Drought Stress Induces Morpho-Physiological and Proteome Changes of Pandanus amaryllifolius
Abstract
:1. Introduction
2. Results
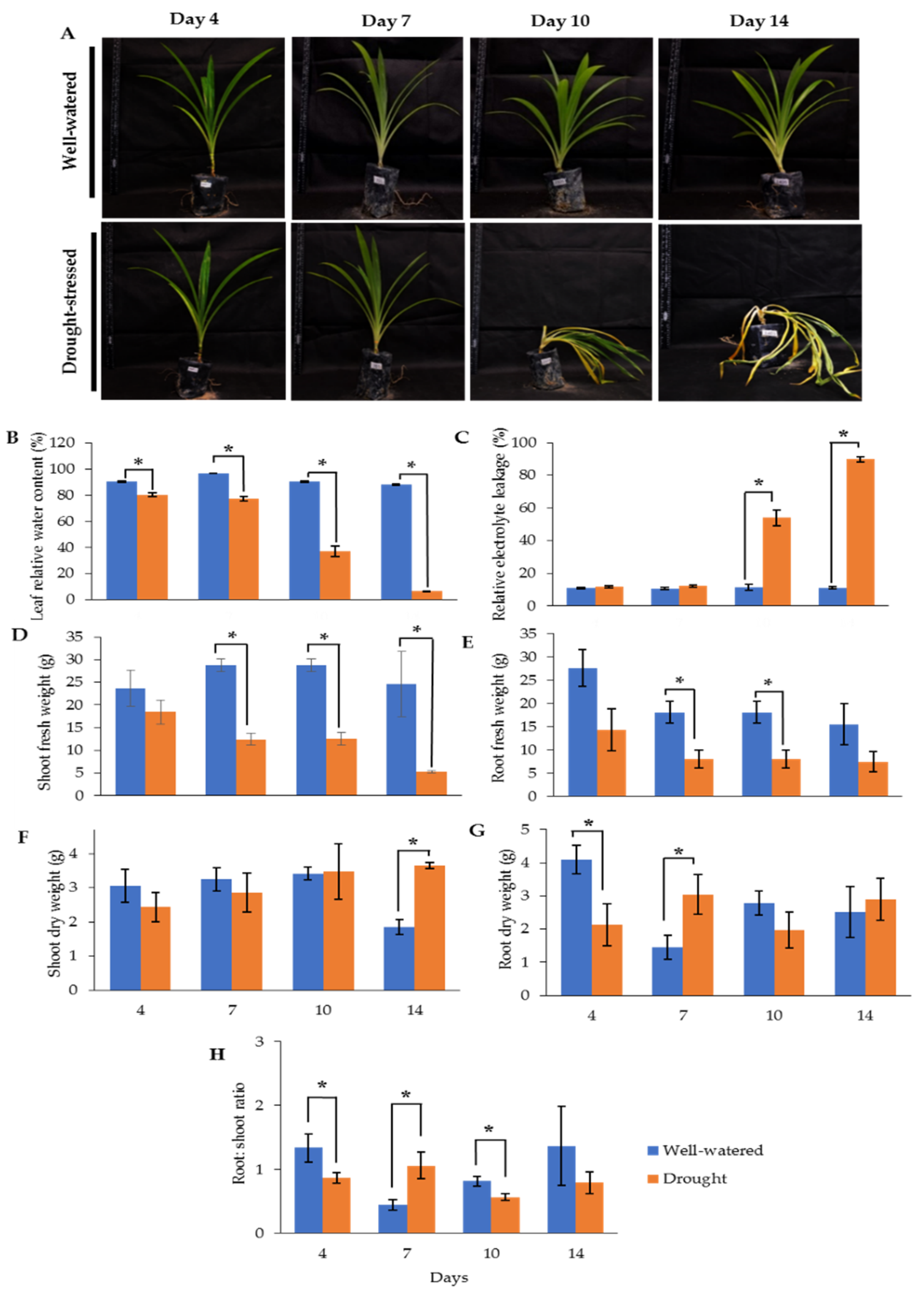
2.1. Morphological Changes of Drought-Stressed Pandanus amaryllifolius
2.2. Changes of Proline and Malondialdehyde Contents in Pandanus Plants under Drought Stress
2.3. Antioxidant Enzyme Changes in Pandanus Plants in Response to Drought Stress
2.4. Protein Changes in Well-Watered, Drought-Stressed, and Recovered Pandanus Plants
3. Discussion
3.1. Drought Stress Affected the Growth and Antioxidant Enzyme Activities of Pandanus Plants
3.2. Stress and Defense Protein Abundance under Drought Stress
3.3. Cell Membrane-Related Proteins Increased under Drought Stress
3.4. Carbohydrate Metabolism-Related Protein Abundance under Drought Stress
4. Materials and Methods
4.1. Plant Material and Drought Treatment
4.2. Determination of Leaf Relative Water Content
4.3. Measurement of Relative Electrolyte Leakage
4.4. Measurement of Plant Weight
4.5. Automated Colorimetric Assay
4.6. Measurement of Chlorophyll Content
4.7. Determination of Malondialdehyde Content
4.8. Measurement of Proline Content
4.9. Antioxidant Enzymatic Assays
4.9.1. Superoxide Dismutase
4.9.2. Catalase
4.9.3. Ascorbate Peroxidase
4.9.4. Peroxidase
4.9.5. Glutathione Reductase
4.9.6. Hydrogen Peroxide
4.10. Protein Extraction and Quantification
4.11. Protein Preparation
4.12. Protein TMT-Labelling and Fractionation
4.13. Liquid Chromatography-Tandem Mass Spectroscopy
4.14. Proteomics Data Analysis
4.15. Functional Classification of Proteins
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security: 2017; FAO: Rome, Italy, 2018; ISBN 978-92-5-130359-7. [Google Scholar]
- Foreign Agricultural Service USDA. Available online: https://www.fas.usda.gov/data/thailand-impact-drought-agriculture-2020 (accessed on 21 September 2021).
- Zulkarami, B.; Razi, I.M.; Halimi, M.S.; Mondal, M.A.; Panhwar, Q.A.; Islam, M.R. Effectiveness of different phytohormones on grain filling and yield of rice (Oryza sativa L.) under drought stress. J. Food Agri. Environ. 2014, 12, 697–700. [Google Scholar]
- Nalina, M.; Saroja, S.; Chakravarthi, M.; Rajkumar, R.; Radhakrishnan, B.; Chandrashekara, K.N. Water deficit-induced oxidative stress and differential response in antioxidant enzymes of tolerant and susceptible tea cultivars under field condition. Acta. Physiol. Plant. 2021, 43, 10. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Shi, W.; David-Schwartz, R.; Li, S.; Yang, F.; Lin, Z. Transcriptome profiling reveals the effects of drought tolerance in giant juncao. BMC Plant. Biol. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wang, S.; Jing, D.; Liang, G. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2021, 181, 104291. [Google Scholar] [CrossRef]
