Overexpression of Nicotianamine Synthase (AtNAS1) Increases Iron Accumulation in the Tuber of Potato
Abstract
:1. Introduction
2. Results
2.1. Generation of 35S::AtNAS-Overexpression Potato
2.2. AtNAS1 Overexpression Had No Influence on Shoot Growth and Tuber Yield
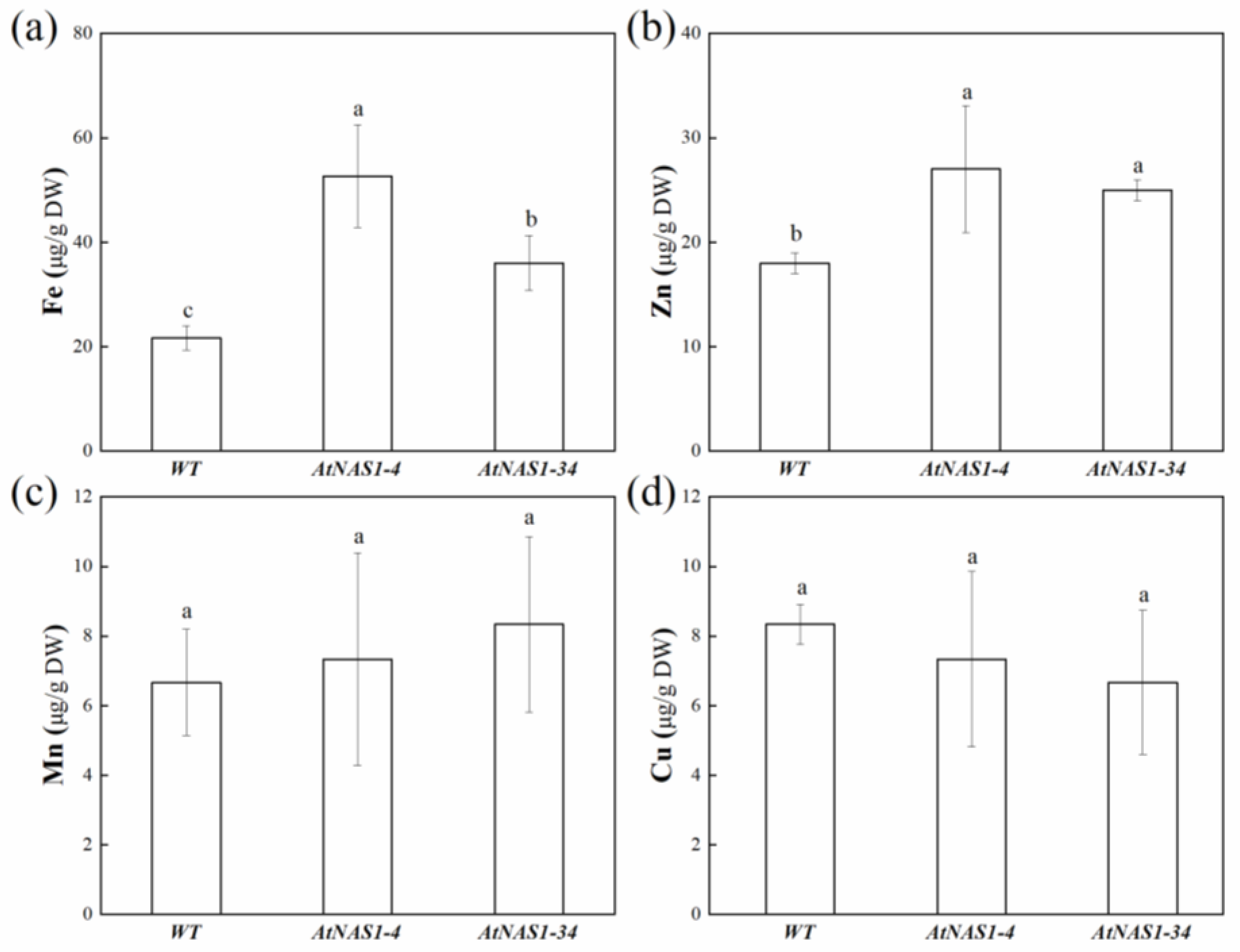
2.3. Constitutive AtNAS1 Expression Alters Fe and Zn Accumulation in Potato Tubers
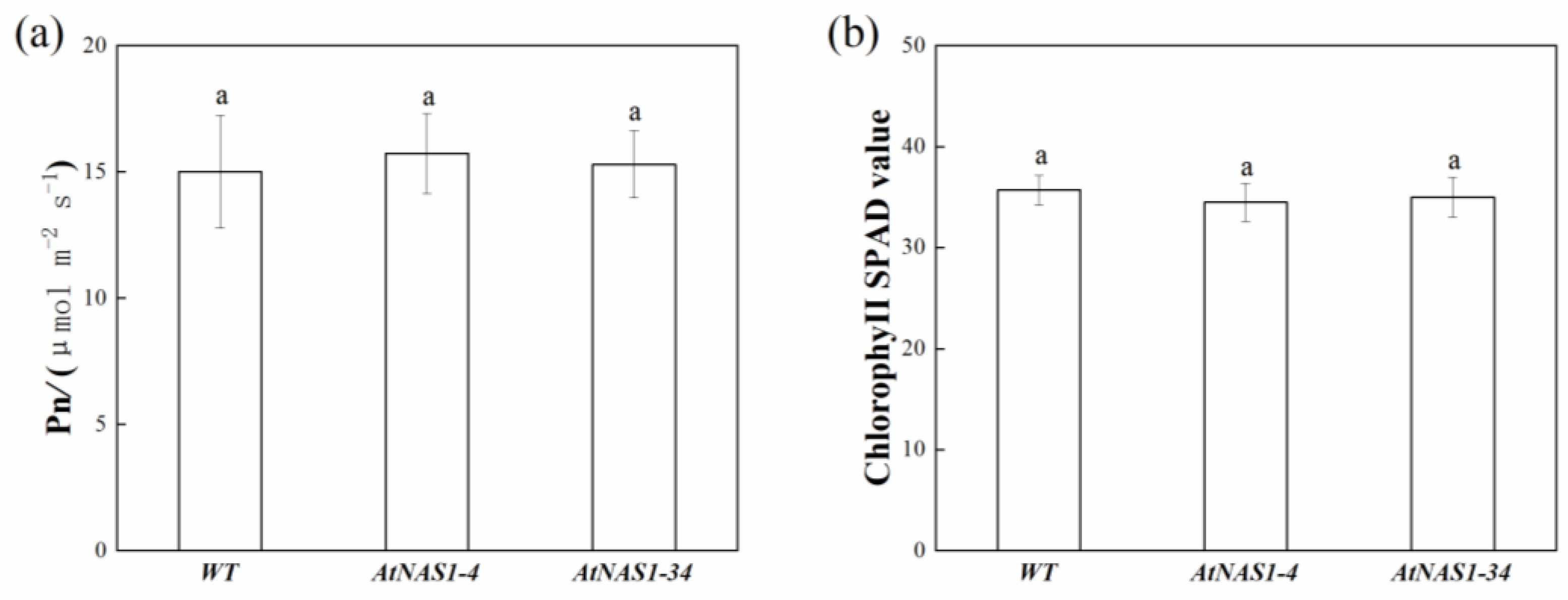
2.4. The Photosynthesis Rate and Chl Content in Potato Leaves Were Not Changed
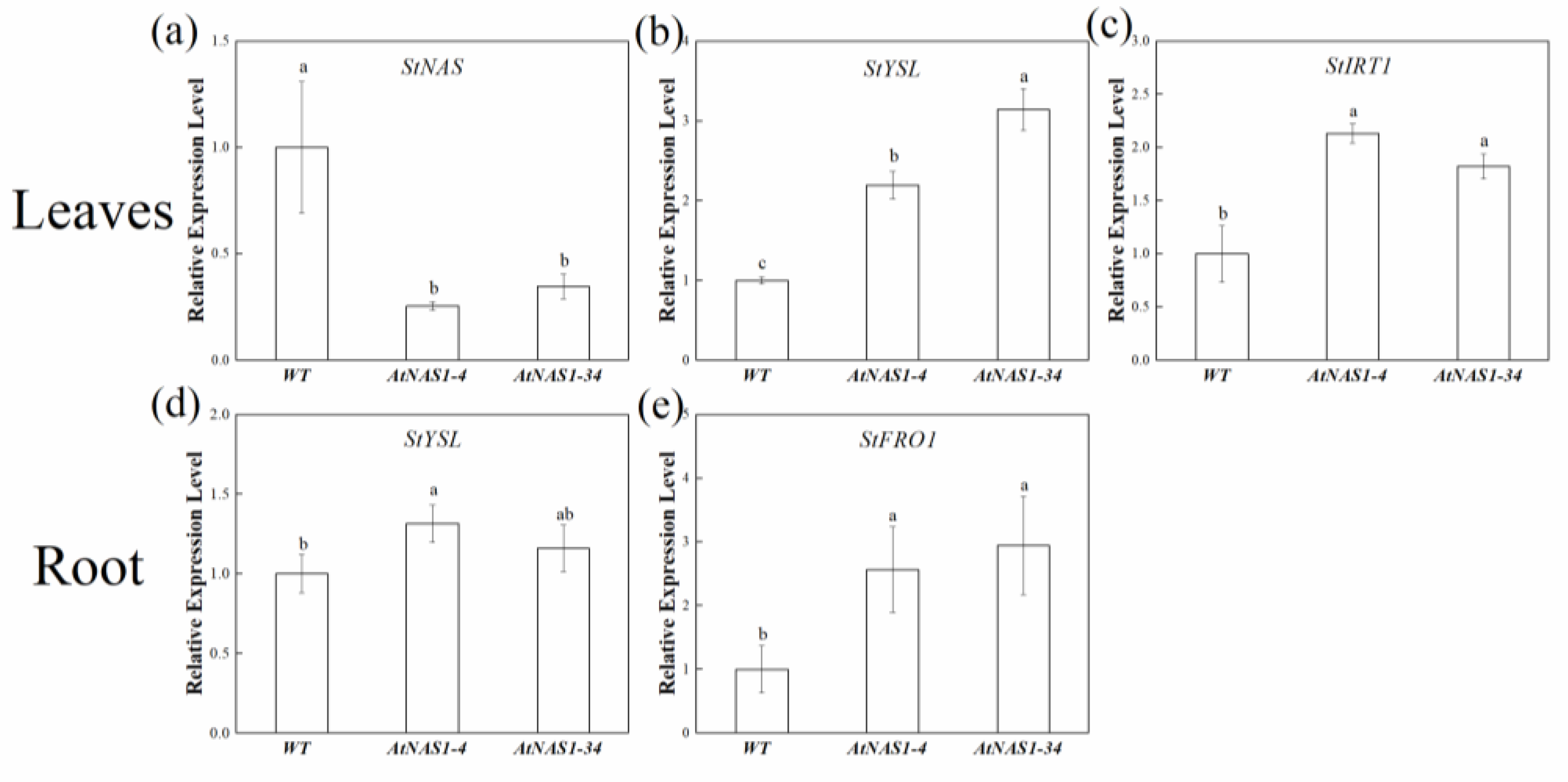
2.5. Expression of Genes Related to Fe Uptake and Homeostasis Was Altered
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Plasmid Construction, and Transformation of Potato
3.3. RNA Extraction and Quantitative RT-PCR
3.4. Elemental Analysis
3.5. Determination of Photosynthesis Rate and Chlorophyll Concentration
3.6. Statistical Calculations
4. Discussion
5. Conclusions and Indications for the Future
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Camprubi, E.; Jordan, S.F.; Vasiliadou, R.; Lane, N. Iron catalysis at the origin of life. IUBMB Life 2017, 69, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Functions of Mineral Nutrients: Macronutrients—Sciencedirect. In Mineral Nutrition of Higher Plants, 2nd ed.; Annual Review Inc.: Palo Alto, CA, USA, 1995; pp. 229–312. [Google Scholar]
- Broadley, M.R.; White, P.J. Plant Mineralse. In Phytonutrients; Salter, A., Wiseman, H., Tucker., G., Eds.; John Wiley & Sons: New York, NY, USA, 2012; pp. 254–298. [Google Scholar]
