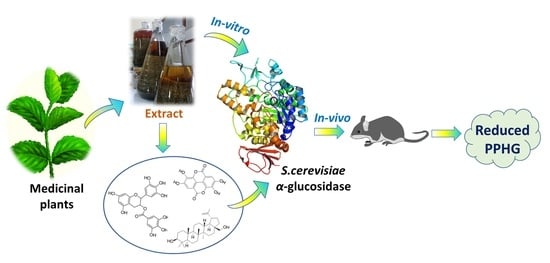
Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes
Abstract
:1. Introduction
2. Alpha-Glucosidases Structure and Mechanism of Action

3. Plant Extracts as α-Glucosidase Inhibitor Sources
4. Plant-Derived Bioactive Compounds as Potential α-Glucosidase Inhibitors
4.1. Flavonoids
4.2. Terpenoids
4.3. Phenolic Acids and Their Derivatives
4.4. Polysaccharides
4.5. Tannins
4.6. Other Secondary Metabolites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alrefai, H.; Allababidi, H.; Levy, S.; Levy, J. The Endocrine System in Diabetes Mellitus. Endocrine 2002, 18, 105–120. [Google Scholar] [CrossRef]
- IDF. International Diabetes Federation IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 1 August 2022).
- Bae, J.H.; Han, K.-D.; Ko, S.-H.; Yang, Y.S.; Choi, J.H.; Choi, K.M.; Kwon, H.-S.; Won, K.C. Diabetes Fact Sheet in Korea 2021. Diabetes Metab. J. 2022, 46, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar] [PubMed]
- Kumar Tripathi, B.; Srivastava, A.K. Diabetes mellitus: Complications and therapeutics RA130. Med. Sci. Monit. 2006, 12, 130–147. [Google Scholar]
- Li, M.; Song, L.; Qin, X. Advances in the cellular immunological pathogenesis of type 1 diabetes. J. Cell. Mol. Med. 2014, 18, 749–758. [Google Scholar] [CrossRef]
- Rashid, K.; Chowdhury, S.; Ghosh, S.; Sil, P.C. Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochem. Pharmacol. 2017, 143, 140–155. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Esser, N.; Paquot, N.; Scheen, A.J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs 2015, 24, 283–307. [Google Scholar] [CrossRef]
- Association, A.D. Standards of Medical Care in Diabetes—2014. Diabetes Care 2013, 37, S11–S66. [Google Scholar] [CrossRef] [Green Version]
- Bello, N.A.; Pfeffer, M.A.; Skali, H.; McGill, J.B.; Rossert, J.; Olson, K.A.; Weinrauch, L.; Cooper, M.E.; de Zeeuw, D.; Rossing, P.; et al. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ Open Diabetes Res. Care 2014, 2, e000011. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Hua, D.; Huang, D.; Zhang, Q.; Yan, C. Characterization of a new heteropolysaccharide from green guava and its application as an α-glucosidase inhibitor for the treatment of type II diabetes. Food Funct. 2018, 9, 3997–4007. [Google Scholar] [CrossRef]
- Chiasson, J.-L. Acarbose for the Prevention of Diabetes, Hypertension, and Cardiovascular Disease in Subjects with Impaired Glucose Tolerance: The Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (Stop-Niddm) Trial. Endocr. Pract. 2006, 12, 25–30. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Shen, Y. Voglibose (Basen, AO-128), One of the Most Important α-Glucosidase Inhibitors. Curr. Med. Chem. 2006, 13, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Krentz, A.J.; Bailey, C.J. Oral Antidiabetic Agents. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef]
- Sugihara, H.; Nagao, M.; Harada, T.; Nakajima, Y.; Tanimura-Inagaki, K.; Okajima, F.; Tamura, H.; Inazawa, T.; Otonari, T.; Kawakami, M.; et al. Comparison of three α-glucosidase inhibitors for glycemic control and bodyweight reduction in Japanese patients with obese type 2 diabetes. J. Diabetes Investig. 2014, 5, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Basak, P.; Sadhukhan, P.; Sarkar, P.; Sil, P.C. Perspectives of the Nrf-2 signaling pathway in cancer progression and therapy. Toxicol. Rep. 2017, 4, 306–318. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sinha, M.; Sil, P.C. Arsenic-induced oxidative cerebral disorders: Protection by taurine. Drug Chem. Toxicol. 2009, 32, 93–102. [Google Scholar] [CrossRef]
- Ghosh, S.; Basak, P.; Dutta, S.; Chowdhury, S.; Sil, P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. 2017, 103, 41–55. [Google Scholar] [CrossRef]
- Manna, P.; Sinha, M.; Sil, P.C. Cadmium induced testicular pathophysiology: Prophylactic role of taurine. Reprod. Toxicol. 2008, 26, 282–291. [Google Scholar] [CrossRef]
- Manna, P.; Sinha, M.; Sil, P.C. Prophylactic role of arjunolic acid in response to streptozotocin mediated diabetic renal injury: Activation of polyol pathway and oxidative stress responsive signaling cascades. Chem. Biol. Interact. 2009, 181, 297–308. [Google Scholar] [CrossRef]
- Manna, P.; Ghosh, J.; Das, J.; Sil, P.C. Streptozotocin induced activation of oxidative stress responsive splenic cell signaling pathways: Protective role of arjunolic acid. Toxicol. Appl. Pharmacol. 2010, 244, 114–129. [Google Scholar] [CrossRef]
- Manna, P.; Ghosh, M.; Ghosh, J.; Das, J.; Sil, P.C. Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: Role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology 2012, 6, 1–21. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim. Biophys. Acta—Gen. Subj. 2016, 1860, 2065–2075. [Google Scholar] [CrossRef]
- Sinha, M.; Manna, P.; Sil, P.C. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. J. Nat. Med. 2007, 61, 251–260. [Google Scholar] [CrossRef]
- Kashtoh, H.; Hussain, S.; Khan, A.; Saad, S.M.; Khan, J.A.J.; Khan, K.M.; Perveen, S.; Choudhary, M.I. Oxadiazoles and thiadiazoles: Novel α-glucosidase inhibitors. Bioorg. Med. Chem. 2014, 22, 5454–5465. [Google Scholar] [CrossRef]
- Niaz, H.; Kashtoh, H.; Khan, J.A.J.; Khan, A.; Wahab, A.T.; Alam, M.T.; Khan, K.M.; Perveen, S.; Choudhary, M.I. Synthesis of diethyl 4-substituted-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylates as a new series of inhibitors against yeast α-glucosidase. Eur. J. Med. Chem. 2015, 95, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kashtoh, H.; Muhammad, M.T.; Khan, J.J.A.; Rasheed, S.; Khan, A.; Perveen, S.; Javaid, K.; Atia-Tul-Wahab; Khan, K.M.; Choudhary, M.I. Dihydropyrano [2,3-c] pyrazole: Novel in vitro inhibitors of yeast α-glucosidase. Bioorg. Chem. 2016, 65, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.L.; Breslin, P.A.S. High Endogenous Salivary Amylase Activity Is Associated with Improved Glycemic Homeostasis following Starch Ingestion in Adults. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyrot des Gachons, C.; Breslin, P.A.S. Salivary Amylase: Digestion and Metabolic Syndrome. Curr. Diabetes Rep. 2016, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, S.A.K.; Withers, S.G. Unusual enzymatic glycoside cleavage mechanisms. Acc. Chem. Res. 2014, 47, 226–235. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Sotomayor, M.; Quezada-Calvillo, R.; Avery, S.E.; Chacko, S.K.; Yan, L.; Lin, A.H.-M.; Ao, Z.; Hamaker, B.R.; Nichols, B.L. Maltase-Glucoamylase Modulates Gluconeogenesis and Sucrase-Isomaltase Dominates Starch Digestion Glucogenesis. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 704–712. [Google Scholar] [CrossRef]
- Nichols, B.L.; Avery, S.; Sen, P.; Swallow, D.M.; Hahn, D.; Sterchi, E. The maltase-glucoamylase gene: Common ancestry to sucrase-isomaltase with complementary starch digestion activities. Proc. Natl. Acad. Sci. USA 2003, 100, 1432–1437. [Google Scholar] [CrossRef] [Green Version]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-amylase and starch digestion: An interesting marriage. Starch—Stärke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of starch digestion by α -amylase—Structural basis for kinetic properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Sim, L.; Quezada-Calvillo, R.; Sterchi, E.E.; Nichols, B.L.; Rose, D.R. Human Intestinal Maltase–Glucoamylase: Crystal Structure of the N-Terminal Catalytic Subunit and Basis of Inhibition and Substrate Specificity. J. Mol. Biol. 2008, 375, 782–792. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C.; DeLano, W. PyMOL Molecular Graphic System Version 2020, 2. Available online: https://pymol.org/2/support.html? (accessed on 1 August 2022).