- Bankaji, I.; Sleimi, N.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Identification and expression of the cucurbita WRKY transcription factors in response to water deficit and salt stress. Sci. Hortic. 2019, 256, 108562. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef]
- Santos, J.; Oliveira, L.E.; Coelho, V.T.; Lopes, G.; Souza, T.; Porto, A.C.; Lira, J.; Massote, R.; Rocha, C.; Gomes, M.P. Performance of hevea brasiliensis under drought conditions on osmoregulation and antioxidant activity through evaluation of vacuolar invertase and reducing sugars. Plant. Sci. Today 2021, 8, 312–323. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and proteomic responses of mulberry trees (Morus alba L.) to combined salt and drought stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Liu, Y.; Li, C. Tandem mass tag-based (TMT) quantitative proteomics analysis reveals the response of fine roots to drought stress in cotton (Gossypium hirsutum L.). BMC Plant. Biol. 2020, 20, 328. [Google Scholar] [CrossRef]
- Goche, T.; Shargie, N.G.; Cummins, I.; Brown, A.P.; Chivasa, S.; Ngara, R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020, 10, 11835. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, S.K.; Misra, S.; Pandey, V.; Agrawal, L.; Nautiyal, C.S.; Chauhan, P.S. Revealing the complexity of protein abundance in chickpea root under drought-stress using a comparative proteomics approach. Plant. Physiol. Biochem. 2020, 151, 88–102. [Google Scholar] [CrossRef]
- Azri, W.; Cosette, P.; Guillou, C.; Rabhi, M.; Nasr, Z.; Mliki, A. Physiological and proteomic responses to drought stress in leaves of two wild grapevines (Vitis sylvestris): A comparative study. Plant. Growth Regul. 2020, 91, 37–52. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, F.; Zou, R.; Liu, J.; Yan, Y. Integrated physiological and chloroplast proteome analysis of wheat seedling leaves under salt and osmotic stresses. J. Proteomics 2021, 234, 104097. [Google Scholar] [CrossRef] [PubMed]
- Amnan, M.A.M.; Pua, T.-L.; Lau, S.-E.; Tan, B.C.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. Osmotic stress in banana is relieved by exogenous nitric oxide. PeerJ 2021, 9, e10879. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13, 341. [Google Scholar] [CrossRef] [Green Version]
- Reshidan, N.H.; Abd Muid, S.; Mamikutty, N. The effects of Pandanus amaryllifolius (Roxb.) leaf water extracts on fructose-induced metabolic syndrome rat model. BMC Complement. Altern. Med. 2019, 19, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agroforestry.org. Available online: https://agroforestry.org/images/pdfs/P.tectorius-pandanus.pdf (accessed on 13 January 2022).
