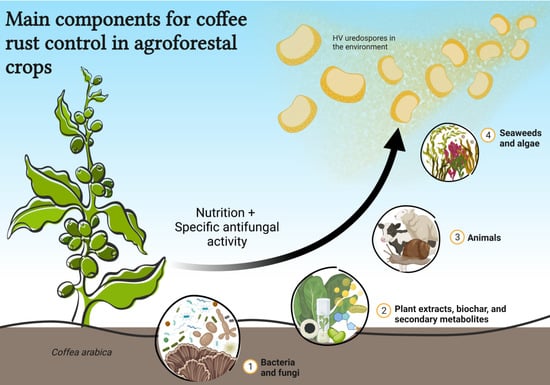
Towards an Eco-Friendly Coffee Rust Control: Compilation of Natural Alternatives from a Nutritional and Antifungal Perspective
Abstract
:1. Introduction
2. Defense Mechanisms of High Mountain Coffee and the Transition for Sustainable Production
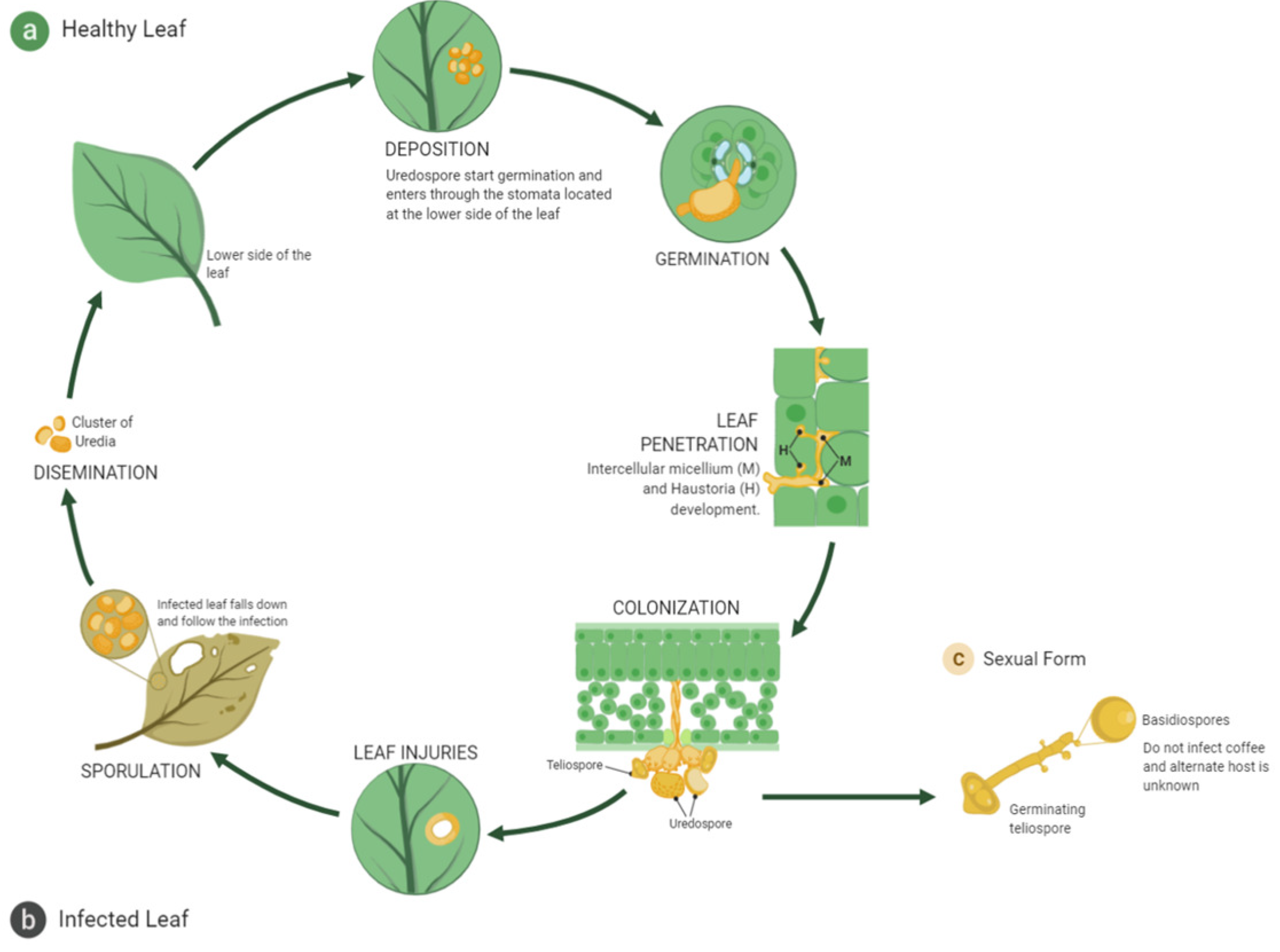
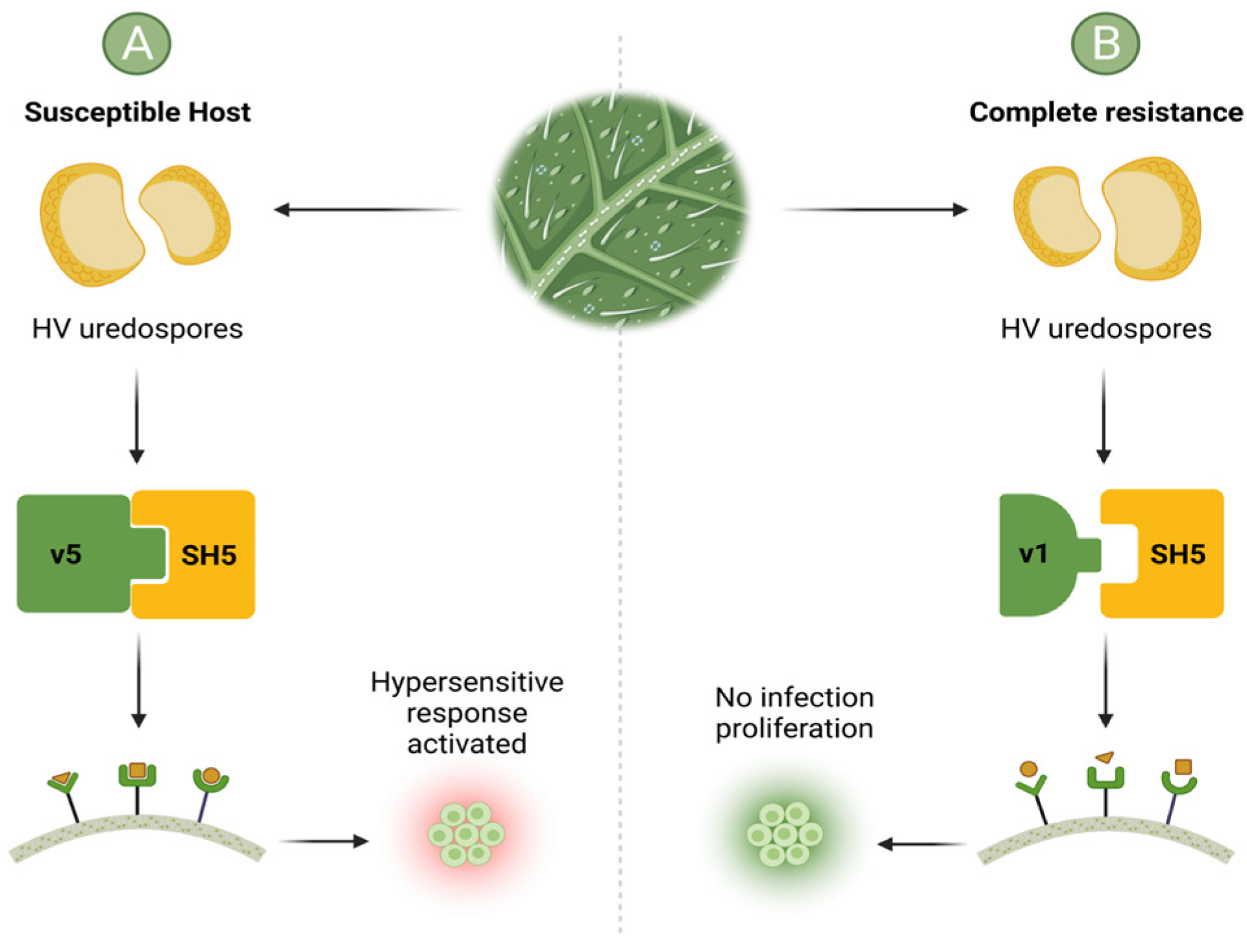
2.1. Virulence Factors of Hemileia vastatrix and Defense Mechanisms of High Mountain Coffee Plants
2.2. New Directions for Coffee Production: Relevance of Empowering Shaded Crops
3. Botanical Bioactive Compounds for CLR Control Suitable for Shaded Production Systems
| Plant | Class/Compounds | Efficacy of the Assay on Spore Germination Inhibition | Reference |
|---|---|---|---|
| Baccharis glutinosa | Flavonoids: Multijuginol, (Z)-3-hydroxy-1-(2-hydroxyphenyl)-3-phenyl prop-2-en-1-one, 3′-Methoxyquercetin and 12aβ-hydroxydeguelin. | Leaves treated with MEBs 1 significantly decreased the germination percentage of uredospores up to <5% as the dose increased (p < 0.05). | [66] |
| Camellia sinesis | Monoterpenes: Limonene, linalool, geraniol and Sesquerpitene: β-caryophyllene. | A significant reduction of severity was observed in the treatments with C. sinensis; they provide a fungicidal effect and growth suppressor of the causal agents. | [63] |
| Bassica nigra- Piper nigerium | Alkaloid: Piperine. Monoterpenes: sabinene, limonene, and β-pinene. Sesquerpitenes: β-caryophyllene, α-selinene, and germacrene. | No significant reduction of severity was observed in the treatments, therefore is recommended just as a preventive alternative. | [63] |
| Cymbopogon sp., Thymus sp. and Cynamomum sp. | Monoterpenes: D-limonene, cineole, β-myrcene, anethole, p-anisaldehyde, carvacrol, carvone, limonene, felandrene, pinene. | All the essential oils inhibited the germination of urediniospores at increasing concentrations. | [42] |
