Impact of Storage Controlled Atmosphere on the Apple Phenolic Acids, Flavonoids, and Anthocyanins and Antioxidant Activity In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Variation in the Quantitative Composition of Phenolic Acids in Apple Samples before and after Storage in Controlled Atmospheric Conditions
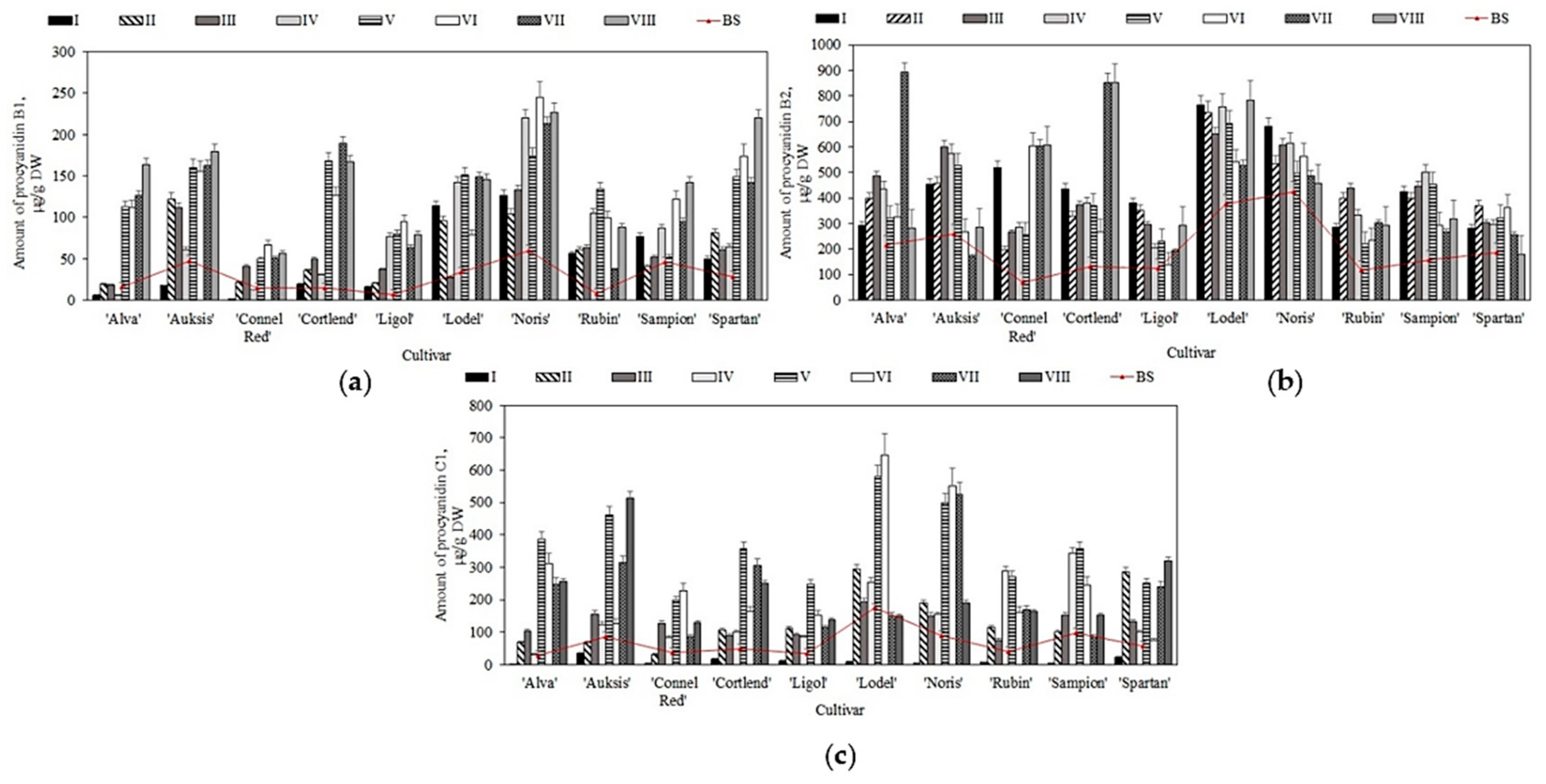
2.2. Variation in the Quantitative Composition of Flavan-3-ols in Apple Samples before and after Storage in Controlled Atmospheric Conditions
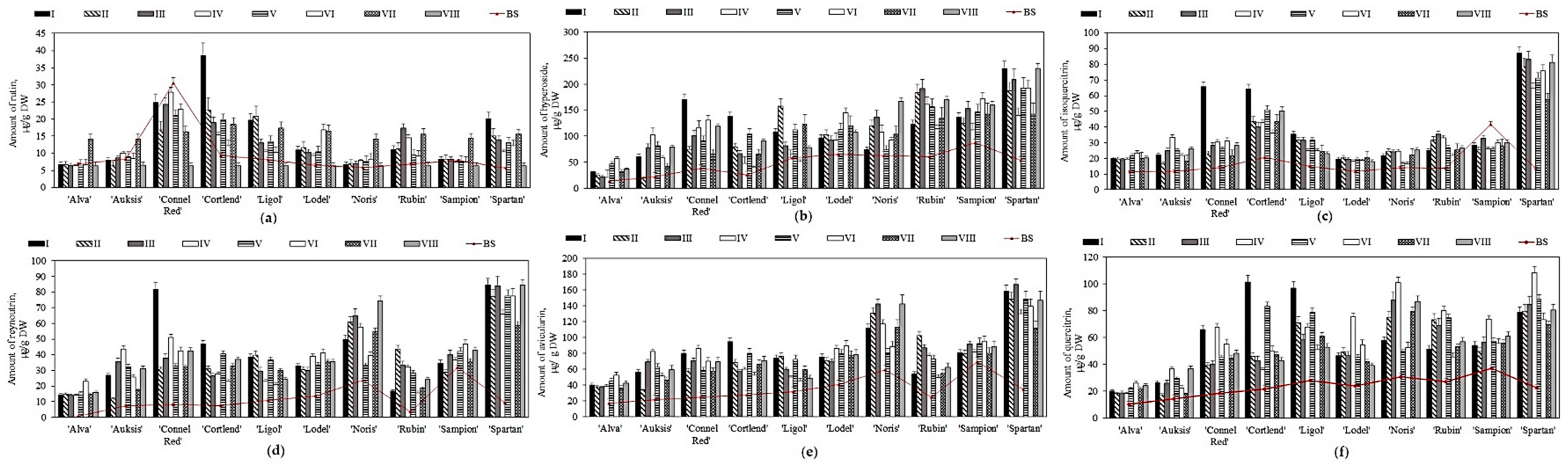
2.3. Variation in the Quantitative Composition of Flavonols in Apple Samples before and after Storage in Controlled Atmospheric Conditions
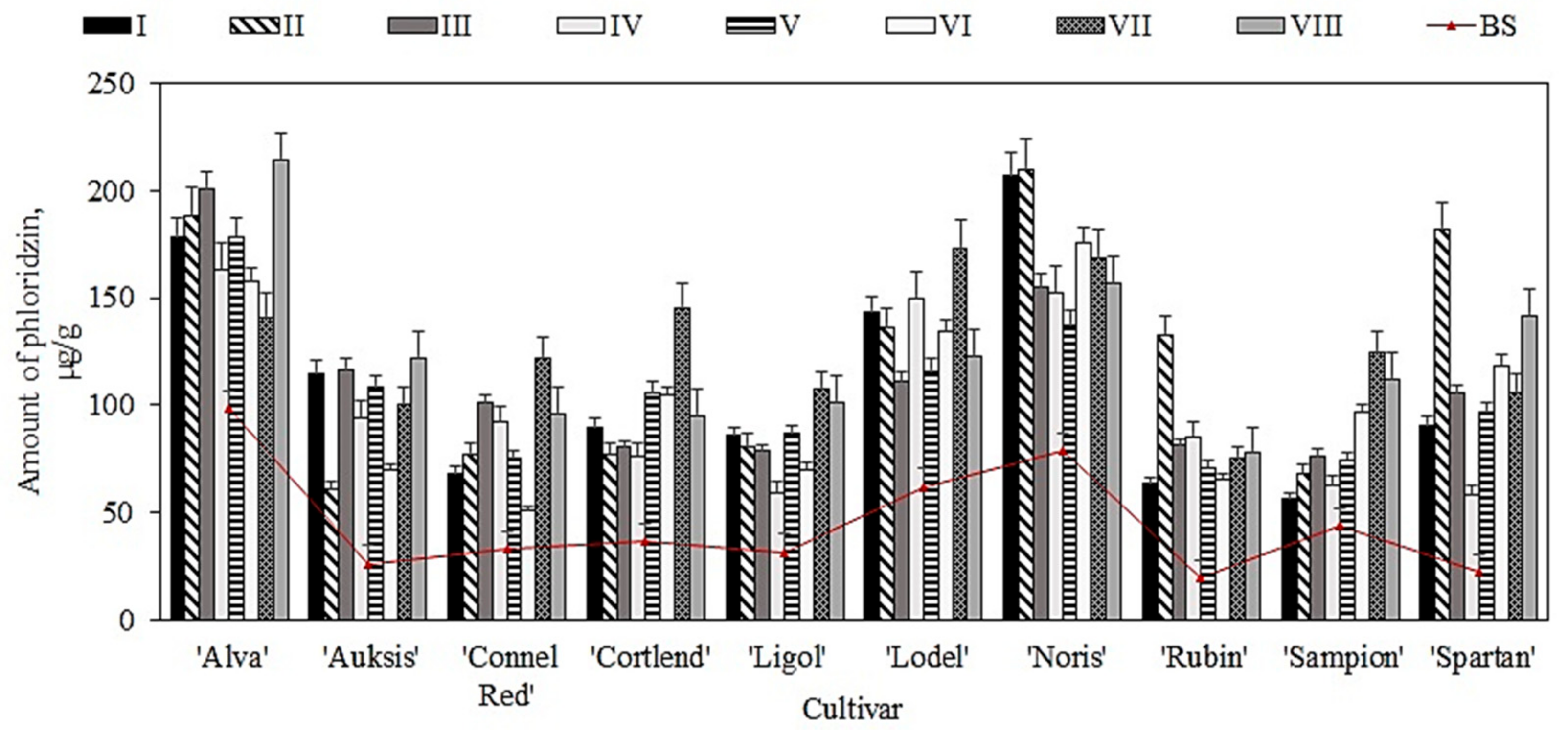
2.4. Variation in the Quantitative Cmposition of Dihydrochalcone in Apple Samples before and after Storage in Controlled Atmospheric Conditions
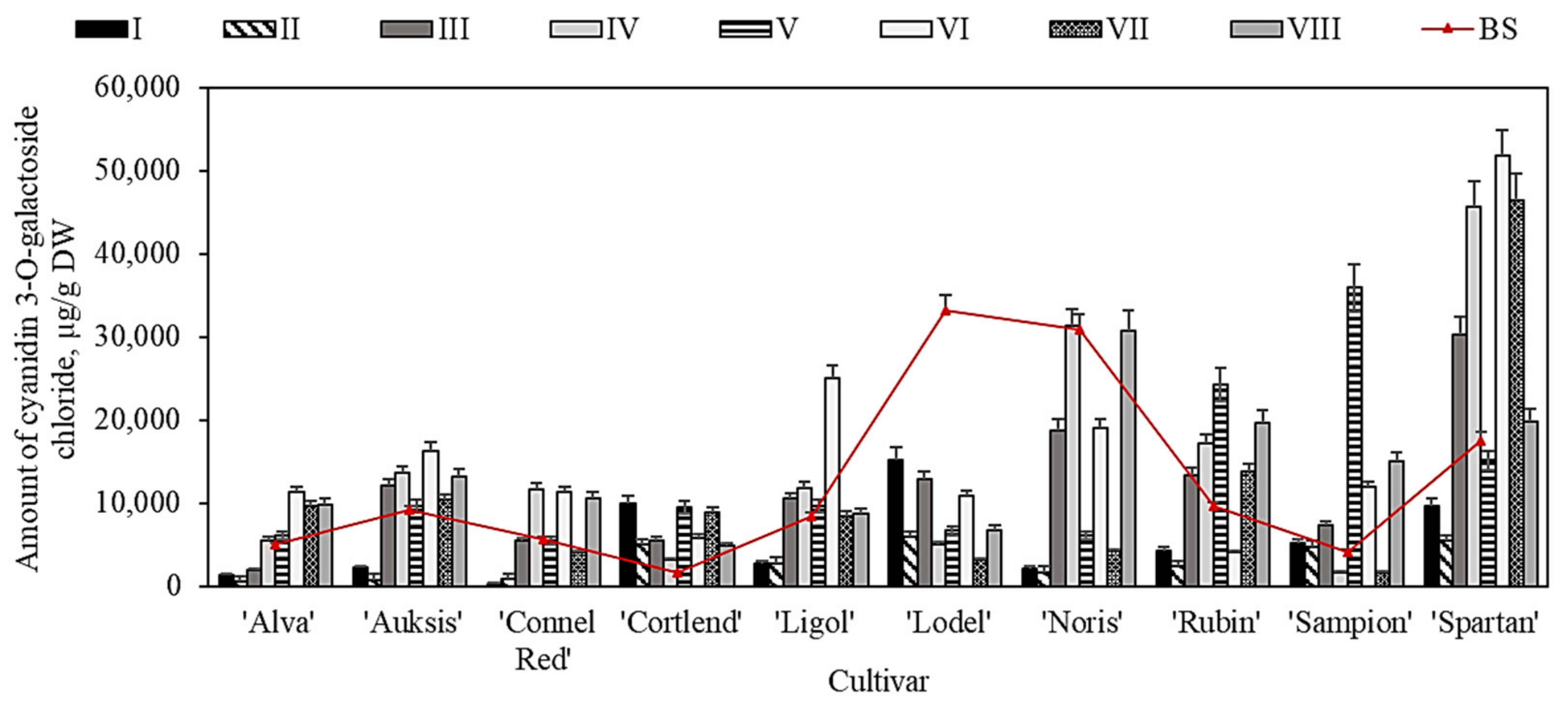
2.5. Variation in the Quantitative Composition of Anthocyanin in Apple Peel Samples before and after Storage in Controlled Atmospheric Conditions
2.6. Hierarchical Cluster Analysis of Phenolic Compounds
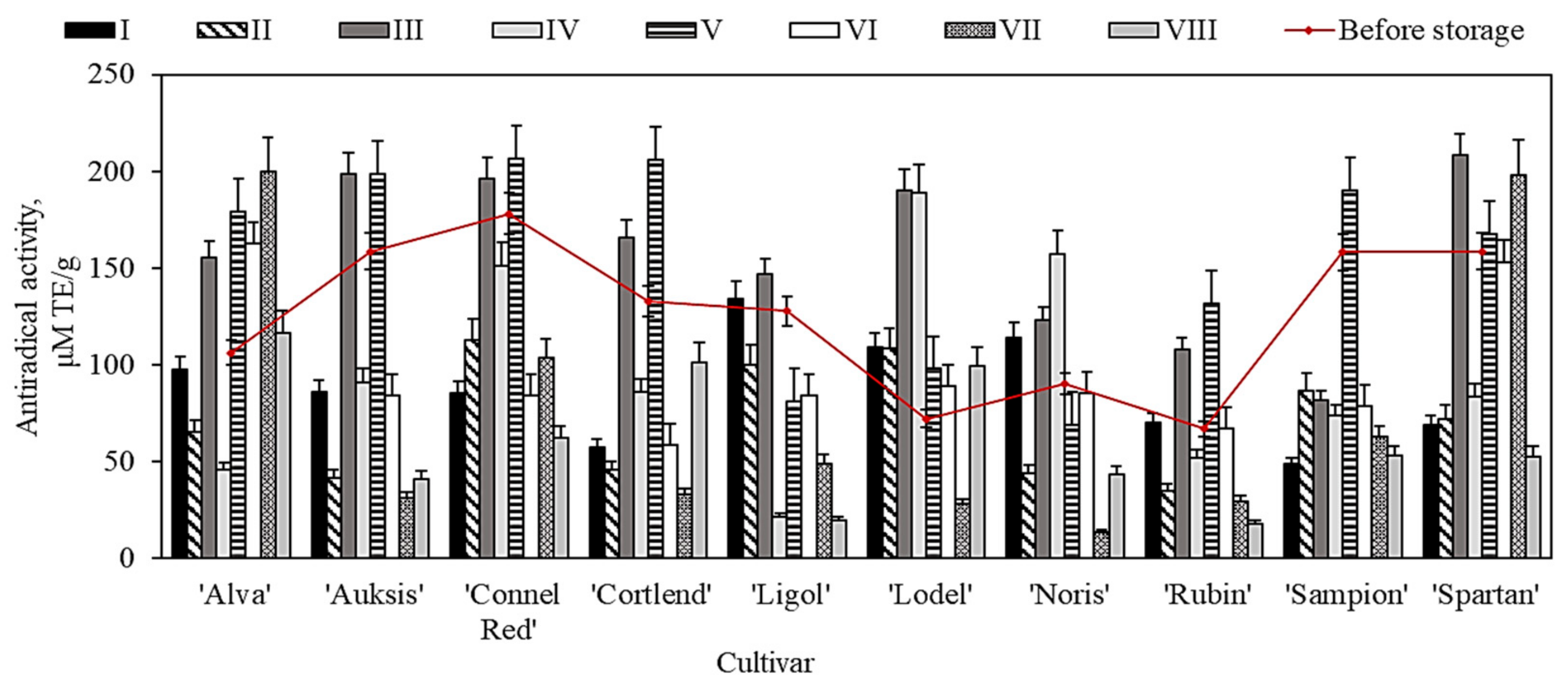
2.7. Variation of In Vitro Antioxidant Activity of Apple Extracts before and after Storage in Controlled Atmospheric Conditions
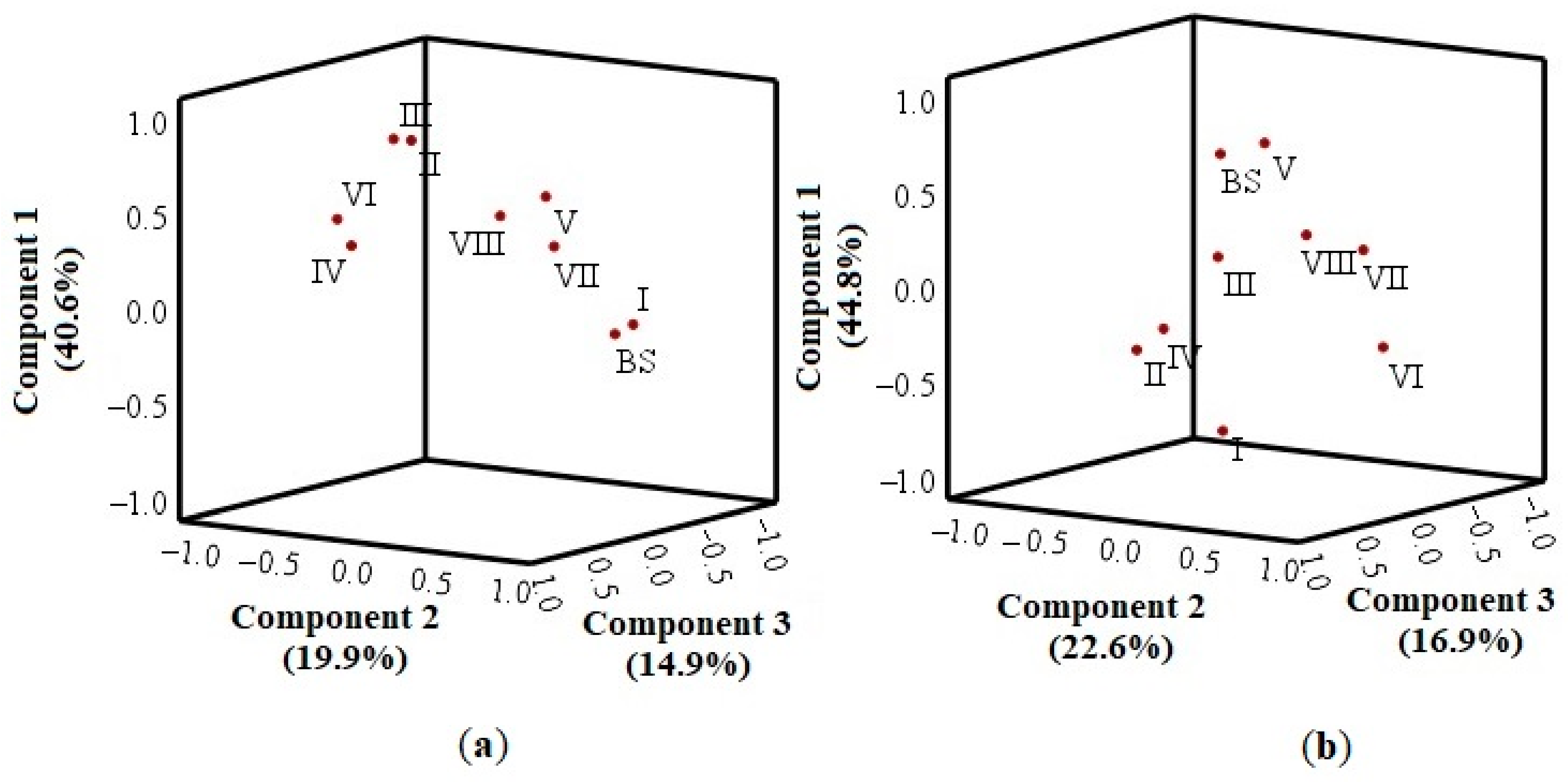
2.8. Principal Component Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Solvents
3.3. Controlled Atmospheric Conditions during Apple Storage
3.4. Preparation of Apple and Apple Peel Extracts
3.5. Evaluation of Phenolic Acid and Flavonoids by HPLC-PDA
3.6. Evaluation of Anthocyanins by HPLC-PDA
3.7. Antioxidant Activity Assays
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serra, A.T.; Rocha, J.; Sepodesc, B.; Matiasa, A.A.; Feliciano, R.P.; de Carvalho, A.; Bronze, M.R.; Duarte, C.M.; Figueira, M.E. Evaluation of cardiovascular protective effect of different apple varieties—Correlation of response with composition. Food Chem. 2012, 135, 2378–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maragò, E.; Iacopini, P.; Camangi, F.; Scattino, C.; Ranieri, A.; Stefani, A.; Sebastiani, L. Phenolic profile and antioxidant activity in apple juice and pomace: Effects of different storage conditions. Fruits 2015, 70, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Preti, R.; Tarola, A.M. Study of polyphenols, antioxidant capacity and minerals for the valorisation of ancient apple cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Kevers, C.; Pincemail, J.; Tabart, J.; Defraigne, J.O.; Dommes, J. Influence of cultivar, harvest time, storage conditions, and peeling on the antioxidant capacity and phenolic and ascorbic acid contents of apples and pears. J. Agric. Food Chem. 2011, 59, 6165–6171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. J. Funct. Foods 2015, 18, 1086–1094. [Google Scholar] [CrossRef]