- Sim, L.; Jayakanthan, K.; Mohan, S.; Nasi, R.; Johnston, B.D.; Pinto, B.M.; Rose, D.R. New Glucosidase Inhibitors from an Ayurvedic Herbal Treatment for Type 2 Diabetes: Structures and Inhibition of Human Intestinal Maltase-Glucoamylase with Compounds from Salacia reticulata. Biochemistry 2010, 49, 443–451. [Google Scholar] [CrossRef]
- Elferink, H.; Bruekers, J.P.J.; Veeneman, G.H.; Boltje, T.J. A comprehensive overview of substrate specificity of glycoside hydrolases and transporters in the small intestine. Cell. Mol. Life Sci. 2020, 77, 4799–4826. [Google Scholar] [CrossRef]
- Sim, L.; Willemsma, C.; Mohan, S.; Naim, H.Y.; Mario Pinto, B.; Rose, D.R. Structural Basis for Substrate Selectivity in Human Maltase-Glucoamylase and Sucrase-Isomaltase N-terminal Domains. J. Biol. Chem. 2010, 285, 17763. [Google Scholar] [CrossRef]
- Ren, L.; Qin, X.; Cao, X.; Wang, L.; Bai, F.; Bai, G.; Shen, Y. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell 2011, 2, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Tesar, C.; Wilton, R.; Keigher, L.; Babnigg, G.; Joachimiak, A. Novel α-glucosidase from human gut microbiome: Substrate specificities and their switch. FASEB J. 2010, 24, 3939–3949. [Google Scholar] [CrossRef] [Green Version]
- Bishoff, H. Pharmacological properties of the novel glucosidase inhibitors BAY m 1099 (miglitol) and BAY o 1248. Diabetes Res. Clin. Pract. 1985, 1, S53. [Google Scholar]
- Horii, S.; Fukase, H.; Matsuo, T.; Kameda, Y.; Asano, N.; Matsui, K. Synthesis and a-D-Glucosidase Inhibitory Activity of N-Substituted Valiolamine Derivatives as Potential Oral Antidiabetic Agents. J. Med. Chem. 1986, 29, 1038–1046. [Google Scholar] [CrossRef]
- Englyst, H.N.; Hay, S.; Macfarlane, G.T. Polysaccharide breakdown by mixed populations of human faecal bacteria. FEMS Microbiol. Ecol. 1987, 3, 163–171. [Google Scholar] [CrossRef]
- Weaver, G.A.; Tangel, C.T.; Krause, J.A.; Parfitt, M.M.; Jenkins, P.L.; Rader, J.M.; Lewis, B.A.; Miller, T.L.; Wolin, M.J. Acarbose Enhances Human Colonic Butyrate Production. J. Nutr. 1997, 127, 717–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, S.; Reddy, G.D.; Reddy, B.M.; Ramesh, M.; Rao, A.V.N.A. Antihyperglycemic activity of Caralluma attenuata. Fitoterapia 2003, 74, 274–279. [Google Scholar] [CrossRef]
- Suba, V.; Murugesan, T.; Arunachalam, G.; Mandal, S.C.; Saha, B.P. Anti-diabetic potential of Barleria lupulina extract in rats. Phytomedicine 2004, 11, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Hafeez, J.; Khalifa, A.S.; Naeem, M.; Ali, T.; Eed, E.M. In vitro and in vivo study of inhibitory potentials of α-glucosidase and acetylcholinesterase and biochemical profiling of M. charantia in alloxan-induced diabetic rat models. Am. J. Transl. Res. 2022, 14, 3824–3839. [Google Scholar] [PubMed]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M.; et al. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M.; et al. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Alqahtani, Y.S.; Mahnashi, M.H.; Alyami, B.A.; Alqarni, A.O.; Huneif, M.A.; Nahari, M.H.; Ali, A.; Javed, Q.; Ilyas, H.; Rafiq, M. Preparation of Spice Extracts: Evaluation of Their Phytochemical, Antioxidant, Antityrosinase, and Anti-α-Glucosidase Properties Exploring Their Mechanism of Enzyme Inhibition with Antibrowning and Antidiabetic Studies in Vivo. Biomed. Res. Int. 2022, 2022, 9983124. [Google Scholar] [CrossRef]
- Kim, H.R.; Paulrayer, A.; Kwon, Y.G.; Ryu, D.G.; Baek, D.G.; Geum, J.H.; Lee, J.H.; Lee, G.S.; Kwon, K.B. Acute effects of Amomum villosum Lour. fruit extract on postprandial glycemia and insulin secretion: A single-blind, placebo-controlled, crossover study in healthy subjects. Saudi J. Biol. Sci. 2020, 27, 2968–2971. [Google Scholar] [CrossRef]
- Kim, H.R.; Antonisamy, P.; Kim, Y.S.; Lee, G.; Ham, H.D.; Kwon, K.B. Inhibitory effect of Amomum villosum water extracts on α-glucosidase activity. Physiol. Mol. Plant Pathol. 2022, 117, 101779. [Google Scholar] [CrossRef]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef]
- Li, M.; Luo, X.; Ho, C.-T.; Li, D.; Guo, H.; Xie, Z. A new strategy for grading of Lu’an guapian green tea by combination of differentiated metabolites and hypoglycaemia effect. Food Res. Int. 2022, 159, 111639. [Google Scholar] [CrossRef]
- Xie, L.; Yu, D.; Li, Y.; Ju, H.; Chen, J.; Hu, L.; Yu, L. Characterization, Hypoglycemic Activity, and Antioxidant Activity of Methanol Extracts from Amomum tsao-ko: In vitro and in vivo Studies. Front. Nutr. 2022, 9, 869749. [Google Scholar] [CrossRef]
- Naseem, S.; Ismail, H. In vitro and in vivo evaluations of antioxidative, anti-Alzheimer, antidiabetic and anticancer potentials of hydroponically and soil grown Lactuca sativa. BMC Complement. Med. Ther. 2022, 22, 30. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Alqahtani, Y.S.; Alqarni, A.O.; Alyami, B.A.; Alqahtani, O.S.; Jan, M.S.; Hussain, F.; Islam, Z.U.; Ullah, F.; Ayaz, M.; et al. Phytochemistry, anti-diabetic and antioxidant potentials of Allium consanguineum Kunth. BMC Complement. Med. Ther. 2022, 22, 154. [Google Scholar] [CrossRef]
- Medha, M.M.; Devnath, H.S.; Biswas, B.; Bokshi, B.; Sadhu, S.K. In silico profiling of analgesic and antihyperglycemic effects of ethanolic leaves extract of Amischotolype mollissima: Evidence from in vivo studies. Saudi J. Biol. Sci. 2022, 29, 103312. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shakouri Khomartash, M.; Gorgani-Firuzjaee, S.; Vahidi, M.; Motevalli Khiavi, F.; Taslimi, P. α-glucosidase inhibitory, antioxidant activity, and GC/MS analysis of Descurainia sophia methanolic extract: In vitro, in vivo, and in silico studies. Arab. J. Chem. 2022, 15, 104055. [Google Scholar] [CrossRef]