| Cymbopogon citratus, Aloe barbadensis, Moringa oleifera, Nicotiana tabacum | Monoterpeno: Citral (C. citratus) Anthranonic glycoside: Aloin (A. barbadensis) Tannins (M. oleifera) Alkaloid: Nicotine (N. tabacum) | The plant extracts are effective in inhibiting fungal spore germination. Extracts from M. oleifera and C. citratus proved to be the most effective, compared with A. barbadensis | [68] |
| Piper aduncum L. | Monoterpene: Piperitone | It can reduce uredospore mycelium germination in laboratory conditions. | [69] |
| Ardisia compressa | NC 2 | Significant inhibition of the uredospore germination in vitro | [70] |
| Eriobotrya japonica Ardisia compressa, and Ocimun basilicum | Alkaloids, flavonoids, coumarins, and terpenes. | The aqueous extracts from the plants reduced the inhibition of the germination of uredospore at 0.12, 037, and 0.38 %, respectively | [70] |
4. Novel Approaches: Use of Organisms and Biochar for the Management of Hemileia vastatrix
4.1. Strategies Based on Animals Implementation
| Animal | Use | Experimentation Details | Reference |
|---|---|---|---|
| Mycodiplosis larvae | Predator | There is a positive correlation between the severity caused by the rust of coffee (HV) and the number of Mycodiplosis spp. larvae. | [83] |
| Bradybaena similaris and Bulimulus guadalupensis | Predator | Experiments showed that after 24 h B. similaris cleared the coffee leaves of CLR spores while B. guadalupensis failed to consume any CLR uredospores. | [82] |
| Cattle | Urine | Treatments consisting of three concentrations of cow urine (10, 20, and 30%) reduced the incidence of CLR; however, they decreased the number of leaf injuries and enriched coffee crops. | [84] |