- Gurmeet, S.; Amrita, P. Unique Pandanus—Flavour, Food and Medicine. J. Pharma. Phytochem. 2015, 3, 8–14. [Google Scholar]
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 1–25. ISBN 978-981-10-9029-5. [Google Scholar]
- Reyes, J.A.O.; Carpentero, A.S.; Santos, P.J.A.; Delfin, E.F. Effects of water regime, genotype, and formative stages on the agro-physiological response of sugarcane (Saccharum officinarum L.) to drought. Plants 2020, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Júnior, S.D.O.; de Andrade, J.R.; dos Santos, C.M.; Silva, A.L.J.; Endres, L.; Silva, J.V.; dos Santos Silva, L.K. Osmoregulators’ accumulation minimizes the effects of drought stress in sugarcane and contributes to the recovery of photochemical efficiency in photosystem II after rewatering. Acta. Physiol. Plant. 2020, 42, 62. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, K.; Wang, Y.Q.; Zhang, Z.P.; Lu, F.; Yu, H.Q.; Zou, J.Q. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Sanjari, S.; Shobbar, Z.-S.; Ghanati, F.; Afshari-Behbahanizadeh, S.; Farajpour, M.; Jokar, M.; Khazaei, A.; Shahbazi, M. Molecular, chemical, and physiological analyses of sorghum leaf wax under post-flowering drought stress. Plant. Physiol. Biochem. 2021, 159, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Oraee, A.; Tehranifar, A. Evaluating the potential drought tolerance of pansy through its physiological and biochemical responses to drought and recovery periods. Sci. Hortic. 2020, 265, 109225. [Google Scholar] [CrossRef]
- Dinç, E.; Ceppi, M.G.; Tóth, S.Z.; Bottka, S.; Schansker, G. The chl a fluorescence intensity is remarkably insensitive to changes in the chlorophyll content of the leaf as long as the chl a/b ratio remains unaffected. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 770–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Feng, L.; Liu, Q.; Wu, X.; Fan, Y.; Raza, M.A.; Cheng, Y.; Chen, J.; Wang, X.; Yong, T.; et al. Effect of interactions between light intensity and red-to-far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ. Exp. Bot. 2018, 150, 79–87. [Google Scholar] [CrossRef]
- Lau, S.E.; Hamdan, M.F.; Pua, T.L.; Saidi, N.B.; Tan, B.C. Plant nitric oxide signaling under drought stress. Plants 2021, 10, 360. [Google Scholar] [CrossRef]
- Fiasconaro, M.L.; Lovato, M.E.; Antolín, M.C.; Clementi, L.A.; Torres, N.; Gervasio, S.; Martín, C.A. Role of proline accumulation on fruit quality of pepper (Capsicum annuum L.) grown with a K-Rich compost under drought conditions. Sci. Hortic. 2019, 249, 280–288. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Xie, Y.; Su, L.; Zhang, R.; Wang, H.; Li, C.; Long, S. Drought resistance mechanisms of Phedimus aizoon L. Sci. Rep. 2021, 11, 13600. [Google Scholar] [CrossRef] [PubMed]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta. Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline protects plants against abiotic oxidative stress: Biochemical and molecular mechanisms. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 477–522. ISBN 978-0-12-799963-0. [Google Scholar]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Nagaraju, M.; Kumar, A.; Rajasheker, G.; Manohar Rao, D.; Kavi Kishor, P.B. DnaJs, the critical drivers of Hsp70s: Genome-wide screening, characterization, and expression of DnaJ family genes in Sorghum bicolor. Mol. Biol. Rep. 2020, 47, 7379–7390. [Google Scholar] [CrossRef]