- Zhang, L.L.; Han, L.; Yang, S.Y.; Meng, X.M.; Ma, W.F.; Wang, M. The mechanism of interactions between flavan-3-ols against a-glucosidase and their in vivo antihyperglycemic effects. Bioorg. Chem. 2019, 85, 364–372. [Google Scholar] [CrossRef]
- Rynjah, C.V.; Devi, N.N.; Khongthaw, N.; Syiem, D.; Majaw, S. Evaluation of the antidiabetic property of aqueous leaves extract of Zanthoxylum armatum DC. using in vivo and in vitro approaches. J. Tradit. Complement. Med. 2018, 8, 134–140. [Google Scholar] [CrossRef]
- Yang, S.-E.; Lin, Y.-F.; Liao, J.-W.; Chen, J.-T.; Chen, C.-L.; Chen, C.-I.; Hsu, S.-L.; Song, T.-Y. Insulin sensitizer and antihyperlipidemic effects of Cajanus cajan (L.) millsp. root in methylglyoxal-induced diabetic rats. Chin. J. Physiol. 2022, 65, 125. [Google Scholar] [CrossRef]
- Yue, H.; Wang, L.; Jiang, S.; Banma, C.; Jia, W.; Tao, Y.; Zhao, X. Hypoglycemic effects of Rhodiola crenulata (HK. f. et. Thoms) H. Ohba in vitro and in vivo and its ingredient identification by UPLC-triple-TOF/MS. Food Funct. 2022, 13, 1659–1667. [Google Scholar] [CrossRef]
- Zhang, X.F.; Tang, Y.J.; Guan, X.X.; Lu, X.; Li, J.; Chen, X.L.; Deng, J.L.; Fan, J.M. Flavonoid constituents of Amomum tsao-ko Crevost et Lemarie and their antioxidant and antidiabetic effects in diabetic rats—In vitro and in vivo studies. Food Funct. 2022, 13, 437–450. [Google Scholar] [CrossRef]
- Abu-Odeh, A.; Shehadeh, M.; Suaifan, G.A.R.Y.; Karameh, N.; Rahman, D.A.; Kandil, Y. In Vitro and In Vivo Antidiabetic Activity, Phenolic Content and Microscopical Characterization of Terfezia claveryi. Molecules 2022, 27, 4843. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Kong, F.; He, L.; Fang, L.; Shu, Q. Quantitative analysis of resveratrol derivatives in the seed coats of tree peonies and their hypoglycemic activities in vitro/vivo. Food Funct. 2022, 13, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Bouknana, S.; Daoudi, N.E.; Bouhrim, M.; Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Bnouham, M. Ammodaucus leucotrichus Coss. & Durieu: Antihyperglycemic activity via the inhibition of α-amylase, α-glucosidase, and intestinal glucose absorption activities and its chemical composition. J. Pharm. Pharmacogn. Res. 2022, 10, 94–103. [Google Scholar] [CrossRef]
- Ortega, R.; Valdés, M.; Alarcón-Aguilar, F.J.; Fortis-Barrera, Á.; Barbosa, E.; Velazquez, C.; Calzada, F. Antihyperglycemic Effects of Salvia polystachya Cav. and Its Terpenoids: α-Glucosidase and SGLT1 Inhibitors. Plants 2022, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Amin, E.; Abdel-Bakky, M.S.; Darwish, M.A.; Mohammed, H.A.; Chigurupati, S.; Qureshi, K.A.; Hassan, M.H.A. The Glycemic Control Potential of Some Amaranthaceae Plants, with Particular Reference to In Vivo Antidiabetic Potential of Agathophora alopecuroides. Molecules 2022, 27, 973. [Google Scholar] [CrossRef]
- Zhang, X.; Rehman, R.U.; Wang, S.; Ji, Y.; Li, J.; Liu, S.; Wang, H. Blue honeysuckle extracts retarded starch digestion by inhibiting glycosidases and changing the starch structure. Food Funct. 2022, 13, 6072–6088. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Li, J.; Zhang, Z.; He, Y.; Yang, H.; Zhou, P. Inhibition on α-Glucosidase Activity and Non-Enzymatic Glycation by an Anti-Oxidative Proteoglycan from Ganoderma lucidum. Molecules 2022, 27, 1457. [Google Scholar] [CrossRef]
- Teng, B.S.; Wang, C.D.; Yang, H.J.; Wu, J.S.; Zhang, D.; Zheng, M.; Fan, Z.H.; Pan, D.; Zhou, P. A protein tyrosine phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. J. Agric. Food Chem. 2011, 59, 6492–6500. [Google Scholar] [CrossRef]
- Abd El Hafeez, M.S.; El Gindi, O.; Hetta, M.H.; Aly, H.F.; Ahmed, S.A. Quality Control, Anti-Hyperglycemic, and Anti-Inflammatory Assessment of Colvillea racemosa Leaves Using In Vitro, In Vivo Investigations and Its Correlation with the Phytoconstituents Identified via LC-QTOF-MS and MS/MS. Plants 2022, 11, 830. [Google Scholar] [CrossRef]
- Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B.; Singh, R.; Andola, H.C.; Joshi, S.K.; Semwal, D.K. Antidiabetic effect of aqueous-ethanol extract from the aerial parts of Artemisia roxburghiana. Nat. Prod. Res. 2020, 36, 1300–1305. [Google Scholar] [CrossRef]
- Saadullah, M.; Asif, M.; Uzair, M.; Afzal, S.; Rashid, S.A.; Rashad, M.; Bashir, R.; Mahmood, S.; Batool, J.A. Pharmacological evaluation of the hypoglycemic and anti-Alzheimer’s activities of aerial parts of Breynia distachia (Phyllanthaceae). Trop. J. Pharm. Res. 2022, 21, 579–587. [Google Scholar] [CrossRef]
- Muddatstsir, I.; Risky, S.E.; Setyo Purnomo, A.; Fahimah, M.; Sri, F. Antidiabetic, cytotoxic and antioxidant activities of Rhodomyrtus tomentosa leaf extracts. RSC Adv. 2022, 12, 25697–25710. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Hu, X.; Li, C.; Wang, L. Bio-affinity ultra-filtration combined with HPLC-ESI-qTOF-MS/MS for screening potential α-glucosidase inhibitors from Cerasus humilis (Bge.) Sok. leaf-tea and in silico analysis. Food Chem. 2022, 373, 131528. [Google Scholar] [CrossRef]
- Yuca, H.; Özbek, H.; Demirezer, L.Ö.; Güvenalp, Z. Assessment of the α-glucosidase and α-amylase inhibitory potential of Paliurus spina-christi Mill. and its terpenic compounds. Med. Chem. Res. 2022, 31, 1393–1399. [Google Scholar] [CrossRef]
- Divneet Kaur, N.K.; Chopra, A. A comprehensive review on phytochemistry and pharmacological activities of Vernonia amygdalina. Pharmacogn. Phytochem. 2018, 2018, 2629–2636. [Google Scholar] [CrossRef] [Green Version]
- Vonia, S.; Hartati, R.; Insanu, M. In Vitro Alpha-Glucosidase Inhibitory Activity and the Isolation of Luteolin from the Flower of Gymnanthemum amygdalinum (Delile) Sch. Bip ex Walp. Molecules 2022, 27, 2132. [Google Scholar] [CrossRef]
- Xiong, G.; Ma, L.; Zhang, H.; Li, Y.; Zou, W.; Wang, X.; Xu, Q.; Xiong, J.; Hu, Y.; Wang, X. Physicochemical properties, antioxidant activities and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 2022, 194, 484–498. [Google Scholar] [CrossRef]