| ExLom-P® 1 | Extract | It was found that the application of crude ExLom-P® suppressed rust spore germination (0% germinated spores) on coffee leaf discs. Furthermore, they suppress diseases in leaves due to the microbial richness and the abundance of chitinase enzymes and β 1,3 glucanases. In addition, they provide promoters of metabolic defense against fungi, such as abscisic acid, jasmonic acid, and salicylic acid. | [81] |
| ExLom-PCJ® 2 | Extract | It diminished the leaf damage of the coffee rust due to its microbial richness and the abundance of chitinase enzymes and β 1,3 glucanases. Therefore, the authors recommended it as a nutrition additive to increase coffee rust tolerance. | [81] |
4.2. Antifungal Activity and Coffee-Crop Nutrition Properties of Bacteria and Fungi
4.3. From Sand to Land: Macroalgae as a Nutrition Key Factor for Infected Coffee Crops
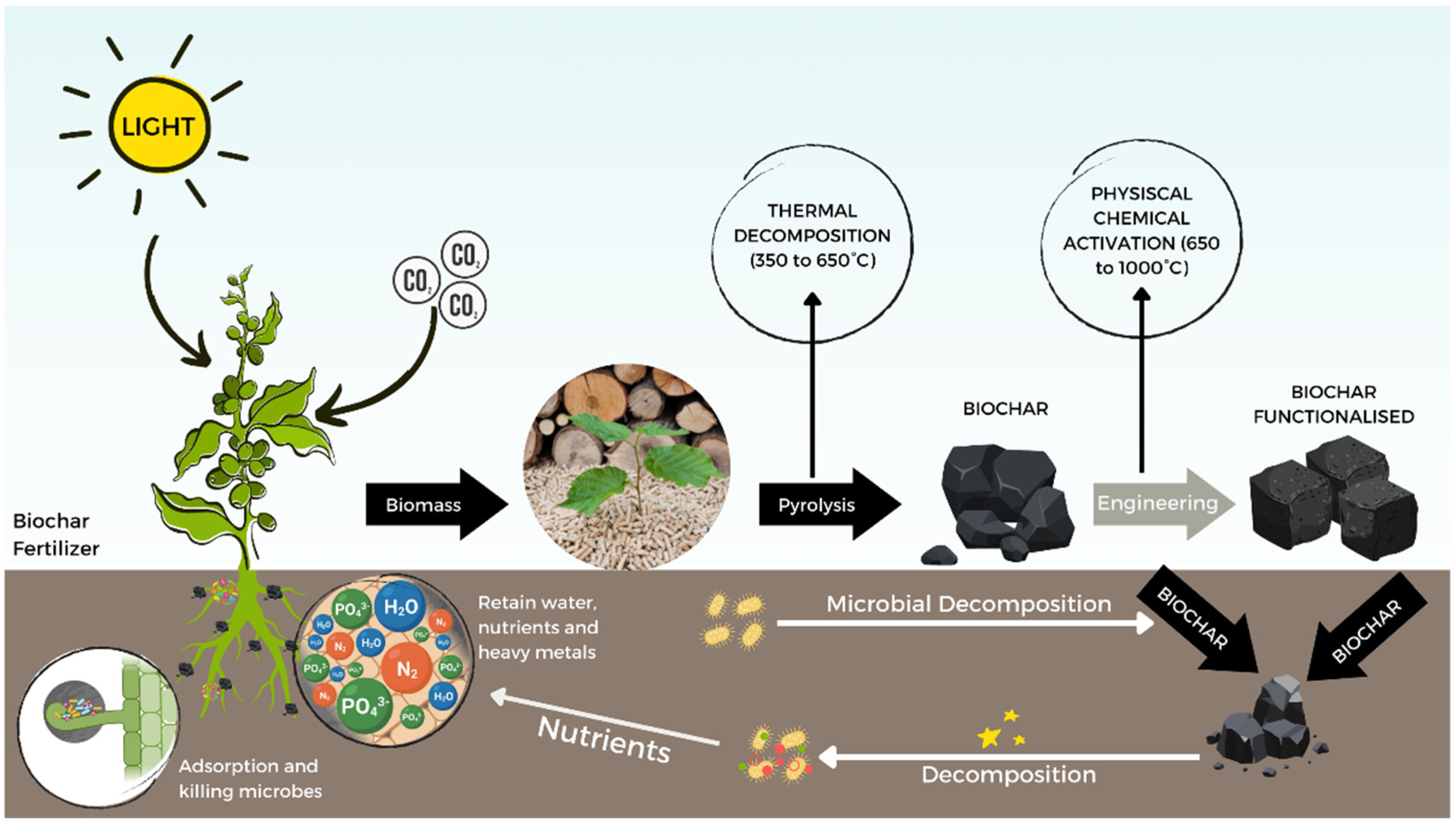
4.4. Biochar Application as a Possible Solution for Rust Diseases Management on Coffee Trees
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization (ICO). Impact of COVID-19 on the Global Coffee Sector: Survey of ICO Exporting Members. Int. Coffee Organ. 2020, 3, 14. [Google Scholar]
- Torres Castillo, N.E.; Melchor-Martínez, E.M.; Ochoa Sierra, J.S.; Ramirez-Mendoza, R.A.; Parra-Saldívar, R.; Iqbal, H.M.N. Impact of Climate Change and Early Development of Coffee Rust—An Overview of Control Strategies to Preserve Organic Cultivars in Mexico. Sci. Total Environ. 2020, 738, 140225. [Google Scholar] [CrossRef] [PubMed]
- Le Cunff, G. How Sales of Certain Types of Coffee Could Transform Economies—World Economic Forum. Available online: https://www.weforum.org/agenda/2020/08/quality-coffee-can-boost-local-economies-and-benefit-farmers/ (accessed on 12 August 2022).