- Wallace, A.; Lunt, O.R. Iron chlorosis in horticultural plants, a review. Proc. Am. Soc. Hort. Sci. 1960, 75, 819–841. [Google Scholar] [CrossRef] [Green Version]
- Welch, R.M.; Graham, R.D. Breeding for Micronutrients in Staple Food Crops from a Human Nutrition Perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, W.; Tittle, J.; Schikora, A. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol. 2000, 122, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, N.J.; Procter, C.M.; Connolly, E.L.; Guerinot, M.L. A ferric-chelate reductase for iron uptake from soils. Nature 1999, 397, 694–697. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Niehus, R.; Picot, A.; Oliveira, N.M.; Mitri, S.; Foster, K.R. The evolution of siderophore production as a competitive trait. Evolution 2017, 6, 1443–1455. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.F.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(Iii) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. The essential basic helix-Loop-Helix protein fit1 is required for the iron deficiency response. Plant Cell. 2004, 16, 3400–3412. [Google Scholar] [CrossRef]
- Jakoby, M.; Wang, H.Y.; Reidt, W.; Weisshaar, B.; Bauer, P. Fru (Bhlh029) is required for induction of iron mobilization genes in arabidopsis thaliana. FEBS Lett. 2004, 577, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Bauer, P.; Ling, H.Q.; Guerinot, M.L. FIT, the FER-LIKE IRON DEFICIENCY INDUCED TRANSCRIPTION FACTOR in Arabidopsis. Plant Physiol. Biochem. 