- Carmo, L.S.T.; Martins, A.C.Q.; Martins, C.C.C.; Passos, M.A.S.; Silva, L.P.; Araujo, A.C.G.; Brasileiro, A.C.M.; Miller, R.N.G.; Guimarães, P.M.; Mehta, A. comparative proteomics and gene expression analysis in Arachis duranensis reveal stress response proteins associated to drought tolerance. J. Proteom. 2019, 192, 299–310. [Google Scholar] [CrossRef]
- Wang, T.; Ye, C.; Wang, M.; Chu, G. Identification of cold-stress responsive proteins in Anabasis aphylla seedlings via the ITRAQ proteomics technique. J. Plant. Interact. 2017, 12, 505–519. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, T.; Li, M.-X.; Zhao, C.-L.; Jia, N.; Wang, X.-X.; Sun, Y.-Z.; Li, G.-L.; Xu, M.; Zhou, R.-G.; et al. The Arabidopsis j-protein atdjb1 facilitates thermotolerance by protecting cells against heat-induced oxidative damage. New Phytol. 2012, 194, 364–378. [Google Scholar] [CrossRef]
- Sirohi, P.; Yadav, B.S.; Afzal, S.; Mani, A.; Singh, N.K. Identification of drought stress-responsive genes in rice (Oryza sativa) by meta-analysis of microarray data. J. Genet. 2020, 99, 35. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Fang, B.; Wang, W.; Yang, Y.; Rao, L.; Zhang, C. Genome-wide analysis of the rice j-protein family: Identification, genomic organization, and expression profiles under multiple stresses. 3 Biotech 2019, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Chakraborty, S.; Datta, A.; Chakraborty, N. Proteomics approach to identify dehydration responsive nuclear proteins from chickpea (Cicer arietinum L.). Mol. Cell. Proteom. 2008, 7, 88–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, M.K.; Basu, D.; Datta, A.; Chakraborty, N.; Chakraborty, S. Dehydration-Responsive nuclear proteome of rice (Oryza sativa L.) illustrates protein network, novel regulators of cellular adaptation, and evolutionary perspective. Mol. Cell. Proteom. 2009, 8, 1579–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, F.; Kiani-Pouya, A.; Shabala, L.; Li, L.; Tahir, A.; Yu, M.; Hedrich, R.; Chen, Z.; Wilson, R.; Zhang, H.; et al. Salinity effects on guard cell proteome in Chenopodium quinoa. Int. J. Mol. Sci. 2021, 22, 428. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; van der Graaff, E.; Albacete, A.; Eom, S.H.; Großkinsky, D.K.; Böhm, H.; Janschek, U.; Rim, Y.; Ali, W.W.; Kim, S.Y.; et al. The Arabidopsis PLAT domain protein1 is critically involved in abiotic stress tolerance. PLoS ONE 2014, 9, e112946. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Blanchoin, L.; Staiger, C.J. Signaling to actin stochastic dynamics. Annu. Rev. Plant. Biol. 2015, 66, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Liu, S.-D.; Ge, C.-W.; Zhang, X.-M.; Zhang, S.-P.; Chen, J.; Shen, Q.; Ju, F.-Y.; Yang, Y.-F.; Li, Y.; et al. Association analysis of drought tolerance and associated traits in upland cotton at the seedling stage. Int. J. Mol. Sci. 2019, 20, 3888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, T.J.; Deeks, M.J.; Wang, P.; Hussey, P.J. The evolution of the actin binding NET superfamily. Front. Plant. Sci. 2014, 5, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.C. α-l-Arabinofuranosidases: Biochemistry, molecular biology and application in biotechnology. Biotechnol. Adv. 2000, 18, 403–423. [Google Scholar] [CrossRef]
- Zhu, J.; Alvarez, S.; Marsh, E.L.; LeNoble, M.E.; Cho, I.-J.; Sivaguru, M.; Chen, S.; Nguyen, H.T.; Wu, Y.; Schachtman, D.P.; et al. Cell wall proteome in the maize primary root elongation zone. ii. region-specific changes in water soluble and lightly ionically bound proteins under water deficit. Plant. Physiol. 2007, 145, 1533–1548. [Google Scholar] [CrossRef] [Green Version]