- Al-Abdulkader, A.M.; Al-Namazi, A.A.; AlTurki, T.A.; Al-Khuraish, M.M.; Al-Dakhil, A.I. Optimizing Coffee Cultivation and Its Impact on Economic Growth and Export Earnings of the Producing Countries: The Case of Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Agricultura y Desarrollo Rural Café. La Bebida Que Despierta a México. Available online: https://www.gob.mx/agricultura/articulos/cafe-la-bebida-que-despierta-a-mexico?idiom=es (accessed on 8 March 2022).
- SENASICA. Roya Del Cafeto (Hemileia Vastatrix Berkeley & Broome) Ficha Técnica No. 40. Available online: https://prod.senasica.gob.mx/SIRVEF/ContenidoPublico/Roya%20cafeto/Fichas%20tecnicas/Ficha%20T%C3%A9cnica%20de%20Roya%20del%20cafeto.pdf (accessed on 8 March 2022).
- Prezoto, F.; Maciel, T.T.; Detoni, M.; Mayorquin, A.Z.; Barbosa, B.C. Pest Control Potential of Social Wasps in Small Farms and Urban Gardens. Insects 2019, 10, 192. [Google Scholar] [CrossRef] [Green Version]
- McKenna, M. Coffee Rust Is Going to Ruin Your Morning. Available online: https://www.theatlantic.com/science/archive/2020/09/coffee-rust/616358/ (accessed on 8 March 2022).
- Jacques, A.; Rivas, G. La Roya Anaranjada Del Cafeto. Galileo Rivas to Cite This Version: HAL Id: Hal-01071036. 2013. Available online: https://hal.archives-ouvertes.fr/Hal-01071036/ (accessed on 5 September 2022).
- Toniutti, L.; Breitler, J.C.; Etienne, H.; Campa, C.; Doulbeau, S.; Urban, L.; Lambot, C.; Pinilla, J.C.H.; Bertrand, B. Influence of Environmental Conditions and Genetic Background of Arabica Coffee (C. Arabica L) on Leaf Rust (Hemileia Vastatrix) Pathogenesis. Front. Plant Sci. 2017, 8, 2025. [Google Scholar] [CrossRef] [Green Version]
- de Resende, M.L.V.; Pozza, E.A.; Reichel, T.; Botelho, D.M.S. Strategies for Coffee Leaf Rust Management in Organic Crop Systems. Agronomy 2021, 11, 1865. [Google Scholar] [CrossRef]
- Avelino, J.; Cristancho, M.; Georgiou, S.; Imbach, P.; Aguilar, L.; Bornemann, G.; Läderach, P.; Anzueto, F.; Hruska, A.J.; Morales, C. The Coffee Rust Crises in Colombia and Central America (2008–2013): Impacts, Plausible Causes and Proposed Solutions. Food Secur. 2015, 7, 303–321. [Google Scholar] [CrossRef] [Green Version]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G. dong Plant Defense against Fungal Pathogens by Antagonistic Fungi with Trichoderma in Focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A Review of Plant Leaf Fungal Diseases and Its Environment Speciation. Bioengineered 2019, 10, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Science and Technology; Earthscan: London, UK, 2009; pp. 1–12. ISBN 978-1-84407-658-1. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of Biomass to Produce Fuels and Chemical Feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Bulmau, C.; Badea, A.; Apostol, T.; Gheorghe, C.; Marculescu, C.; Dinca, C. Effect of Pyrolysis Conditions on Bio-Char Production from Biomass. In Proceedings of the Proceedings of the 3rd WSEAS International Conference on Renewable Energy Sources, Brasov, Romania, 26–28 June 2014; Energy Problems and Environmental Engineering: Bucharest Romania, 2009; pp. 239–241. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Renita, A.A. Hybrid Synthesis of Novel Material through Acid Modification Followed Ultrasonication to Improve Adsorption Capacity for Zinc Removal. J. Clean. Prod. 2018, 172, 92–105. [Google Scholar] [CrossRef]
- Matovic, D. Biochar as a Viable Carbon Sequestration Option: Global and Canadian Perspective. Energy 2011, 36, 2011–2016. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Jiang, Y.; Tan, G.; Xu, N.; Xu, Y. Enhanced Power Generation and Wastewater Treatment in Sustainable Biochar Electrodes Based Bioelectrochemical System. Bioresour. Technol. 2017, 241, 841–848. [Google Scholar] [CrossRef]
- Shen, Y. Chars as Carbonaceous Adsorbents/Catalysts for Tar Elimination during Biomass Pyrolysis or Gasification. Renew. Sustain. Energy Rev. 2015, 43, 281–295. [Google Scholar] [CrossRef]
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and Chemical Properties of Swine-Manure-Derived Biochar Pertinent to Its Potential Use as a Soil Amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Zech, W.; Haumaier, L.; Hempfling, R. Ecological Aspects of Soil Organic Matter in Tropical Land Use. In Humic Substances in Soil and Crop Sciences: Selected Readings; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1990; Volume 1, pp. 187–202. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Lehmann, J.; Kuzyakov, Y.; Pan, G.; Ok, Y.S. Biochars and the Plant-Soil Interface. Plant Soil 2015, 395, 1–5. [Google Scholar] [CrossRef]
- Kammann, C.; Glaser, B.; Schmidt, H.P. Combining Biochar and Organic Amendments. In Biochar in European Soils and Agriculture; Routledge: Abingdon-on-Thames, UK, 2016; pp. 136–164. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Graber, E.R.; Elad, Y. Biochar Impact on Plant Resistance to Disease. In Biochar and Soil Biota; CRC Press: London, UK, 2013; pp. 49–76. ISBN 9780429071935. [Google Scholar]