2007, 45, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.A.; Briat, J.F.; Curie, C. Dual regulation of the Arabidopsis high-affinity root iron uptake system by local and long-distance signals. Plant Physiol. 2003, 132, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Jeon, U.S.; Lee, S.J.; Kim, Y.K.; Persson, D.P.; Husted, S.; Schjørring, J.K.; Kakei, Y.; Masuda, H.; Nishizawa, N.K.; et al. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc. Natl. Acad. Sci. USA 2009, 106, 22014–22019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.A.; Kyriacou, B.; Callahan, D.L.; Carruthers, L.; Stangoulis, J.; Lombi, E.; Tester, M. Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 2011, 6, e24476. [Google Scholar] [CrossRef] [Green Version]
- Nozoye, T.; Otani, M.; Senoura, T.; Nakanishi, H.; Nishizawa, N.K. Overexpression of barley nicotianamine synthase 1 confers tolerance in the sweet potato to iron deficiency in calcareous soil. Plant Soil 2016, 418, 75–88. [Google Scholar] [CrossRef]
- Paul, S.; Ali, N.; Gayen, D.; Datta, S.K.; Datta, K. Molecular breeding of Osfer 2 gene to increase iron nutrition in rice grain. GM Crop. Food 2012, 3, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Keller, B.; Gruissem, W.; Bhullar, N.K. Rice NICOTIANAMINE SYNTHASE 2 expression improves dietary iron and zinc levels in wheat. Theor. Appl. Genet. 