- Li, H.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Phenolic profiling of five different Australian grown apples. Appl. Sci. 2021, 11, 2421. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kviklys, D.; Liaudanskas, M.; Janulis, V.; Uselis, N.; Viškelis, J.; Viškelis, P. Lower nitrogen nutrition determines higher phenolic content of organic apples. Hortic. Sci. 2017, 44, 113–119. [Google Scholar]
- Kviklys, D.; Liaudanskas, M.; Janulis, V.; Viškelis, P.; Rubinskienė, M.; Kviklienė, N.; Lanauskas, J.; Uselis, N. Rootstock genotype determines phenol content in apple fruits. Plant Soil Environ. 2014, 60, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do Amarante, C.V.T.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Liu, Y.; Che, F.; Wang, L.; Meng, R.; Zhang, X.; Zhao, Z. Fruit coloration and anthocyanin biosynthesis after bag removal in non-red and red apples (Malus × domestica Borkh.). Molecules 2013, 18, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ikram, M.; Park, T.J.; Kim, M.O. Pathology, risk factors, and oxidative damage related to type 2 diabetes-mediated Alzheimer’s disease and the rescuing effects of the potent antioxidant anthocyanin. Oxid. Med. Cell. Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Alsataf, S.; Başyiğit, B.; Karaaslan, M. Multivariate analyses of the antioxidant, antidiabetic, antimicrobial activity of pomegranate tissues with respect to pomegranate juice. Waste Biomass Valoriz. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [Green Version]
- Szymanowska, U.; Baraniak, B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.M.; Calhau, C.; Pintado, M.M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Pertuzatti, P.B.; Barcia, M.T.; Rebello, L.P.G.; Gómez-Alonso, S.; Duarte, R.M.T.; Duarte, M.C.T.; Godoy, H.T.; Hermosín-Gutiérrez, I. Antimicrobial activity and differentiation of anthocyanin profiles of rabbiteye and highbush blueberries using HPLC–DAD–ESI-MSn and multivariate analysis. J. Funct. Foods 2016, 26, 506–516. [Google Scholar] [CrossRef]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- Gomes, J.V.P.; Rigolon, T.C.B.R.; Souza, M.S.S.; Alvarez-Leite, J.I.; Lucia, C.M.D.; Martino, H.S.D.; Rosa, C.O.B. Antiobesity effects of anthocyanins on mitochondrial biogenesis, inflammation, and oxidative stress: A systematic review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef]