- Summerell, B.A. Resolving Fusarium: Current Status of the Genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar] [CrossRef]
- Elmer, W.H. Effect of Leaf Mold Mulch, Biochar, and Earthworms on Mycorrhizal Colonization and Yield of Asparagus Affected by Fusarium Crown and Root Rot. Plant Dis. 2016, 100, 2507–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanomi, G.; Ippolito, F.; Scala, F. A “Black” Future for Plant Pathology? Biochar as a New Soil Amendment for Controlling Plant Diseases. J. Plant Pathol. 2015, 97, 223–234. [Google Scholar] [CrossRef]
- Segura Escobar, M.B. Efectos de La Sombra de Cashá (Chloroleucon Eurycyclum) En El Cultivo Del Café (Coffea Arabica) Sobre Los Procesos de Esporulación, Dispersión a Través Del Agua y Deposición de Hemileia Vastatrix. Centro Agronómico de Investigación y Enseñanza (CATIE): Turrialba, Costa Rica, 2017. [Google Scholar]
- Hindorf, H.; Omondi, C.O. A Review of Three Major Fungal Diseases of Coffea Arabica L. in the Rainforests of Ethiopia and Progress in Breeding for Resistance in Kenya. J. Adv. Res. 2011, 2, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.; Talhinhas, P.; Loureiro, A.; Duplessis, S. Hemileia Vastatrix Gene Expression during the Infection Process of Coffee Leaves; Australian Securities and Investments Commission: Brisbane, Australian, 2010; pp. 754–758. [Google Scholar]
- Koutouleas, A.; Jørgen Lyngs Jørgensen, H.; Jensen, B.; Lillesø, J.P.B.; Junge, A.; Ræbild, A. On the Hunt for the Alternate Host of Hemileia Vastatrix. Ecol. Evol. 2019, 9, 13619–13631. [Google Scholar] [CrossRef] [Green Version]
- Agrios, G.N. How Plants Defend Themselves Against Pathogens. In Plant Pathology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 207–248. [Google Scholar] [CrossRef]
- Gómez-De La Cruz, I.; Pérez-Portilla, E.; Escamilla-Prado, E.; Martínez-Bolaños, M.; Carrión-Villarnovo, G.L.L.; Hernández-Leal, T.I.; Gómez-De La Cruz, I.; Pérez-Portilla, E.; Escamilla-Prado, E.; Martínez-Bolaños, M.; et al. Selección in Vitro de Micoparásitos Con Potencial de Control Biológico Sobre Roya Del Café (Hemileia Vastatrix). Rev. Mex. Fitopatol. 2018, 36, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Agrios, G.N. Plant Diseases Caused by Fungi. In Plant Pathology; Academic Press: San Diego, CA, USA, 2005; Volume 232, pp. 385–614. ISBN 978-0-12-044565-3. [Google Scholar]
- Flor, H.H. Identification of Races of Flax Rust by Lines with Single Rust-Conditioning Genes; United States Department of Agriculture: Washington, DC, USA, 1954; Volume 25. [Google Scholar]
- Gichuru, E.K.; Ithiru, J.M.; Silva, M.C.; Pereira, A.P.; Varzea, V.M.P. Additional Physiological Races of Coffee Leaf Rust (Hemileia Vastatrix) Identified in Kenya. Trop. Plant Pathol. 2012, 37, 424–427. [Google Scholar] [CrossRef] [Green Version]
- Libert-Amico, A.; Paz-Pellat, F. Del Papel a La Acción En La Mitigación y Adaptación al Cambio Climático: La Roya Del Cafeto En Chiapas. Madera Bosques 2018, 24, 1. [Google Scholar] [CrossRef]
- Tavares, S.; Pires, A.S.; Azinheira, H.G.; Ramos, A.P.; Bispo, C.; Andrade, C.; Loureiro, A.; Schmidt, D.; Link, T.; Voegele, R.T.; et al. Genetics of Five-Needle Pines, Rusts of Forest Trees, and Strobusphere. In Proceedings of the IUFRO Joint Conference, Fort Collins, CO, USA, 15–20 June 2014; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2018; Volume 225, p. 245. [Google Scholar]
- Guerra-Guimarães, L.; Cardoso, S.; Martins, I.; Loureiro, A.; Silva, A.B.d.; Várzea, V.M.P.; Silva, M.D.C. Differential Induction of Superoxide Dismutase in Coffea Arabica-Hemileia Vastatrix Interactions. In 22nd International Conference on Coffee Science, ASIC 2008; Association Scientifique Internationale du Café (ASIC): Campinas, Brazil, 2009; Volume 14, pp. 1036–1039. ISBN 2900212219. [Google Scholar]
- Talhinhas, P.; Batista, D.; Diniz, I.; Vieira, A.; Silva, D.N.; Loureiro, A.; Tavares, S.; Pereira, A.P.; Azinheira, H.G.; Guerra-Guimarães, L.; et al. The Coffee Leaf Rust Pathogen Hemileia Vastatrix: One and a Half Centuries around the Tropics. Mol. Plant Pathol. 2017, 18, 1039–1051. [Google Scholar] [CrossRef] [Green Version]
- Coffee Rust. Available online: https://www.apsnet.org/edcenter/disandpath/fungalbasidio/pdlessons/Pages/CoffeeRust.aspx (accessed on 27 September 2022).
- Lingle, T.R.; Menon, S.N. Cupping and Grading-Discovering Character and Quality. In The Craft and Science of Coffee; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 181–203. ISBN 9780128035580. [Google Scholar]
- Hernández, M.; Pandolph, R.; Sänger, C.; Vos, R. Volatile Coffee Prices: COVID-19 and Market Fundamentals; International Food Policy Research Institute: Washington, DC, USA, 2020. [Google Scholar]
- Koh, I.; Garrett, R.; Janetos, A.; Mueller, N.D. Climate Risks to Brazilian Coffee Production. Environ. Res. Lett. 2020, 15, 104015. [Google Scholar] [CrossRef]
- Davis, M. The Global Challenge of Adapting Coffee to a Changing Climate—SEI. Available online: https://www.sei.org/featured/global-challenge-adapting-coffee-changing-climate/ (accessed on 24 September 2022).