- Bhuyan, P.; Ganguly, M.; Baruah, I.; Borgohain, G. Bioactive compounds isolated from black rice bran: Combined in vitro and in silico evidence supporting the antidiabetic effect of black rice. RSC Adv. 2022, 12, 22650–22661. [Google Scholar] [CrossRef]
- Vinodhini, S.; Rajeswari, V.D. Exploring the antidiabetic and anti-obesity properties of Samanea saman through in vitro and in vivo approaches. J. Cell. Biochem. 2019, 120, 1539–1549. [Google Scholar] [CrossRef]
- Ma, L.F.; Yan, J.J.; Lang, H.Y.; Jin, L.C.; Qiu, F.J.; Wang, Y.J.; Xi, Z.F.; Shan, W.G.; Zhan, Z.J.; Ying, Y.M. Bioassay-guided isolation of lanostane-type triterpenoids as α-glucosidase inhibitors from Ganoderma hainanense. Phytochem. Lett. 2019, 29, 154–159. [Google Scholar] [CrossRef]
- Subramanian, R.; Asmawi, M.Z.; Sadikun, A. In vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Pol. 2008, 55, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-glucosidase activity by selected edible seaweeds and fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef]
- Aslam, H.; Khan, A.; Naureen, H.; Ali, F.; Ullah, F.; Sadiq, A. Potential application of Conyza canadensis (L) Cronquist in the management of diabetes: In vitro and in vivo evaluation. Trop. J. Pharm. Res. 2018, 17, 1287–1293. [Google Scholar] [CrossRef]
- Shihabudeen, H.M.S.; Priscilla, D.H.; Thirumurugan, K.; Mohamed Sham Shihabudeen, H.; Hansi Priscilla, D.; Thirumurugan, K.; Shihabudeen, H.M.S.; Priscilla, D.H.; Thirumurugan, K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Funct. Foods Connect. Nutr. Health Food Sci. 2013, 8, 289–314. [Google Scholar] [CrossRef]
- Agawane, S.B.; Gupta, V.S.; Kulkarni, M.J.; Bhattacharya, A.K.; Koratkar, S.S. Chemo-biological evaluation of antidiabetic activity of Mentha arvensis L. and its role in inhibition of advanced glycation end products. J. Ayurveda Integr. Med. 2019, 10, 166–170. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Zhai, J.; Han, N.; Liu, Z.; Li, S.; Yin, J. Characterization of antioxidant, α-glucosidase and tyrosinase inhibitors from the rhizomes of Potentilla anserina L. and their structure–activity relationship. Food Chem. 2021, 336, 127714. [Google Scholar] [CrossRef]
- Ning, Z.W.; Zhai, L.X.; Huang, T.; Peng, J.; Hu, D.; Xiao, H.T.; Wen, B.; Lin, C.Y.; Zhao, L.; Bian, Z.X. Identification of α-glucosidase inhibitors from: Cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019, 10, 1893–1902. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, C.; Weng, P.; Zhang, X.; Xia, Q.; Wu, Z.; Liu, L.; Xiao, J. In vitro evaluation of digestive enzyme inhibition and antioxidant effects of naked oat phenolic acid compound (OPC). Int. J. Food Sci. Technol. 2020, 55, 2531–2540. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.; Lu, X.; Wang, Y. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Pham, Q.T.; Luong, T.N.H.; Le, H.K.; Vo, V.G. Potential Antidiabetic Activity of Extracts and Isolated Compound from Adenosma bracteosum (Bonati). Biomolecules 2020, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Salahuddin, M.A.H.; Ismail, A.; Kassim, N.K.; Hamid, M.; Ali, M.S.M. Phenolic profiling and evaluation of in vitro antioxidant, α-glucosidase and α-amylase inhibitory activities of Lepisanthes fruticosa (Roxb) Leenh fruit extracts. Food Chem. 2020, 331, 127240. [Google Scholar] [CrossRef]
- Antu, K.A.; Riya, M.P.; Mishra, A.; Anilkumar, K.S.; Chandrakanth, C.K.; Tamrakar, A.K.; Srivastava, A.K.; Raghu, K.G. Antidiabetic property of Symplocos cochinchinensis is mediated by inhibition of alpha glucosidase and enhanced insulin sensitivity. PLoS ONE 2014, 9, e105829. [Google Scholar] [CrossRef] [PubMed]
- Floris, S.; Fais, A.; Medda, R.; Pintus, F.; Piras, A.; Kumar, A.; Kuś, P.M.; Westermark, G.T.; Era, B. Washingtonia filifera seed extracts inhibit the islet amyloid polypeptide fibrils formations and α-amylase and α-glucosidase activity. J. Enzyme Inhib. Med. Chem. 2021, 36, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Lin, H.R.; Yang, C.S.; Liaw, C.C.; Sung, P.J.; Kuo, Y.H.; Cheng, M.J.; Chen, J.J. Antioxidant and Anti-α-Glucosidase Activities of Various Solvent Extracts and Major Bioactive Components from the Fruits of Crataegus pinnatifida. Antioxidants 2022, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, X.; Sun, J.; Wang, Z. Phytochemical Composition, Antioxidant Activity, α-Glucosidase and Acetylcholinesterase Inhibitory Activity of Quinoa Extract and Its Fractions. Molecules 2022, 27, 2420. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Ogi, K.; Sumitani, H. Elucidation of an α-glucosidase inhibitor from the peel of Allium cepa by principal component analysis. Biosci. Biotechnol. Biochem. 2019, 83, 751–754. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Ren, C.; Pan, Y.; Li, J.; Zhao, X.; Xu, C.; Chen, K.; Li, X.; Gao, Z. Two Myricetin-Derived Flavonols from Morella rubra Leaves as Potent α-Glucosidase Inhibitors and Structure-Activity Relationship Study by Computational Chemistry. Oxid. Med. Cell. Longev. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Tian, J.L.; Si, X.; Wang, Y.H.; Gong, E.S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit α-glucosidase and α-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef]
- Ni, M.; Hu, X.; Gong, D.; Zhang, G. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocoll. 2020, 105, 105824. [Google Scholar] [CrossRef]
- Sgariglia, M.A.; Garibotto, F.M.; Soberón, J.R.; Angelina, E.L.; Andujar, S.A.; Vattuone, M.A. Study of polyphenols from Caesalpinia paraguariensis as α-glucosidase inhibitors: Kinetics and structure–activity relationship. New J. Chem. 2022, 46, 11044–11055. [Google Scholar] [CrossRef]