- Liobikas, J.; Skemiene, K.; Trumbeckaite, S.; Borutaite, V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol. Res. 2016, 113, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Patel, D.; Patel, S.; Upadhyay, K.; Thadani, J.; Mandal, R.; Das, S.; Devkar, R. Anthocyanin rich extract of Brassica oleracea L. alleviates experimentally induced myocardial infarction. PLoS ONE 2017, 12, e0182137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef]
- Thewes, F.R.; Brackmann, A.; Both, V.; Weber, A.; Anese, R.O.; Ferrão, T.S.; Wagner, R. The different impacts of dynamic controlled atmosphere and controlled atmosphere storage in the quality attributes of ‘Fuji Suprema’ apples. Postharvest Biol. Technol. 2017, 130, 7–20. [Google Scholar] [CrossRef]
- Klein, B.; Falk, R.B.; Thewes, F.R.; Anese, R.O.; Santos, I.D.; Ribeiro, S.R.; Donadel, J.Z.; Brackmann, A.; Barin, J.S.; Cichoski, A.J.; et al. Dynamic controlled atmosphere: Effects on the chemical composition of cuticular wax of ‘Cripps pink’ apples after long-term storage. Postharvest Biol. Technol. 2020, 164, 111170. [Google Scholar] [CrossRef]
- Buccheri, M.; Picchi, V.; Grassi, M.; Gandin, D.; Bianchi, G.; Lo Scalzo, R. Dynamic changes of antioxidants and fermentative metabolites in apple peel in relation to storage, controlled atmosphere, and initial low oxygen stress. Sci. Hortic. 2021, 288, 110312. [Google Scholar] [CrossRef]
- Mditshwa, A. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Hoang, N.T.T.; Golding, J.B.; Wilkes, M.A. The effect of postharvest 1-MCP treatment and storage atmosphere on ‘Cripps Pink’ apple phenolics and antioxidant activity. Food Chem. 2011, 127, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Zanella, A.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Anese, R.O.; Brackmann, A.; Wendt, L.M.; Thewes, F.R.; Schultz, E.E.; Ludwig, V.; Pasquetti Berghetti, M.R. Interaction of 1-methylcyclopropene, temperature and dynamic controlled atmosphere by respiratory quotient on ‘Galaxy’ apples storage. Food Packag. Shelf Life 2019, 20, 100246. [Google Scholar] [CrossRef]
- Vondráková, Z.; Trávnícková, A.; Malbeck, J.; Haisel, D.; Černý, R.; Cvikrová, M. The effect of storage conditions on the carotenoid and phenolic acid contents of selected apple cultivars. Eur. Food Res. Technol. 2020, 246, 1783–1794. [Google Scholar] [CrossRef]
- Anese, R.O.; Thewes, F.R.; Brackmann, A.; Eliseu Schultz, E.; Wagner, R.; Klein, B.; Roberto, M.; Berghetti, P.; Mallmann Wendt, L. Growth regulators on quality traits and volatile organic compounds profile of ‘Royal Gala’ apple at harvest and after dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2020, 164, 111158. [Google Scholar] [CrossRef]