- CIFOR. How a Coffee Rust Epidemic Escalated in Chiapas, Mexico. Available online: https://forestsnews.cifor.org/61800/how-a-coffee-rust-epidemic-escalated-in-chiapas-mexico?fnl= (accessed on 18 November 2020).
- Silva, M.D.C.; Várzea, V.; Guerra-Guimarães, L.; Azinheira, H.G.; Fernandez, D.; Petitot, A.S.; Bertrand, B.; Lashermes, P.; Nicole, M. Coffee Resistance to the Main Diseases: Leaf Rust and Coffee Berry Disease. Braz. J. Plant Physiol. 2006, 18, 119–147. [Google Scholar] [CrossRef] [Green Version]
- Berndt, R. Species Richness, Taxonomy and Peculiarities of the Neotropical Rust Fungi: Are They More Diverse in the Neotropics? Biodivers. Conserv. 2012, 21, 2299–2322. [Google Scholar] [CrossRef] [Green Version]
- Su, C. Factors Impacting American Leaf Spot Disease Incidence and Intensity in a Shaded Coffee Farm in Chiapas, Mexico. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2021. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Rigal, C.; Vaast, P.; Ramalho, J.C.; et al. Shaded-Coffee: A Nature-Based Strategy for Coffee Production under Climate Change? A Review. Front. Sustain. Food Syst. 2022, 6, 877476. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, E.A.; Almazán-Núñez, R.C.; Corcuera, P.; González-García, F.; Brito-Millán, M.; Alvarado-Castro, V.M. Land Use Cover Changes the Bird Distribution and Functional Groups at the Local and Landscape Level in a Mexican Shaded-Coffee Agroforestry System. Agric. Ecosyst. Environ. 2022, 330, 107882. [Google Scholar] [CrossRef]
- de Melo Virginio Filho, E.; Somarriba, E.; Cerda, R.; Casanoves, F.; Cordero, C.A.; Avelino, J.; Roupsard, O.; Rapidel, B.; Vaast, P.; Harmand, J.-M.; et al. Aportes a La Investigación, Fortalecimiento de Capacidades y Formulación de Políticas Para El Sector Cafetalero En 20 Años de Ensayos de Sistemas Agroforestales Con Café. AgroForestería Las Américas 2021, 51, 107–151. [Google Scholar]
- Villavicencio-Enríquez, L. Agroforestry Characterization in Traditional and Rustic Coffee Systems in San Miguel, Veracruz, México. Rev. Chapingo Ser. Cienc. For. Ambiente 2013, 19, 67–80. [Google Scholar] [CrossRef]
- de Carvalho, A.F.; Fernandes-Filho, E.I.; Daher, M.; Gomes, L.d.C.; Cardoso, I.M.; Fernandes, R.B.A.; Schaefer, C.E.G.R. Microclimate and Soil and Water Loss in Shaded and Unshaded Agroforestry Coffee Systems. Agrofor. Syst. 2020, 95, 119–134. [Google Scholar] [CrossRef]
- Melchor, R.L.A.; Rosales, V.G.; Pérez, M.C.G.; Fernández, S.P.; Álvarez, G.O.; Mastache, J.M.N. Effectiveness of Carboxylic Acids from Pichia Membranifaciens against Coffee Rust. Ciência Agrotecnologia 2018, 42, 42–50. [Google Scholar] [CrossRef]
- Silva, M.d.C.; Guerra-Guimarães, L.; Diniz, I.; Loureiro, A.; Azinheira, H.; Pereira, A.P.; Tavares, S.; Batista, D.; Várzea, V. An Overview of the Mechanisms Involved in Coffee-Hemileia Vastatrix Interactions: Plant and Pathogen Perspectives. Agronomy 2022, 12, 326. [Google Scholar] [CrossRef]
- Cerna Chávez, E.; Magaña Arteaga, R.; Velazquez Guerrero, J.J.; Ochoa Fuentes, Y.M.; Hernandez Bautista, O. Evaluación de Extractos Vegetales Sobre Incidencia y Severidad de Hemileia Vastatrix En Cultivo de Café. Ecosistemas Recur. Agropecu. 2019, 6. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of Emerging Technologies for the Extraction of Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2019, 60, 1826–1841. [Google Scholar] [CrossRef]
- Morales-Antonio, M.A.; Santiago-Martínez, G.M.; Vásquez-López, A.; Rodríguez-Ortiz, G.; Soto-Castro, D.; Lozano-Trejo, S.; Castañeda-Hidalgo, E.; Morales-Antonio, M.A.; Santiago-Martínez, G.M.; Vásquez-López, A.; et al. Uredospore Germination of Hemileia Vastatrix and Its Inhibition by the Effect of Plant Extracts in Vitro. Int. J. Agric. Nat. Resour. 2021, 48, 108–114. [Google Scholar] [CrossRef]
- Lam-Gutiérrez, A.; Winkler, R.; Garrido-Ramírez, E.R.; Rincón-Rosales, R.; Gutiérrez-Miceli, F.A.; Peña-Ocaña, B.A.; Guzmán-Albores, J.M.; Ruíz-Valdiviezo, V.M. Antifungal Activity of Root Extracts from Baccharis Salicina on Germination of Uredospores of Hemileia Vastatrix. Int. J. Agric. Biol. 2021, 25, 1075–1084. [Google Scholar] [CrossRef]