2017, 130, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Brumbarova, T.; Bauer, P. Iron uptake and transport in plants. In CABI Books: CABI International; CABI: Wallingford, UK, 2008. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.T. Nutritional requirements of potatoes. Am. J. Pot. Res. 2005, 82, 301–307. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noma, M.; Noguchi, M.; Tamaki, E. A new amino acid, nicotianamine, from tobacco leaves. Tetrahedron Lett. 1971, 12, 2017–2020. [Google Scholar] [CrossRef]
- Hell, R.; Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta 2003, 216, 541–551. [Google Scholar] [CrossRef]
- Takahashi, M.; Terada, Y.; Nakai, I.; Nakanishi, H.; Yoshimura, E.; Mori, S.; Nishizawa, N.K. Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell. 2003, 15, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Rellán-Álvarez, R.; Fink-Straube, C.; Abadía, J.; Bauer, P. Nicotianamine functions in the Phloem-based transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis. Plant Cell. 2012, 24, 2380–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojima, S.; Nishizawa, N.K.; Fushiya, S.; Nozoe, S.; Irifune, T.; Mori, S. Biosynthesis of Phytosiderophores: In Vitro Biosynthesis of 2′-Deoxymugineic Acid from l-Methionine and Nicotianamine. Plant Physiol. 1990, 93, 1497–1503. [Google Scholar] [CrossRef] [Green Version]