- Thewes, F.R.; Brackmann, A.; Neuwald, D.A. Dynamics of sugars, anaerobic metabolism enzymes and metabolites in apples stored under dynamic controlled atmosphere. Sci. Hortic. 2019, 255, 145–152. [Google Scholar] [CrossRef]
- Bekele, E.A.; Ampofo-Asiama, J.; Alis, R.R.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A.H. Dynamics of metabolic adaptation during initiation of controlled atmosphere storage of ‘Jonagold’ apple: Effects of storage gas concentrations and conditioning. Postharvest Biol. Technol. 2016, 117, 9–20. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which polyphenolic compounds contribute to the total antioxidant activities of apple. J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. J. Food Sci. Technol. 2021, 58, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical profiles of new red-fleshed apple varieties compared with old and new white-fleshed varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Sluis, A.A.; Dekker, M.; Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Saba, K.M.; Watkins, C.B. Flesh browning development of ‘Empire’ apple during a shelf-life period after 1-methylcyclopropene (1-MCP) treatment and controlled atmosphere storage. Sci. Hortic. 2020, 261, 108938. [Google Scholar] [CrossRef]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R.; Horvath, C.R. Postharvest variation in apple (Malus × domestica Borkh.) flavonoids following harvest, storage, and 1-MCP treatment. J. Agric. Food Chem. 2006, 54, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Petriccione, M.; Rega, P.; Scortichini, M.; Napolitano, A. A reappraisal of traditional apple cultivars from Southern Italy as a rich source of phenols with superior antioxidant activity. Food Chem. 2013, 140, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Gacnik, S.; Veberic, R.; Hudina, M.; Marinovic, S.; Halbwirth, H.; Mikulic-Petkovšek, M. Salicylic and methyl salicylic acid affect quality and phenolic profile of apple fruits three weeks before the harvest. Plants 2021, 10, 1807. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Laskowski, P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Belviso, S.; Scursatone, B.; Re, G.; Zeppa, G. Novel data on the polyphenol composition of Italian ancient apple cultivars. Int. J. Food Prop. 2013, 16, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, E.L.; Landi, M.; Massai, R.; Remorini, D.; Conte, G.; Guidi, L. Ancient apple cultivars from Garfagnana (Tuscany, Italy): A potential source for ‘nutrafruit’ production. Food Chem. 2019, 294, 518–525. [Google Scholar] [CrossRef]