- Le, T.-K.-D.K.D.; Danova, A.; Aree, T.; Duong, T.-H.H.; Koketsu, M.; Ninomiya, M.; Sawada, Y.; Kamsri, P.; Pungpo, P.; Chavasiri, W. α-Glucosidase Inhibitors from the Stems of Knema globularia. J. Nat. Prod. 2022, 85, 776–786. [Google Scholar] [CrossRef]
- Ouyang, J.K.; Dong, L.M.; Xu, Q.L.; Wang, J.; Liu, S.B.; Qian, T.; Yuan, Y.F.; Tan, J.W. Triterpenoids with α-glucosidase inhibitory activity and cytotoxic activity from the leaves of Akebia trifoliata. RSC Adv. 2018, 8, 40483–40489. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; Elgamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; Alajmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.L.; Li, S.B.; Yan, M.Q.; Luo, Q.; Wang, L.S.; Shen, L.L.; Liao, M.L.; Lu, C.H.; Liu, X.Y.; Liang, C.Q. Bioactive dammarane triterpenoid saponins from the leaves of Cyclocarya paliurus. Phytochemistry 2021, 183, 112618. [Google Scholar] [CrossRef]
- Tran, C.-L.; Dao, T.-B.-N.; Tran, T.-N.; Mai, D.-T.; Tran, T.-M.-D.; Tran, N.-M.-A.; Dang, V.-S.; Vo, T.-X.; Duong, T.-H.; Sichaem, J.; et al. Alpha-Glucosidase Inhibitory Diterpenes from Euphorbia antiquorum Growing in Vietnam. Molecules 2021, 26, 2257. [Google Scholar] [CrossRef]
- Chen, K.; Liu, X.Q.; Wang, W.L.; Luo, J.G.; Kong, L.Y. Taxumarienes A–G, seven new α-glucosidase inhibitory taxane-diterpenoids from the leaves of Taxus mairei. Bioorg. Chem. 2020, 94, 103400. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Lan, Q.; Su, B.-J.; Chen, Z.-F.; Liang, D. Structurally diverse abietane-type Diterpenoids from the aerial parts of Gaultheria leucocarpa var. yunnanensis. Phytochemistry 2022, 201, 113255. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Wang, G.; Liu, W.; Seeram, N.P.; Mu, Y.; Xu, Y.; Huang, X.; Li, L. Phenolics from Eugenia jambolana seeds with advanced glycation endproduct formation and alpha-glucosidase inhibitory activities. Food Funct. 2018, 9, 4246–4254. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Yu, J.; Chen, X.; Zhang, S.; Cai, Y.; Li, L. A new functionality study of vanillin as the inhibitor for α-glucosidase and its inhibition kinetic mechanism. Food Chem. 2021, 353, 129448. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Cao, J.J.; Liu, R.; Chen, H.Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, R.; Yu, S.; Zhao, W. A novel α-glucosidase inhibitor polysaccharide from Sargassum fusiforme. Int. J. Food Sci. Technol. 2022, 57, 67–77. [Google Scholar] [CrossRef]
- Zheng, Q.; Jia, R.-B.; Ou, Z.-R.; Li, Z.-R.; Zhao, M.; Luo, D.; Lin, L. Comparative study on the structural characterization and α-glucosidase inhibitory activity of polysaccharide fractions extracted from Sargassum fusiforme at different pH conditions. Int. J. Biol. Macromol. 2022, 194, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Y.; Chanu, M.B.; Mondal, G.; Manna, P.; Chattoraj, A.; Chandra Deka, D.; Chandra Talukdar, N.; Chandra Borah, J. Procyanidin A2, an anti-diabetic condensed tannin extracted from Wendlandia glabrata, reduces elevated G-6-Pase and mRNA levels in diabetic mice and increases glucose uptake in CC1 hepatocytes and C1C12 myoblast cells. RSC Adv. 2019, 9, 17211–17219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LEE, S.-H.; PARK, M.-H.; KANG, S.-M.; KO, S.-C.; KANG, M.-C.; CHO, S.; PARK, P.-J.; JEON, B.-T.; KIM, S.-K.; HAN, J.-S.; et al. Dieckol Isolated from Ecklonia cava Protects against High-Glucose Induced Damage to Rat Insulinoma Cells by Reducing Oxidative Stress and Apoptosis. Biosci. Biotechnol. Biochem. 2012, 76, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.-N.; Guo, L.-B.; He, J.; Liu, P.-H.; Tian, H.-Y.; Zhang, W.-K.; Xu, J.-K. Diverse gallotannins with α-glucosidase and α-amylase inhibitory activity from the roots of Euphorbia fischeriana steud. Phytochemistry 2022, 202, 113304. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-B.; Tian, J.-Y.; Dai, Z.; Ye, F.; Ma, S.-C.; Wang, A.-G. A-Glucosidase inhibitors extracted from the roots of Polygonum multiflorum Thunb. Fitoterapia 2017, 117, 65–70. [Google Scholar] [CrossRef]
- Tran, H.H.T.; Nguyen, M.C.; Le, H.T.; Nguyen, T.L.; Pham, T.B.; Chau, V.M.; Nguyen, H.N.; Nguyen, T.D. Inhibitors of α-glucosidase and α-amylase from Cyperus rotundus. Pharm. Biol. 2014, 52, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xie, L.; Xie, J.; Liu, Y.; Chen, W. Pelargonidin-3-O-rutinoside as a novel α-glucosidase inhibitor for improving postprandial hyperglycemia. Chem. Commun. 2019, 55, 39–42. [Google Scholar] [CrossRef]
- Guang, C.J.; Wu, S.F.; Zhang, Q.F.; Yin, Z.P.; Zhang, L. α-Glucosidase inhibitory effect of anthocyanins from Cinnamomum camphora fruit: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 143, 696–703. [Google Scholar] [CrossRef]
- Jung, H.; Ali, M.; Choi, J. Promising Inhibitory Effects of Anthraquinones, Naphthopyrone, and Naphthalene Glycosides, from Cassia obtusifolia on α-Glucosidase and Human Protein Tyrosine Phosphatases 1B. Molecules 2016, 22, 28. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, C.W.; Lee, J.I.; Vinh, L.B.; Kim, K.T.; Cho, I.S. An investigation of the inhibitory mechanism of α-glucosidase by chysalodin from Aloe vera. Int. J. Biol. Macromol. 2020, 147, 314–318. [Google Scholar] [CrossRef]
- Duong, T.H.; Hang, T.X.H.; Le Pogam, P.; Tran, T.N.; Mac, D.H.; Dinh, M.H.; Sichaem, J. α-Glucosidase Inhibitory Depsidones from the Lichen Parmotrema tsavoense. Planta Med. 2020, 86, 776–781. [Google Scholar] [CrossRef]
- Trang, D.T.; Yen, D.T.H.; Cuong, N.T.; Anh, L.T.; Hoai, N.T.; Tai, B.H.; Van Doan, V.; Yen, P.H.; Quang, T.H.; Nhiem, N.X.; et al. Pregnane glycosides from Gymnema inodorum and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2021, 35, 2157–2163. [Google Scholar] [CrossRef]