- López-Hinojosa, M.; de María, N.; Guevara, M.A.; Vélez, M.D.; Cabezas, J.A.; Díaz, L.M.; Mancha, J.A.; Pizarro, A.; Manjarrez, L.F.; Collada, C.; et al. rootstock effects on scion gene expression in maritime pine. Sci. Rep. 2021, 11, 11582. [Google Scholar] [CrossRef]
- Nemati, M.; Piro, A.; Norouzi, M.; Moghaddam Vahed, M.; Nisticò, D.M.; Mazzuca, S. Comparative physiological and leaf proteomic analyses revealed the tolerant and sensitive traits to drought stress in two wheat parental lines and their F6 progenies. Environ. Exp. Bot. 2019, 158, 223–237. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant. Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.-R.; Islam, M.T.; Park, S.-H.; Lee, H.; Bae, D.-W.; Kim, T.-H. Antagonistic shifting from abscisic acid- to salicylic acid-mediated sucrose accumulation contributes to drought tolerance in Brassica napus. Environ. Exp. Bot. 2019, 162, 38–47. [Google Scholar] [CrossRef]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water Status. Plant. Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Quan, W.; Hu, Y.; Mu, Z.; Shi, H.; Chan, Z. Overexpression of AtPYL5 under the control of guard cell specific promoter improves drought stress tolerance in arabidopsis. Plant. Physiol. Biochem. 2018, 129, 150–157. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bresson, J.; Bieker, S.; Riester, L.; Doll, J.; Zentgraf, U. A guideline for leaf senescence analyses: From quantification to physiological and molecular investigations. J. Exp. Bot. 2018, 69, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: San Diego, CA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant. Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: San Diego, CA, USA, 1974; pp. 673–684. ISBN 978-0-12-091302-2. [Google Scholar]
- Chen, G.-X.; Asada, K. Inactivation of ascorbate peroxidase by thiols requires hydrogen peroxide. Plant. Cell Physiol. 1992, 33, 117–123. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Mannervik, B. Measurement of glutathione reductase activity. Curr. Protoc. Tox. 1999, 1, 7.2.1–7.2.4. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant. Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, E.; Wang, W.; Scali, M.; Cresti, M. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nat. Protoc. 2014, 9, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, J.A.; Côté, R.G.; Csordas, A.; Dianes, J.A.; Fabregat, A.; Foster, J.M.; Griss, J.; Alpi, E.; Birim, M.; Contell, J.; et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: Status in 2013. Nucleic Acids Res. 2013, 41, D1063–D1069. [Google Scholar] [CrossRef] [PubMed]







| Accession | Protein | Biological Process | Function | Cluster a |
|---|---|---|---|---|
| F1SWA0 | Zerumbone synthase | Protein synthesis | Oxidoreductase | 1 |
| P49043 | Vacuolar-processing enzyme | Cysteine-type endopeptidase | Hydrolase | 1 |
| P48711 | Ribulose bisphosphate carboxylase large chain | Photorespiration | Magnesium ion binding | 1 |
| Q9FLN4 | 50S ribosomal protein L27, chloroplastic | Ribonucleoprotein | mRNA binding | 1 |
| A0A357 | 30S ribosomal protein S18, chloroplastic | Ribonucleoprotein | rRNA binding | 1 |
| A1E9N5 | 30S ribosomal protein S7, chloroplastic | Ribonucleoprotein | rRNA binding | 1 |
| O23760 | Caffeic acid 3-O-methyltransferase | Lignin biosynthesis | Methyltransferase | 1 |
| B2LMP1 | 30S ribosomal protein S15, chloroplastic | Ribonucleoprotein | Structural constituent of ribosome | 1 |