- Lv, Y. Triterpenes and Phenolic Compounds in Apple Fruit (Malus domestica Borkh.). Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2016; pp. 23–24. [Google Scholar]
- Kanno, H.; Kawakami, Z.; Tabuchi, M.; Mizoguchi, K.; Ikarashi, Y.; Kase, Y. Protective effects of glycoumarinand procyanidin B1, active componentes of traditional Japanese medicine yokukansan, on amyloid β oligomer-induced neuronal death. J. Ethnopharmacol. 2015, 159, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.D.; Lanzi, C.R.; Toblli, J.E.; Elesgaray, R.; Oteiza, P.I.; Fraga, C.G.; Galleano, M. Dietary (–)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal córtex from fructose-fed rats. Free Radic. Biol. Med. 2016, 90, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geană, E.I.; Ciucure, C.T.; Ionete, R.E.; Ciocârlan, A.; Aricu, A.; Ficai, A.; Andronescu, E. Profiling of phenolic compounds and triterpene acids of twelve apple (Malus domestica Borkh.) cultivars. Foods 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Xu, Y.; Mei, X.; Meng, Q.; Gcao, Y.; Li, B.; Tu, Y. Antiobesity and lipid lowering effects of theaflavins on high-fat diet induced obese rats. J. Funct. Foods 2013, 5, 1142–1150. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2019, 246, 437–448. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. J. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, S.; Joshi, A.; Arora, B.; Bhowmik, A.; Sharma, R.R.; Kumar, P. Significance of FRAP, DPPH, and CUPRAC assays for antioxidant activity determination in apple fruit extracts. Eur. Food Res. Technol. 2020, 246, 591–598. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Gomes, M.H.; Almeida, D.P.F.; Pintado, M. Effect of modified atmosphere on polyphenols during storage of pasteurised strawberry purées. Food Sci. Technol. 2015, 60, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Krupa, T.; Tomala, K. Antioxidant capacity, anthocyanin content profile in ‘Bluecrop’ blueberry fruit. Veg. Crop. Res. Bull. 2007, 66, 129–141. [Google Scholar] [CrossRef]
- Khorshidi, S.; Davarynejad, G.; Tehranifar, A.; Fallahi, E. Effect of modified atmosphere packaging on chemical composition, antioxidant activity, anthocyanin, and total phenolic content of cherry fruits. Hortic. Environ. Biotechnol. 2011, 52, 471–481. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, G.; Peng, J.; Salokhe, V.M. Effects of modified atmosphere package on preservation of strawberries. Int. Agrophys. 2003, 17, 143–148. [Google Scholar]