- Choucry, M.A.; Shalabi, A.A.; El Halawany, A.M.; El-Sakhawy, F.S.; Zaiter, A.; Morita, H.; Chaimbault, P.; Abdel-Sattar, E. New Pregnane Glycosides Isolated from Caralluma hexagona Lavranos as Inhibitors of α-Glucosidase, Pancreatic Lipase, and Advanced Glycation End Products Formation. ACS Omega 2021, 6, 18881–18889. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Yu, M.-H.; Xu, L.-J.; Niu, L.-X.; Huang, C.-Y.; Xu, H.; Yang, P.-M.; Hu, X. Diels–Alder type adducts with potent alpha-glucosidase inhibitory activity from Morus macroura. Phytochem. Lett. 2018, 26, 149–153. [Google Scholar] [CrossRef]
- Quan, Y.S.; Zhang, X.Y.; Yin, X.M.; Wang, S.H.; Jin, L.L. Potential α-glucosidase inhibitor from Hylotelephium erythrostictum. Bioorg. Med. Chem. Lett. 2020, 30, 127665. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Li, J.-B.; Zhang, X.; Zhou, N.; Zhang, W.-Y.; Lu, H. Prenylated xanthones with α-glucosidase and α-amylase inhibitory effects from the pericarp of Garcinia mangostana. J. Asian Nat. Prod. Res. 2022, 24, 624–633. [Google Scholar] [CrossRef]
- Proença, C.; Ribeiro, D.; Freitas, M.; Fernandes, E. Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3137–3207. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [Green Version]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Biotechnol. Isoprenoids 2015, 148, 63–106. [Google Scholar]
- Greay, S.J.; Hammer, K.A. Recent developments in the bioactivity of mono- and diterpenes: Anticancer and antimicrobial activity. Phytochem. Rev. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Kim, K.-H.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phyther. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Novel polysaccharide from Chaenomeles speciosa seeds: Structural characterization, α-amylase and α-glucosidase inhibitory activity evaluation. Int. J. Biol. Macromol. 2020, 153, 755–766. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary characterization, antioxidant and α-glucosidase inhibitory activities of polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef]
- Wang, B.-H.; Cao, J.-J.; Zhang, B.; Chen, H.-Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef]
- Gong, P.; Guo, Y.; Chen, X.; Cui, D.; Wang, M.; Yang, W.; Chen, F. Structural Characteristics, Antioxidant and Hypoglycemic Activities of Polysaccharide from Siraitia grosvenorii. Molecules 2022, 27, 4192. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Szczurek, A. Perspectives on Tannins. Biomolecules 2021, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-products of agri-food industry as tannin-rich sources: A review of tannins’ biological activities and their potential for valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]








| Name | Extract/Part Used | Model | Type of Study | Tested Substance Dosage | Administration Route | Assessing Criterion | Effect on Animal Blood Glucose Level | Ref. |
|---|---|---|---|---|---|---|---|---|
| Momordica charantia | Methanol extract | Male albino Wistar rats | Alloxan-induced diabetes | 200 mg/kg BW | Oral | Fasting blood glucose (FBG) and insulin levels | Hypoglycemic | [52] |
| Artemisia absinthium L. | Aqueous extract/leaves | Wistar rats | Alloxan-induced diabetes | 200 mg/kg BW | Oral | PBGL | Hypoglycemic | [56] |
| Star anise | Ethyl acetate extract/ fruit | Rabbits | Alloxan-mono-hydrate-induced diabetes | 250 mg/kg BW | Injection | Blood glucose levels (BGL) and body weight | Hypoglycemic | [57] |
| Amomum villosum | Water extracts/ fruit | Male SD rats | Sucrose loading test (SLT) (2 g/kg BW) | 250 and 500 mg/kg BW | Oral | BGL | Hypoglycemic | [59] |
| Merremia tridentata (L.) | Ethanol extract (SE) and flavonoid-rich fraction (FF)/stem | Mice | Alloxan-induced diabetic | SE (100 mg/kg BW) and FF (50, 75 mg/kg BW) | Oral | BGL and body weight | Hypoglycemic | [60] |
| Lu’an guapian green tea | Methanol extract | Male mice | GTT and ITT | - | Oral | Post prandial hyperglycemia effect | Hypoglycemic | [61] |
| Amomum tsao-ko | Methanol extract | Mice | STZ-induced diabetes | 100 and 200 mg/kg BW | Oral | FBG | Hypoglycemic | [62] |
| Lactuca sativa | Methanol extract | Male SD rats | STZ-induced diabetes | 50, 100 and 200 mg/kg BW | Oral | BGL | Hypoglycemic | [63] |
| Allium consanguineum | Compounds 1 and 2 isolated from the plant | Albino mice | Alloxan-induced diabetic oral glucose tolerance test (OGTT) | 500, 250, 125, 62.5 and 31.25 μg/kg BW | Oral | Postprandial effect | Hypoglycemic | [64] |
| Amischotolype mollissima | Ethanolic leaves extract | Swiss albino mice | OGTT (2 gm/kg BW) | 250 and 500 mg/kg BW | Oral | FBG No cytotoxicity of the extract until 4000 mg/kg BW | Hypoglycemic | [65] |
| Descurainia sophia | Methanolic flower extract | Male Wistar rats | Alloxan-induced diabetic | 2.25 and 4.50 g/kg BW | Oral | Blood glucose level | Hypoglycemic | [66] |
| Catechin and epicatechin | Phenolic extract | Male SD rats | SLT (2 g/kg BW) | 20 mg/kg BW | Oral | PBG level | Hypoglycemic | [67] |
| Zanthoxylum armatum | Aqueous leaves extract | Female Swiss albino mice | Alloxan-induced diabetes | 100–4000 mg/kg BW | Oral | Hypoglycemic activity | Hypoglycemic | [68] |
| Lethal dose | LD50 5000 mg/kg | |||||||
| Cajanus cajan (L.) | Ethanol extract | Wistar rats | Methylglyoxal (MGO)-induced insulin resistance | 10, 50 and 100 mg/kg BW | Oral | (OGTT), (ITT)/BGL | Hypoglycemic/ dose-dependent | [69] |
| Rhodiola crenulata | Ethanol extract/ root | Male SD Rat/male Kunming (KM) mice | Alloxan-induced diabetes in mice/OSTT in mice | and 400 mg/kg BW | Oral | Post carb. glucose level | Hypoglycemic | [70] |
| Amomum tsao-ko Crevost and Lemarie | Methanol extract flavonoid constituent | Male SD Rats | STZ-induced diabetes | 100 mg/kg BW | Oral | Postprandial glucose level (OGTT)/FBG | Hypoglycemic | [71] |
| Terfezia claveryi | Aqueous extract Phenolic content | Male BALB/c mice | High-fat diet alloxan-induced diabetic mice | 250 and 500 mg/kg BW | Oral | Blood glucose level | Hypoglycemic/ dose-dependant | [72] |