- Rodas Arzét, A.M.; García Alvares, R.J.; Salomón Miranda, G.; Nufio Barillas, J.S. Evaluación y Caracterización Extractos Botánicos Supercríticos de Orégano (Lippia Graveolens) Para El Control de La Roya (Hemileia Vastatrix) En El Cultivo de Café (Coffea Arabica L.) En Las Zonas Productoras Del Municipio de La Unión; Departamento de Zacapa—Repositorio Institucional USAC, Universidad de San Carlos de Guatemala: Zacapa, Guatemala, 2016. [Google Scholar]
- Mudyiwa, R.; Mwatsiya, N.; Manenji, B.; Chidoko, P.; Mahoya, C. Evaluation of Different Botanicals for the Control of Coffee Leaf Rust (Hemileia Vastatrix Berkeley and Broome). Int. J. Plant Soil. Sci. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Alvarado-Castillo, G.; Benítez-Badillo, G.; José, V.L.-G.A.; Ortiz-Ceballos, G.C.; Aguirre Beltrán, G. Uredospores’ Mycelium Germination Inhibition of Coffee Rust (Hemileia Vastatrix) through Three Alternative Compounds: First Study. Wulfenia J. 2017, 24, 2. [Google Scholar]
- Antonio García-Pérez, J.; Alarcón-Gutiérrez, E.; Viayne Del, R.P. Extractos Acuosos de Plantas Como Inhibidores de La Germinación de Urediniosporas de Hemileia Vastatrix; La Roya Anaranjada Del Café. AyTBUAP 2021, 6, 45–60. [Google Scholar] [CrossRef]
- Salustiano, M.E.; Carlos, A.; Filho, F.; Pozza, E.A.; Antônio De Castro, H. Extratos de Candeia (Eremanthus Erythropappus (Dc.) Macleish) Na Inibição in Vitro de Cylindrocladium Scoparium e de Quatro Espécies de Ferrugens. CERNE 2006, 12, 189–193. [Google Scholar]
- Silva, J.L.; Souza, P.E.; Monteiro, F.P.; Freitas, M.L.O.; Silva Junior, M.B.; Belan, L.L. Antifungal Activity Using Medicinal Plant Extracts against Pathogens of Coffee Tree. Rev. Bras. Plantas Med. 2014, 16, 539–544. [Google Scholar] [CrossRef] [Green Version]
- Osorio Echeverri, V.M. Evaluación Del Efecto Inhibitorio de Los Aceites Esenciales de Tomillo (Thymus Vulgaris) Frente a Los Hongos Colletotrichum Sp. Causante de La Enfermedad de Las Cerezas Del Café (CBD) y Hemileia Vastatrix Causante de Roya. Available online: https://ipt.biodiversidad.co/cr-sib/resource.do?r=1467_tomillo_20190922 (accessed on 9 March 2022).
- Valencia, V.; García-Barrios, L.; Sterling, E.J.; West, P.; Meza-Jiménez, A.; Naeem, S. Smallholder Response to Environmental Change: Impacts of Coffee Leaf Rust in a Forest Frontier in Mexico. Land Use Policy 2018, 79, 463–474. [Google Scholar] [CrossRef]
- Tawfeeq Al-Ani, L.K.; Aguilar-Marcelino, L.; Fiorotti, J.; Sharma, V.; Sarker, M.S.; Furtado, E.L.; Wijayawardene, N.N.; Herrera-Estrella, A. Biological Control Agents and Their Importance for the Plant Health. In Microbial Services in Restoration Ecology; Singh, J.S., Vimal, S.R., Eds.; Elsevier: Cambridge, MA, USA, 2020; Volume 1, pp. 13–36. [Google Scholar] [CrossRef]
- Zullim, J.; Zullim Ortega-Enriquez, J. Control Biológico de Compuestos Bioactivos a Partir de Hongos Endófitos Contra Plagas de Insectos. INVURNUS 2020, 15, 24–29. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Shahid, M.; Zaidi, A.; Khan, M.S.; Rizvi, A.; Saif, S.; Ahmed, B. Recent Advances in Management Strategies of Vegetable Diseases. In Microbial Strategies for Vegetable Production; Springer: Cham, Switzerland, 2017; pp. 197–226. [Google Scholar] [CrossRef]
- Subroto, G.; Kusbianto, D.E.; Avivi, S.; Slameto, S.; Setiyono, S. Correlation Between Secondary Metabolites of Leaf and the Resistance to Leaf Rust (Hemileia Vastatrix) on Several Arabica Coffee Clones. Ilmu Pertan. (Agric. Sci.) 2019, 4, 71–75. [Google Scholar] [CrossRef]
- Pereira, R.B.; Lucas, G.C.; Perina, F.J.; Alves, E. Óleos Essenciais No Controle Da Ferrugem Em Cafeeiro. Ciência Agrotecnologia 2012, 36, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Hernández, H.E. El Extracto Acuoso de Lombricomposta: Generalidades, Caracterización y Potencial Alternativa Para El Control Biológico de Hemileia Vastatrix. Available online: https://agritrop.cirad.fr/591518/1/23.%20El%20extracto%20acuoso%20de%20lombricomposta.%20Generalidades%2C%20caracterizaci%C3%B3n%20y%20potencial%20alternativa%20para%20el%20control%20biol%C3%B3gico%20de%20H.%20vastatrix.pdf (accessed on 8 March 2022).