- Dziedzic, E.; Błaszczyk, J.; Bieniasz, M.; Dziadek, K.; Kopeć, A. Effect of modified (MAP) and controlled atmosphere (CA) storage on the quality and bioactive compounds of blue honeysuckle fruits (Lonicera caerulea L.). Sci. Hortic. 2020, 265, 109226. [Google Scholar] [CrossRef]
- Vilas-Boas, E.V.B.; Kader, A.A. Efect of atmospheric modifcation, 1-MCP and chemicals on quality of fresh-cut banana. Postharvest Biol. Technol. 2006, 39, 155–162. [Google Scholar] [CrossRef]
- Buccheri, M.; Lovati, F.; Petriccione, M.; Rega, P.; Grassi, M.; Lo Scalzo, R. Control of superficial scald and analysis of α-farnesene and conjugated trienols in ‘Annurca’ apple. Acta Hortic. 2018, 1194, 1443–1450. [Google Scholar] [CrossRef]
- Horvitz, S. Postharvest Handling of Berries; InTech: London, UK, 2017; Volume 6, pp. 107–123. [Google Scholar]
- Zevallos, L.C. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Senica, M.; Bavec, M.; Stampar, F.; Mikulic-Petkovsek, M. Blue honeysuckle (Lonicera caerulea subsp. edulis (Turcz. Ex Herder) Hultén.) berries and changes in their ingredients across different locations. J. Sci. Food Agric. 2018, 98, 3333–3342. [Google Scholar] [CrossRef]
- Harb, J.; Saleh, O.; Kittemann, D.; Neuwald, D.; Hoffmann, T.; Reski, R.; Schwab, W. Changes in polyphenols and expression levels of related genes in ‘Duke’ blueberries stored under high CO2 levels. J. Agric. Food Chem. 2014, 62, 7460–7467. [Google Scholar] [CrossRef]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Podestá, F.E. The functional organization and control of plant respiration. CRC Crit. Rev. Plant Sci. 2006, 25, 160–189. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of storage temperature and duration on physiological responses of pomegranate fruit. Ind. Crops Prod. 2013, 47, 300–309. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Espin, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agris. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Haffner, K.; Rosenfeld, H.J.; Skrede, G.; Wang, L. Quality of red raspberry Rubus idaeus L. cultivars after storage in controlled and normal atmospheres. Postharvest Biol Technol. 2002, 24, 279–289. [Google Scholar] [CrossRef]
- Veazie, P.P.; Collins, J.K. Quality of erect-type blackberry fruit after short intervals of controlled atmosphere storage. Postharvest Biol. Technol. 2002, 25, 35–239. [Google Scholar]