| Paeonia species | Ethanol extract (resveratrol derivatives (vateriferol or VT and trans-ε-viniferin or VF))/Seed coats | Male KM mice | Alloxan-induced diabetic mice | 5, 15 and 30 mg kg BW | Oral | Oral starch tolerance test for PBG level | Hypoglycemic/ dose-dependent | [73] |
| Ammodaucus leucotrichus Coss. and Durieu | Aqueous extract/fruit | Albino Wistar rats | Alloxan diabetic rats | 150 mg/kg BW | Oral | OGTT | Hypoglycemic | [74] |
| Salvia polystachya Cav. | Ethanolic extract/Terpenoid content | BALB/c mice | streptozocin–nicotinamide (STZ–NA) induced diabetes | 50, 100 and 200 mg/kg BW | Oral | Oral sucrose and starch tolerance tests (OSuTT and OStTT)/OGTT and galactose tolerance test (OGaTT)/glucose load (1.5 g/kg−1) | Hypoglycemic/ dose-dependent | [75] |
| Agathophora alopecuroides | Methanol extract | BALB/c male albino mice | STZ-induced diabetic mice | 100 and 200 mg/kg BW | Oral | RBGL and FBGL | Hypoglycemic | [76] |
| Lonicera caerulea L. | Blue honeysuckle extract | Male mice | Oral starch and maltose (2 g kg−1) tolerance assay | 100 and 200 mg kg BW | Oral | PBG level | Hypoglycemic | [77] |
| Ganoderma lucidum | Aqueous extract of fruiting bodies (FYGL) | BKS-db (db/db) diabetic mice | OSTT (2.5 g/kg sucrose) | 225, 450 and 900 mg/kg bw FYGL | Oral | PBG concentration | Hypoglycemic | [78,79] |
| Colvillea racemosa | Ethanol extract (n-butanol fraction)/leaves | Male albino rats | STZ-induced diabetes | 500 mg/kg BW | Oral | FBG | Hypoglycemic | [80] |
| Artemisia roxburghiana | Aqueous ethanol extract/aerial parts | Wistar rats | STZ-NA-induced diabetes | 200 and 400 mg/kg BW in a dose-dependent manner | Oral | BGL | Hypoglycemic/ dose-dependent | [81] |
| Breynia distachia | Methanol extract/aerial parts | SD rats | Alloxan-induced diabetes | 150 and 300 mg/kg BW | Oral | BGL | Hypoglycemic | [82] |
| Rhodomyrtus tomentosa | Methanol extract/Leaf | Male albino Wistar rats | STZ-induced diabetes | 200, 400 and 600 mg/kg BW | Oral | BGL | Hypoglycemic/ dose-dependent | [83] |
| Name of Plants/Compounds | Extract/Class | Source | IC50 | IC50 of Positive Control (Acarbose) | Mode or Type of Inhibition | Ref. |
|---|---|---|---|---|---|---|
| Samanea saman | Methanol extract | Samanea saman (leaves) | 172.25 (50% inhibition) | 115.2 (50% inhibition) | - | [90] |
| Ganoderma hainanense | Chloroform residue | Ganoderma hainanense (Fruiting body) | 0.409 ± 0.041 mg/mL | - | - | [91] |
| Andrographis paniculata | Ethanolic extract | Andrographis paniculata (leaves) | 17.2 ± 0.15 mg/mL | 6.2 ± 0.33 mg/mL | - | [92] |
| Undaria pinnatifida | Acetone extract | Undaria pinnatifida | 0.08 ± 0.002 mg/mL | 0.6 ± 0.01 mg/mL | - | [93] |
| Conyza canaden- sis | Methanolic extract | Conyza canadensis (whole plant) | 107 µg/mL | 23 µg/mL | - | [94] |
| Cinnamon extract | Methanolic extract | Cinnamomum zeylanicum (Bark) | 5.83 µg/mL | 36.89 µg/mL | - | [95] |
| Zanthoxylum armatum | Plant extract | Zanthoxylum armatum (leaves) | 79.82% at 0.8 mg/mL | 23.83% at 0.8 mg/mL | - | [68] |
| Mentha arvensis | Methanolic extract | Mentha arvensis (leaves) | 68% at 50 µg/µl | 85% at 50 µg/µl | - | [96] |
| Black rice | Ethyl acetate extract | Black rice bran | 47.79 ± 2.28 µg/mL | 56.42 ± 4.17 µg/mL | - | [89] |
| Methanolic extract | 48.50 ± 0.83 µg/mL | - | ||||
| Hexane extract | 52.80 ± 1.65 µg/mL | - | ||||
| Potentilla anserine | Butyl alcohol fraction | Potentilla anserine (rhizome) | 14.18 ± 0.95 µg/mL | 19.15 ± 1.57 µg/mL | - | [97] |
| Cyclocarya paliurus | Plant extract | Cyclocarya paliurus tea (leaves) | 31.5 ± 1.05 µg/mL | 296.6 ± 1.06 µg/mL | - | [98] |
| Bound phenolic acid | Plant extract | Naked oats | 0.580 ± 0.010 mg/mL | 0.503 ± 0.017 mg/mL | competitive | [99] |
| Free phenolic acid | 0.721 ± 0.014 mg/mL | 0.503 ± 0.017 mg/mL | mixed | |||
| Nelumbo nucifera (total flavonoids) | Nelumbo nucifera leaf flavonoids | Nelumbo nucifera (leaves) | 1.86 ± 0.018 mg/mL | 0.69 ± 0.047 mg/mL | - | [100] |
| Evodiae fructus (polysaccharides) | Water extract | Evodiae fructus | 84.6% at 4 mg/mL | 99.6% at 4 mg/mL | - | [88] |
| Adenosma bracteosum | Ethanolic extract | Adenosma bracteosum (aerial part) | 26.55 µg/mL | 87.94 µg/mL | - | [101] |
| Lepisanthes fruticosa | Ethanolic extract | Lepisanthes fruticosa (seeds) | 1.873 ± 0.421 mg/mL | 0.064 ± 0.002 mg/mL | - | [102] |
| Symplocos cochinchinensis | Ethanolic extract | Symplocos cochinchinensis (Bark) | 82.07 ± 2.1 µg/mL | 45 ± 1.12 µg/mL | - | [103] |
| Cerasus humilis | 70% methanolic extract | Cerasus humilis (Sok leaf tea) | 36.57 μg/mL | 189.57 μg/mL | - | [84] |
| Paliurus spina-christi Mill | n-hexane sub-extract | Paliurus spina-christi Mill. (fruit) | 445.7 ± 8.5 µg/mL | 4212.6 ± 130.0 µg/mL | - | [85] |
| Gymnanthemum amygdalinum | Ethyl acetate fraction | Gymnanthemum amygdalinum (flower) | 19.24 ± 0.12 µg/mL | 73.36 ± 3.05 µg/mL | - | [87] |
| Washingtonia filifera | Methanolic extract | Washingtonia filifera (Seeds) | 0.53 ± 0.014 µg/mL | 90 ± 7.3 µg/mL | Mixed | [104] |
| Crataegus pinnatifida | Acetone extract | Crataegus pinnatifida (fruits) | 42.35 ± 2.48 µg/mL | 317.8 ± 16.36 µg/mL | - | [105] |
| Chenopodium quinoa Willd. | Ethyl acetate fraction | Chenopodium quinoa Willd. (Quinoa) | 99.66 ± 6.0 µg/mL | 336.25 ± 56.88 µg/mL | - | [106] |
| Name of Plants/Compounds | Extract/ Class | Source | IC50 | IC50 of Positive Control (Acarbose) | Mode or Type of Inhibition | Ref. |
|---|---|---|---|---|---|---|
| Catechin | Flavonoid | Commercial | 1.12 ± 0.03 µM | 1250 ± 35.63 µM | Competitive and reversible | [67] |
| Epicatechin | 0.95 ± 0.02 µM | 1250 ± 35.63 µM | ||||
| Naringenin | Flavonoid | Commercial | 6.51 µM | 49.65 µM | Competitive | [107] |