- Cassin, G.; Mari, S.; Curie, C.; Briat, J.F.; Czernic, P. Increased sensitivity to iron deficiency in Arabidopsis thaliana overaccumulating nicotianamine. J. Exp. Bot. 2009, 60, 1249–1259. [Google Scholar] [CrossRef]
- Beasley, J.T.; Bonneau, J.P.; Sánchez-Palacios, J.T.; Moreno-Moyano, L.T.; Callahan, D.L.; Tako, E.; Glahn, R.P.; Lombi, E.; Johnson, A.A.T. Metabolic engineering of bread wheat improves grain iron concentration and bioavailability. Plant Biotechnol. J. 2019, 17, 1514–1526. [Google Scholar] [CrossRef]
- Zhou, X.; Zha, M.; Huang, J.; Li, L.; Imran, M.; Zhang, C. StMYB44 negatively regulates phosphate transport by suppressing expression of PHOSPHATE1 in potato. J. Exp. Bot. 2017, 68, 1265–1281. [Google Scholar] [CrossRef] [Green Version]
- Chronis, D.; Chen, S.; Lu, S.; Hewezi, T.; Carpenter, S.C.; Loria, R.; Baum, T.J.; Wang, X. A ubiquitin carboxyl extension protein secreted from a plant-parasitic nematode Globodera rostochiensis is cleaved in planta to promote plant parasitism. Plant J. 2013, 74, 185–196. [Google Scholar] [CrossRef]
- Lee, S.; Chiecko, J.C.; Kim, S.A.; Walker, E.L.; Lee, Y.; Guerinot, M.L.; An, G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 2009, 150, 786–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron Deficiency Anaemia Revisited. J. Int. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization Malnutrition. Available online: https://www.who.int/en/news-room/fact-sheets/detail/malnutrition (accessed on 4 February 2019).