| A2WXD9 | Photosystem II 22 kDa protein 1, chloroplastic | Photosynthesis | Non-photochemical quenching | 1 |
| Q9XF91 | Photosystem II 22 kDa protein, chloroplastic | Photosynthesis | Non-photochemical quenching | 1 |
| Q32RY4 | 30S ribosomal protein S4, chloroplastic | Ribonucleoprotein | rRNA binding | 1 |
| O24461 | Ras-related protein Rab7 | Protein transport | GTPase activity | 1 |
| O22925 | Vacuolar-sorting receptor 2 | Protein transport | Calcium ion binding | 2 |
| Q940M2 | Alanine-glyoxylate aminotransferase 2 homolog 1, mitochondrial | Photorespiration | Aminotransferase | 2 |
| Q9LUI2 | Protein NETWORKED 1A | Cytoskeleton | Actin binding protein | 2 |
| P43644 | DnaJ protein homolog ANJ1 | Stress response | Chaperone | 2 |
| P11143 | Heat shock 70 kDa protein | Stress response | Chaperone | 2 |
| A4QLY6 | Photosystem I iron-sulfur center | Photosynthesis (ET) | Oxidoreductase | 2 |
| Q05737 | GTP-binding protein YPTM2 | Protein transport | GTPase activity | 2 |
| Q04960 | DnaJ protein homolog | Stress response | Chaperone | 2 |
| Q9XIM0 | CCG-binding protein 1 | Cellular response to hypoxia | Mediator complex binding | 3 |
| P81370 | Thaumatin-like protein | Plant defence | Pathogenesis | 3 |
| Q6DBP4 | Pectin acetylesterase 8 | Cell wall biogenesis/degradation | Hydrolase | 3 |
| Q9FLC0 | Peroxidase 52 | Hydrogen peroxide | Oxidoreductase | 3 |
| Q96520 | Peroxidase 12 | Hydrogen peroxide | Oxidoreductase | 3 |
| P48980 | Beta-galactosidase | Carbohydrate metabolism | Glycosidase | 3 |
| Q01289 | Protochlorophyllide reductase, chloroplastic | Chlorophyll biosynthesis | Oxidoreductase | 3 |
| P26792 | Beta-fructofuranosidase, insoluble isoenzyme 1 | Carbohydrate metabolism | Glycosidase | 3 |
| Q0DM51 | DEAD-box ATP-dependent RNA helicase 3, chloroplastic | Ribosome biogenesis | Hydrolase | 3 |
| F6H7K5 | Thiamine thiazole synthase 2, chloroplastic | Thiamine biosynthesis | Transferase | 3 |
| Q9LN49 | 3-ketoacyl-CoA synthase 4 | Acyltransferase | Fatty acid biosynthesis | 3 |
| Q75LR2 | Phospho-2-dehydro-3-deoxyheptonate aldolase 1, chloroplastic | Amino acid biosynthesis | Transferase | 3 |
| O82627 | Granule-bound starch synthase 1, chloroplastic/amyloplastic | Starch biosynthesis | Glycosyltransferase | 3 |
| Q8W0A1 | Beta-galactosidase 2 | Carbohydrate metabolism | Glycosidase | 3 |
| O23787 | Thiamine thiazole synthase, chloroplastic | Thiamine biosynthesis | Transferase | 3 |
| Q9ZQ94 | UDP-glycosyltransferase 73C5 | Brassinosteroid metabolism | Glycosyltransferase | 3 |
| O80731 | Pectin acetylesterase 3 | Cell wall biogenesis/degradation | Hydrolase | 3 |
| Q9C992 | 3-ketoacyl-CoA synthase 7 | Acyltransferase | Fatty acid biosynthesis | 3 |
| O22436 | Magnesium-chelatase subunit ChlI, chloroplastic | Chlorophyll biosynthesis | Ligase | 3 |
| Q84P54 | Gamma aminobutyrate transaminase 1, mitochondrial | Biotin biosynthesis | Aminotransferase | 3 |
| A5JTQ2 | Beta-xylosidase/alpha-L-arabinofuranosidase 1 (Fragment) | Carbohydrate metabolism | Glycosidase | 3 |
| Q42850 | Protochlorophyllide reductase B, chloroplastic | Chlorophyll biosynthesis | Oxidoreductase | 3 |
| Q9SD46 | Peroxidase 36 | Hydrogen peroxide | Oxidoreductase | 3 |
| O04931 | Alpha-glucosidase | Carbohydrate metabolism | Glycosidase | 3 |
| Q08937 | 29 kDa ribonucleoprotein B, chloroplastic | mRNA processing | Ribonucleoprotein | 3 |
| Q5ZE07 | Multicopper oxidase LPR1 homolog 2 | Phosphate homeostasis | Oxidoreductase | 3 |
| A4S6Y4 | Lon protease homolog, mitochondrial | Oxidative stress | DNA binding | 3 |