| Apigenin | Flavonoid | Commercial | (1.43 ± 0.02) × 10−5 M | (37.65 ± 0.44) × 10−5 M | Non-competitive | [108] |
| Scutellarein | (0.24 ± 0.02) × 10−5 M | (37.65 ± 0.44) × 10−5 M | Mixed | |||
| Hispidulin | (3.21 ± 0.03) × 10−5 M | (37.65 ± 0.44) × 10−5 M | ||||
| Nepetin | (1.18 ± 0.02) × 10−5 M | (37.65 ± 0.44) × 10−5 M | ||||
| Quercetin-3-O-α-L-rhamnopyranoside-2″-gallate | Flavonoid | Potentilla anserine (rhizome) | 1.05 ± 0.03 µM | 28.06 ± 0.82 µM | Competitve | [97] |
| Quercetin-4′-O-glucoside | Flavonoid | Allium cepa (peel) | 31.4 ± 0.8 | 51.8 ± 10.3 | - | [109] |
| Myricetin-3-O-(2″-O-galloyl)-α-L-rhamnoside | Flavonoid | Morella rubra (leaves) | 1.32 ± 0.17 µM | 369.15 ± 6.18 µM | - | [110] |
| Myricetin-3-O-(4″-O-galloyl)-α-L-rhamnoside | 1.77 ± 0.19 µM | |||||
| Quercetagetin-7-O-β-D-glucopyranoside | Flavonoid | Rubus corchorifolius (fruit) | 4.96 ± 0.54 μM | 1.93 ± 0.08 μM | Non-competitive | [111] |
| Vitexin | Flavonoid | Natural | 52.80 ± 1.65 µM | 375 ± 12.5 μM | Non-competitive | [112] |
| (-) epigallocatechin-gallate | Flavonoid | Caesalpinia paraguariensis (bark) | 5.20 ± 0.15 µM | 1400.00 ± 0.51 µM | Non-competitive | [113] |
| Calodenin A | Flavonoid | Knema globularia (stem) | 0.4 ± 0.1 μM | 93.6 ± 0.5 μM | Non-competitive | [114] |
| Globunone A | 2.0 ± 0.1 μM | |||||
| Globunone B | 1.6 ± 0.2 μM | |||||
| Globunone C | 1.4 ± 0.1 μM | |||||
| Globunone F | 26.6 ± 1.8 μM | |||||
| Dehydrolophirone C | 3.2 ± 0.2 μM | |||||
| Lophirone P | 5.6 ± 0.9 μM | |||||
| Scolopianate A | Triterpenoid | Ganoderma hainanense | 3.4 ± 0.16 µM | 489.6 ± 51.4 µM | - | [91] |
| Akebonoic acid | Triterpenoid | Akebia trifoliata | 9 μM | 409 μM | - | [115] |
| 3-oxolupenal | Triterpenoid | Nuxia oppositifolia | 62.3 ± 2.4 µg/mL | 38.1 ± 3.1 µg/mL | - | [116] |
| Katononic acid | 88.6 ± 6.2 µg/mL | |||||
| Cypaliuruside J | Triterpenoid Saponin | Cyclocarya paliurus (leaves) | 2.22 ± 0.13 μM t | - | Non-competitive | [117] |
| Betulin and betulinic acid mixture | Triterpenes | Paliurus spina-christi Mill. (fruit) | 248 ± 2 µM | 6561 ± 207 µM | - | [85] |
| Andrographolide | Diterpenoid | Commercial | 11.0 ± 0.28 mg/mL | 6.2 ± 0.33 mg/mL | - | [92] |
| Ent-atisane-3-oxo-16β,17-acetonide | Diterpenoid | Euphorbia antiquorum | 69.62 µM | 332.5 µM | Non-competitive | [118] |
| Taxumariene F | Diterpenoid | Taxus mairei | 3.7 ± 0.75 μM | 155.86 ± 4.12 µM | - | [119] |
| Gauleucin E | Diterpenoid | Gaultheria leucocarpa var. yunnanensis | 319.3 μM | 387.8 μM | - | [120] |
| Margoclin | 327.9 μM | |||||
| Tergallic acid dilactone | Polyphenols | Eugenia jambo-lana (seeds) | 5.0 ± 0.34 µM | 289.9 ± 6.67 µM | - | [121] |
| ellagic acid | Phenolic acid and its derivatives | Caesalpinia paraguariensis (bark) | 87.30 ± 0.78 µM | 1400.00 ± 0.51 µM | Mixed | [113] |
| 3-O-methylellagic | 65.10 ± 0.56 µM | Mixed | ||||
| 3,3′-O-dimethylellagic acid | 73.03 ± 0.1 µM | Non-competitive | ||||
| 3,3′-O-dimethylellagic- 4-O-β-D-xylopyranoside | 263.05 ± 0.12 µM | Competitive | ||||
| Vanilin | Phenolic aldehyde | Commercial | 28.34 ± 0.89 mg/mL | 0.52 ± 0.08 mg/mL | Mixed | [122] |
| AXA-1 | Polysaccharides | Wheat bran | 0.38 mg/mL | 0.14 mg/mL | Mixed type non-competitive | [123] |
| WXA-1 | 1.17 mg/mL | 0.14 mg/mL | - | |||
| S. fusiforme polysaccharide (SFP-1) | Polysaccharides | Sargassum fusiforme | 0.681 mg/mL | 1.308 mg/mL | Mixed | [124] |
| S. fusiforme polysaccharide (SFP-7-40) | Polysaccharides | Sargassum fusiforme | 0.304 mg/mL | 0.657 mg/mL | Non-competitive | [125] |
| Procyanidin A2 | Tannin | Wendlandia glabrata | 0.47 μM | 586.6 μM | - | [126] |
| Dieckol | Tannin | Ecklonia cava | 0.24 ± 0.056 mM | 1.05 ± 0.03 mM | - | [127] |
| 1,2,3-tri-O-galloyl-β-D-glucopyranose | Gallotannins | Euphorbia fischeriana | 15.48 ± 0.60 μM | - | Mixed | [128] |
| Rhaponticin | Stilbene | Polygonum multiflorum | 0.3 μM | 50.04 μM | - | [129] |
| Scirpusin B | Stilbene | Cyperus rotundus (rhizome) | 94.3 ± 6.8 µM | 2060 ± 97.5 µM | - | [130] |
| Pelargonidin-3-O-rutinoside | Anthocyanin | strawberries | 1.69 µM | 356.26 µM | Mixed | [131] |
| Cyanidin | Anthocyanin | Cinnamomum camphora (fruit) | 5.291 × 10−3 mM | 1.644 mM | Non-competitive | [132] |
| Alaternin | Anthraquinone | Cassia obtusefolia | 3.45 μM | 191.4 μM | - | [133] |
| Chysalodin | Anthraquinone | Aloe vera | 13.4 ± 1.5 μM | 124.0 ± 3.1 μM | Competitive | [134] |
| Parmosidone I | Depsidone | Parmotrema tsavoense | 10.7 μM | 449 μM | - | [135] |
| Gymnepregoside F | Pregnane glycoside | Gymnema inodorum (leaves) | 63.7 ± 3.9% at 200 μM | - | - | [136] |
| 3β,8β,14β,20-tetrahydroxy-(20S)-pregn-5-ene-3-O-β-D-glucopyranosyl-(1→4)-O-β-D-digitaloside-20-O-3-isoval-β-D-glucopyranoside | Pregnane glycoside | Caralluma hexagona | 0.67 ± 0.01 mM | 0.81 ± 0.86 mM | - | [137] |
| Mulberrofuran K | Chalcone derivatives | Morus macroura | 1.25 μM | 1428 μM | - | [138] |
| 2-(3′,4′-dihydroxyphenyl)-2,3-dihydro-4,6-dihydroxy-2-(methoxy)-3-benzofuranone | Benzofuranone | Hylotelephium erythrostictum | 1.8 μM | 822.9 μM | - | [139] |
| Fucoxanthin | Xanthophyll | Undaria pinnatifida | 0.047 ± 0.001 mg/mL | 0.6 ± 0.01 mg/mL | Mixed type | [93] |
| Mangoxanthone A | Xanthones | Garcinia mangostana (pericarp) | 29.06 ± 1.86 μM | - | - | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. https://doi.org/10.3390/plants11202722
Kashtoh H, Baek K-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants. 2022; 11(20):2722. https://doi.org/10.3390/plants11202722
Chicago/Turabian StyleKashtoh, Hamdy, and Kwang-Hyun Baek. 2022. "Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes" Plants 11, no. 20: 2722. https://doi.org/10.3390/plants11202722
APA StyleKashtoh, H., & Baek, K.-H. (2022). Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants, 11(20), 2722. https://doi.org/10.3390/plants11202722