal |
| 3,3′,4-Tri-O-methyl-4′-O-rutinosyl-ellagic acid (11) | −21.2640 | 2.2× 10−7 | 2.05 2.29 1.83 2.11 1.83 1.41 | Ser341 Lys226 Lys226 Gly107 Lys344 MG1002 | H-donor H-acceptor H-acceptor H-acceptor H-acceptor Metal |
| Luteolin-7-O-glucoside (12) (cynaroside) | −18.9900 | 1.3 × 10−5 | 2.74 2.09 2.81 1.13 | Arg173 Arg383 Asp231 MG1002 | H-acceptor H-acceptor H-donor Metal |
| Suramin | −25.3326 | 2.9 × 10−10 | 1.82 2.04 2.18 1.80 2.12 2.24 2.26 2.00 1.00 | Arg173 Arg173 Arg173 Ser341 Asn343 Asn343 Arg18 Pro175 MG1002 | H-donor H-acceptor H-acceptor H-donor H-acceptor H-acceptor H-acceptor H-donor Metal |
| Properties | Compound 10 | Suramin | |
|---|---|---|---|
| Absorption | Caco-2 permeability (log Papp in 10−6 cm/s) | −1.682 | −3.097 |
| HIA (% Absorbed) | 15.64% | 0 | |
| P-glycoprotein substrate | Yes | Non | |
| P-glycoprotein I inhibitor | Non | Non | |
| P-glycoprotein II inhibitor | Non | Non | |
| Pure water solubility (log mol/L) | −2.895 | −2.892 | |
| Skin Permeability (log Kp) | −2.735 | −2.735 | |
| Distribution | BBB Permeability (log BB) | −2.435 | −4.438 |
| CNS permeability (log PS) | −4.668 | −4.991 | |
| VDss human (log L/kg) | 1.614 | −0.007 | |
| Fraction unbound human (Fu) | 0.347 | 0.379 | |
| Metabolism | CYP 2C19 inhibitor | Non | Non |
| CYP 2C9 inhibitor | Non | Non | |
| CYP 2D6 inhibitor | Non | Non | |
| CYP 2D6 substrate | Non | Non | |
| CYP 3A4 inhibitor | Non | Non | |
| CYP 3A4 substrate | Non | Non | |
| CYP 1A2 inhibitor | Non | Non | |
| Excretion | Total Clearance (log mL/min/kg) | 0.47 | −4.065 |
| Renal OCT2 substrate | Non | Non | |
| Toxicity | Ames test | non-mutagen | non-mutagen |
| Max. tolerated dose human (log mg/kg/day) | 0.49 | 0.438 | |
| Oral Rat Acute Toxicity LD50 (mol/kg) | 2.515 | 2.482 | |
| Oral Rat Chronic Toxicity LOAEL (log mg/kg-bw/day) | 3.491 | 6.817 | |
| hERG I inhibitor | Non | Non | |
| hERG II inhibitor | Yes | Yes | |
| T. pyriformis toxicity (log μg/L) | 0.285 | 0.285 | |
| minnow toxicity (log mM) | 5.837 | 6.162 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hawary, S.S.; Mohammed, R.; Lithy, N.M.; AbouZid, S.F.; Mansour, M.A.; Almahmoud, S.A.; Huwaimel, B.; Amin, E. Digalloyl Glycoside: A Potential Inhibitor of Trypanosomal PFK from Euphorbia abyssinica J.F. Gmel. Plants 2022, 11, 173. https://doi.org/10.3390/plants11020173
El-Hawary SS, Mohammed R, Lithy NM, AbouZid SF, Mansour MA, Almahmoud SA, Huwaimel B, Amin E. Digalloyl Glycoside: A Potential Inhibitor of Trypanosomal PFK from Euphorbia abyssinica J.F. Gmel. Plants. 2022; 11(2):173. https://doi.org/10.3390/plants11020173
Chicago/Turabian StyleEl-Hawary, Seham S., Rabab Mohammed, Nadia M. Lithy, Sameh Fekry AbouZid, Mostafa A. Mansour, Suliman A. Almahmoud, Bader Huwaimel, and Elham Amin. 2022. "Digalloyl Glycoside: A Potential Inhibitor of Trypanosomal PFK from Euphorbia abyssinica J.F. Gmel" Plants 11, no. 2: 173. https://doi.org/10.3390/plants11020173