| Q40147 | Glutamate-1-semialdehyde 2,1-aminomutase, chloroplastic | Chlorophyll biosynthesis | Isomerase | 3 |
| Q9LIK0 | Plastidial pyruvate kinase 1, chloroplastic | Glycolysis | Kinase | 3 |
| Q6STH5 | Fe-S cluster assembly factor HCF101, chloroplastic | iron-sulphur cluster assembly | 4Fe-4S cluster binding | 3 |
| Q0E3C8 | Chaperone protein ClpB3, mitochondrial | Stress response | Chaperone | 3 |
| Q94LW3 | Homeobox protein knotted-1-like 3 | Mucilage biosynthesis | DNA binding | 3 |
| Q42600 | Cytochrome P450 84A1 | Phenylpropanoid biosynthesis | Monooxygenase | 3 |
| Q56UD0 | Beta-fructofuranosidase, insoluble isoenzyme 6 | Carbohydrate metabolism | Glycosidase | 3 |
| Q8L7S6 | Beta-hexosaminidase 3 | Carbohydrate metabolism | Glycosidase | 3 |
| Q39613 | Peptidyl-prolyl cis-trans isomerase | Protein folding | Chaperone | 3 |
| Q9SJ20 | Ribonucleoside-diphosphate reductase large subunit | DNA replication | Oxidoreductase | 3 |
| Q75GT3 | Chaperone protein ClpB2, chloroplastic | Stress response | Chaperone | 3 |
| Q9ZUU4 | RNA-binding protein CP29B, chloroplastic | mRNA processing | Ribonucleoprotein | 3 |
| Q9M591 | Magnesium-protoporphyrin IX monomethyl ester [oxidative] cyclase, chloroplastic | Chlorophyll biosynthesis | Oxidoreductase | 3 |
| Q9CA67 | Geranylgeranyl diphosphate reductase, chloroplastic | Chlorophyll biosynthesis | Oxidoreductase | 3 |
| P50246 | Adenosylhomocysteinase | One-carbon metabolism | Hydrolase | 3 |
| Q6ZIV7 | Hypersensitive-induced response protein 1 | Potassium ion channel regulation | Histidine kinase binding | 3 |
| Q9SI75 | Elongation factor G, chloroplastic | Protein biosynthesis | Elongation factor | 3 |
| P24846 | 4-hydroxy-tetrahydrodipicolinate synthase 1, chloroplastic | Amino acid biosynthesis | Allosteric enzyme | 3 |
| Q41932 | Oxygen-evolving enhancer protein 3-2, chloroplastic | Photosynthesis (ET) | Calcium ion binding | 4 |
| P25795 | Aldehyde dehydrogenase family 7 member A1 | Stress response | Oxidoreductase | 4 |
| Q9AXH0 | Catalase | Hydrogen peroxide | Oxidoreductase | 4 |
| O65660 | PLAT domain-containing protein 1 | Stress response | Catalase | 4 |
| A2YH64 | Catalase isozyme B | Hydrogen peroxide | Oxidoreductase | 4 |
| Q0E4K1 | Catalase isozyme A | Hydrogen peroxide | Oxidoreductase | 4 |
| O04932 | Probable sucrose-phosphate synthase 1 | Glycosyltransferase | Sucrose biosynthesis | 4 |
| Q570C8 | 3-ketoacyl-CoA thiolase 5, peroxisomal | Acyltransferase | Fatty acid biosynthesis | 4 |
| Q9SG80 | Alpha-L-arabinofuranosidase 1 | L-arabinose metabolic | Hydrolase | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amnan, M.A.M.; Aizat, W.M.; Khaidizar, F.D.; Tan, B.C. Drought Stress Induces Morpho-Physiological and Proteome Changes of Pandanus amaryllifolius. Plants 2022, 11, 221. https://doi.org/10.3390/plants11020221
Amnan MAM, Aizat WM, Khaidizar FD, Tan BC. Drought Stress Induces Morpho-Physiological and Proteome Changes of Pandanus amaryllifolius. Plants. 2022; 11(2):221. https://doi.org/10.3390/plants11020221
Chicago/Turabian StyleAmnan, Muhammad Asyraf Mohd, Wan Mohd Aizat, Fiqri Dizar Khaidizar, and Boon Chin Tan. 2022. "Drought Stress Induces Morpho-Physiological and Proteome Changes of Pandanus amaryllifolius" Plants 11, no. 2: 221. https://doi.org/10.3390/plants11020221
APA StyleAmnan, M. A. M., Aizat, W. M., Khaidizar, F. D., & Tan, B. C. (2022). Drought Stress Induces Morpho-Physiological and Proteome Changes of Pandanus amaryllifolius. Plants, 11(2), 221. https://doi.org/10.3390/plants11020221