- Romero, I.; Sanchez-Ballesta, M.T.; Maldonado, R.; Escribano, M.I.; Merodio, C. Anthocyanin, antioxidant activity and stress-induced gene expression in high CO2-treated table grapes stored at low temperature. J. Plant Physiol. 2008, 165, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, S.; Pacifico, S.; Yadollahi, A.; Lettieri, A.; Nocera, P.; Piccolella, S. Red-fleshed apples: Old autochthonous fruits as a novel source of anthocyanin antioxidants. Plant Foods Hum. Nutr. 2015, 70, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Smanalieva, J.; Iskakova, J.; Oskonbaeva, Z.; Wichern, F.; Darr, D. Investigation of nutritional characteristics and free radical scavenging activity of wild apple, pear, rosehip, and barberry from the walnut-fruit forests of Kyrgyzstan. Eur. Food Res. Technol. 2020, 246, 1095–1104. [Google Scholar] [CrossRef]
- Ma, Y.; Ban, Q.; Shi, J.; Dong, T.; Jiang, C.Z.; Wang, W. 1-Methylcyclopropene (1-MCP), storage time, and shelf life and temperature affect phenolic compounds and antioxidant activity of ‘Jonagold’ apple. Postharvest Biol. Technol. 2019, 150, 71–79. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Feygenberg, O.; Maurer, D.; Alkan, N. Improved cold tolerance of mango fruit with enhanced anthocyanin and flavonoid contents. Molecules 2018, 23, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lata, B. Apple peel antioxidant status in relation to genotype, storage type and time. Sci. Hortic. 2008, 117, 45–52. [Google Scholar] [CrossRef]
- Monk, L.S.; Braendle, R.; Crawford, R.M.M. Catalase activity and post-anoxic injury in monocotyledonous species. J. Exp. Bot. 1987, 38, 233–246. [Google Scholar] [CrossRef]
- Feng, S.; Yi, J.; Li, X.; Wu, X.; Zhao, Y.; Ma, Y.; Bi, J. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Dekempeneer, E.; Keulemans, J. Rocket-powered high-performance liquid chromatographic analysis of plant ascorbate and glutathione. Anal. Biochem. 2003, 316, 74–81. [Google Scholar] [CrossRef]
- Li, M.J.; Ma, F.W.; Zhang, M.; Pu, F. Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala). Plant Sci. 2008, 174, 606–612. [Google Scholar] [CrossRef]
- Piretti, M.V.; Gallerani, G.; Brodnik, U. Polyphenol polymerisation involvement in apple superficial scald. Postharvest Biol. Technol. 1996, 8, 11–18. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Wojdyło, A.; Markowski, J.; Siucińska, K. 1-Methylcyclopropene postharvest treatment and their effect on apple quality during long-term storage time. Eur. Food Res. Technol. 2014, 239, 603–612. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Yang, Z.F.; Chen, X.H. Effect of high oxygen atmospheres on fruit decay and quality in Chinese bayberries, strawberries and blueberries. Food Control 2008, 19, 470–474. [Google Scholar] [CrossRef]
- Fawbush, F.; Nock, J.F.; Watkins, C.B. Antioxidant contents and activity of 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apples in air and controlled atmosphere storage. Postharvest Biol. Technol. 2009, 52, 30–37. [Google Scholar] [CrossRef]