- Pivina, L.; Semenova, Y.; Doşa, M.D.; Dauletyarova, M.; Bjørklund, G. Iron Deficiency, Cognitive Functions, and Neurobehavioral Disorders in Children. J. Mol. Neuroence 2019, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Connorton, J.M.; Balk, J. Iron Biofortification of Staple Crops: Lessons and Challenges in Plant Genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; An, G. Over-Expression of Osirt1 Leads to Increased Iron and Zinc Accumulations in Rice. Plant Cell Environ. 2010, 32, 408–416. [Google Scholar] [CrossRef]
- Oliva, N.; Chadha-Mohanty, P.; Poletti, S.; Abrigo, E.; Atienza, G.; Torrizo, L.; Garcia, R.; Dueñas, C.J.; Poncio, M.A.; Balindong, J.; et al. Large-scale production and evaluation of marker-free indica rice IR64 expressing phytoferritin genes. Mol. Breed. 2014, 33, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solís, E.; Gehan, J.; Butts, P.; Siritunga, D.; Okwuonu, I.; Woll, A.; Jiménez-Aguilar, D.M.; et al. Biofortification of field-grown cassava by engineering expression of an iron transporter and ferritin. Nat. Biotechnol. 2019, 37, 144–151. [Google Scholar] [CrossRef]
- Goto, F.; Yoshihara, T.; Shigemoto, N.; Toki, S.; Takaiwa, F. Iron fortification of rice seed by the soybean ferritin gene. Nat. Biotechnol. 1999, 17, 282–286. [Google Scholar] [CrossRef]
- Masuda, H.; Usuda, K.; Kobayashi, T.; Ishimaru, Y.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the Barley Nicotianamine Synthase Gene HvNAS1 Increases Iron and Zinc Concentrations in Rice Grains. Rice 2009, 2, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Reid, S.; Malumphy, C. Arthropods Intercepted on Fresh Sweet Potato (Ipomoea Batatas) Produce Imported into England and Wales. Entomol. Gaz. 2006, 57, 183–194. [Google Scholar] [CrossRef]
- Knapp, S. Potatoes and poverty. Nature 2008, 455, 170–171. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.D. Multiple signaling pathways control tuber induction in potato. Plant Physiol. 1999, 119, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, E.E.; Struik, P.C. Tuber Formation in Potato: Induction, Initiation and Growth. Hortic. Rev. 1992, 14, 89–198. [Google Scholar] [CrossRef]
- Lalonde, S.; Tegeder, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2010, 26, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Legay, S.; Guignard, C.; Ziebel, J.; Evers, D. Iron Uptake and Homeostasis Related Genes in Potato Cultivated in Vitro under Iron Deficiency and Overload. Plant Physiol. Biochem. 2012, 60, 180–189. [Google Scholar] [CrossRef]
- Barberon, M.; Zelazny, E.; Robert, S.; Conéjéro, G.; Curie, C.; Friml, J.; Vert, G. Monoubiquitin-Dependent Endocytosis of the Iron-Regulated Transporter 1 (Irt1) Transporter Controls Iron Uptake in Plants. Proc. Natl. Acad. Sci. USA 2011, 108, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Connolly, E.L. Iron Uptake Mechanisms in Plants: Functions of the Fro Family of Ferric Reductases. Plant Sci. 2009, 176, 709–714. [Google Scholar] [CrossRef]
- Kanobe, M.N.; Rodermel, S.R.; Bailey, T.; Scott, M.P. Changes in endogenous gene transcript and protein levels in maize plants expressing the soybean ferritin transgene. Front. Plant Sci. 2013, 4, 196. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, M.; Li, X.; Li, R.; Huang, J.; Fan, J.; Zhang, J.; Wang, Y.; Zhang, C. Overexpression of Nicotianamine Synthase (AtNAS1) Increases Iron Accumulation in the Tuber of Potato. Plants 2022, 11, 2741. https://doi.org/10.3390/plants11202741
Zha M, Li X, Li R, Huang J, Fan J, Zhang J, Wang Y, Zhang C. Overexpression of Nicotianamine Synthase (AtNAS1) Increases Iron Accumulation in the Tuber of Potato. Plants. 2022; 11(20):2741. https://doi.org/10.3390/plants11202741
Chicago/Turabian StyleZha, Manrong, Xin Li, Rui Li, Jing Huang, Jinping Fan, Jing Zhang, Yan Wang, and Cankui Zhang. 2022. "Overexpression of Nicotianamine Synthase (AtNAS1) Increases Iron Accumulation in the Tuber of Potato" Plants 11, no. 20: 2741. https://doi.org/10.3390/plants11202741