- Awad, M.A.; de Jager, A. Flavonoid and chlorogenic acid concentrations in skin of ‘Jonagold’ and ‘Elstar’ apples during and after regular and ultra-low oxygen storage. Postharvest Biol. Technol. 2000, 20, 15–24. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Bobinas, C.; Janulis, V. Variation of triterpenes in apples stored in a controlled atmosphere. Molecules 2021, 26, 3639. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Liaudanskas, M.; Kviklys, D.; Zymone, Z.; Raudonis, R.; Viskelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef] [Green Version]
- Butkeviciute, A.; Liaudanskas, M.; Kviklys, D.; Gelvonauskiene, D.; Janulis, V. The qualitative and quantitative compositions of phenolic compounds in fruits of Lithuanian heirloom apple cultivars. Molecules 2020, 25, 5263. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskas, M.; Viskelis, P.; Kviklys, D.; Raudonis, R.; Janulis, V. A comparative study of phenolic content in apple fruits. Int. J. Food Prop. 2015, 18, 945–953. [Google Scholar] [CrossRef]
- Williams, W.B.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Cekanavicius, V.; Murauskas, G. Applied Regression Analysis in Social Research; Vilnius University Press: Vilnius, Lithuania, 2014; p. 124. [Google Scholar]




| Variant | Amount of Oxygen (O2), % | Amount of Carbon Dioxide (CO2), % | Amount of Nitrogen (N2), % | Relative Humidity, % | Temperature, °C |
|---|---|---|---|---|---|
| I | 21 | 0.03 | 78.97 | 95 ± 3 | +1.5 ± 0.5 |
| II | 5 | 1 | 94 | ||
| III | 5 | 3 | 92 | ||
| IV | 5 | 5 | 90 | ||
| V | 5 | 7 | 88 | ||
| VI | 1 | 3 | 96 | ||
| VII | 10 | 3 | 87 | ||
| VIII | 20 | 3 | 77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Janulis, V. Impact of Storage Controlled Atmosphere on the Apple Phenolic Acids, Flavonoids, and Anthocyanins and Antioxidant Activity In Vitro. Plants 2022, 11, 201. https://doi.org/10.3390/plants11020201
Butkeviciute A, Viskelis J, Liaudanskas M, Viskelis P, Janulis V. Impact of Storage Controlled Atmosphere on the Apple Phenolic Acids, Flavonoids, and Anthocyanins and Antioxidant Activity In Vitro. Plants. 2022; 11(2):201. https://doi.org/10.3390/plants11020201
Chicago/Turabian StyleButkeviciute, Aurita, Jonas Viskelis, Mindaugas Liaudanskas, Pranas Viskelis, and Valdimaras Janulis. 2022. "Impact of Storage Controlled Atmosphere on the Apple Phenolic Acids, Flavonoids, and Anthocyanins and Antioxidant Activity In Vitro" Plants 11, no. 2: 201. https://doi.org/10.3390/plants11020201