- Hajian-Forooshani, Z.; Vandermeer, J.; Perfecto, I. Insights from Excrement: Invasive Gastropods Shift Diet to Consume the Coffee Leaf Rust and Its Mycoparasite. Ecology 2020, 101, e02966. [Google Scholar] [CrossRef]
- Santiago-Elena, E.; Zamora-Macorra, E.J.; Zamora-Macorra, M.; Elizalde-Gaytan, K.G. Interaction between Mycodiplosis and Hemileia Vastatrix in Three Scenarios of Coffee Crop Management (Coffea Arabica). Mex. J. Phytopathol. 2020, 38, 320–336. [Google Scholar] [CrossRef]
- Roseli dos Reis, G.; Itamar Bachiao de, L.; Angélico De Mendonça, J.M.; Washington Pereira, B.S. Efeito de Biofertilizante Associado à Urina de Vaca Na Incidência de Ferrugem e Cercosporiose Do Cafeeiro. In Proceedings of the VIII Simpósio de Pesquisa dos Cafés do Brasil, Salvador, Brazil, 25–28 November 2013; p. 5. [Google Scholar]
- Shiomi, H.F.; Silva, H.S.A.; de Melo, I.S.; Nunes, F.V.; Bettiol, W. Bioprospecting Endophytic Bacteria for Biological Control of Coffee Leaf Rust. Sci. Agric. 2006, 63, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Hernández, C.; López, L.; Sánchez, L. Agentes de Control Biológico de La Roya Del Café ¿Cómo Funcionan y Qué Tan Efectivos Son? Available online: https://smbb.mx/wp-content/uploads/2021/05/Hernandez-et-al.-2021.pdf (accessed on 8 March 2022).
- Herrera-Estrella, A.; Chet, I. The Biological Control Agent Trichoderma From Fundamentals To Applications. In Handbook of Fungal Biotechnology; Arora, D., Ed.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends. Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Gómez-Hernández, M.; Rodríguez-García, C.M.; Peraza-Echeverría, L.; Peraza-Sánchez, S.R.; Torres-Tapia, L.W.; Pérez-Brito, D.; Vargas-Coronado, R.F.; Cauich-Rodríguez, J.V. In Vitro Antifungal Activity Screening of Beach-Cast Seaweeds Collected in Yucatan, Mexico. J. Appl. Phycol. 2021, 33, 1229–1237. [Google Scholar] [CrossRef]
- Zeriouh, H.; de Vicente, A.; Pérez-García, A.; Romero, D. Surfactin Triggers Biofilm Formation of Bacillus Subtilis in Melon Phylloplane and Contributes to the Biocontrol Activity. Environ. Microbiol. 2014, 16, 2196–2211. [Google Scholar] [CrossRef]
- Daivasikamani, R.S. Biological Control of Coffee Leaf Rust Pathogen, Hemileia Vastatrix Berkeley and Broome Using Bacillus Subtilis and Pseudomonas Fluorescens. J. Biopestic. 2009, 2, 94–98. [Google Scholar]
- Rettinassababady, C.; Jeyalakshmi, C. Bio-Fungicides: The Best Alternative for Sustainable Food Security and Ecosystem. Microbial Diversity and Biotechnology in Food Security; Springer: New Delhi, India, 2014; pp. 401–411. [Google Scholar] [CrossRef]
- Nagórska, K.; Bikowksi, M.; Obuchowski, M. Multicellular Behaviour and Production of a Wide Variety of Toxic Substances Support Usage of Bacillus Subtilis as a Powerful Biocontrol Agent—PubMed. Acta Biochim. Pol. 2007, 54, 495–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacefo, V.; de Araújo, F.F.; Pacheco, A.C. Biological Control of Hemileia Vastatrix Berk. & Broome with Bacillus Subtilis Cohn and Biochemical Changes in the Coffee. Coffee Sci. 2017, 11, 567–574. [Google Scholar]
- Haddad, F.; Maffia, L.A.; Mizubuti, E.S.G.; Teixeira, H. Biological Control of Coffee Rust by Antagonistic Bacteria under Field Conditions in Brazil. Biol. Control 2009, 49, 114–119. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Barreto, R.W.; Rodriguez, M.C.H.; Matei, P.M.; Martín-Gil, J. Control of Coffee Leaf Rust by Chitosan Oligomers and Propolis. Agric. Life Life Agric. Conf. Proc. 2018, 1, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Haddad, F.; Saraiva, R.M.; Mizubuti, E.S.G.; Romeiro, R.S.; Maffia, L.A. Isolation and Selection of Hemileia Vastatrix Antagonists. Eur. J. Plant Pathol. 2014, 139, 763–772. [Google Scholar] [CrossRef]
- Arroyo-Esquivel, J.; Sanchez, F.; Barboza, L.A. Infection Model for Analyzing Biological Control of Coffee Rust Using Bacterial Anti-Fungal Compounds. Math. Biosci. 2019, 307, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambot, C.; Herrera, J.C.; Bertrand, B.; Sadeghian, S.; Benavides, P.; Gaitán, A. Cultivating Coffee Quality-Terroir and Agro-Ecosystem. In The Craft and Science of Coffee; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128035580. [Google Scholar]
- Ennis, K.K. Climate Consequences for Ecosystem Functions, Production And Producer Responses in Coffee Agroecosystems. Ph.D. Dissertation, University of California Santa Cruz, Santa Cruz, CA, USA, 2019. [Google Scholar]
- Virginio Filho, E.D.M.; Astorga, C. Prevention and Control of Coffee Leaf Rust Handbook of Best Practices for Extension Agents and Facilitators Prevention and Control of Coffee Leaf Rust Handbook of Best Practices for Extension Agents and Facilitators; Tropical Agricultural Research and Higher Education Center (CATIE): Cartago, Costa Rica, 2019. [Google Scholar]
- Paudel, N.; Kang, W.H. Establishment of Algae as Bio-Fertilizer for Coffee Plant. Int. J. Sci. Rep. 2018, 4, 153–157. [Google Scholar] [CrossRef]
- Pérez-Madruga, Y.; López-Padrón, I.; Reyes-Guerrero, Y. Las Algas Como Alternativa Natural Para La Producción de Diferentes Cultivos. Cultiv. Trop. 2020, 41. [Google Scholar]
- Felui Sendra, F. Extractos de Algas En La Agricultura. Available online: https://aefa-agronutrientes.org/extractos-de-algas-en-la-agricultura (accessed on 8 March 2022).
- Canales López, B. Enzimas—Algas: Posibilidades de Su Uso Para Estimular La Producción Agrícola y Mejorar Los Suelos. Terra Latinoam. 1999, 17, 271–276. [Google Scholar]
- Chacón-Villalobos, Y.; Chacón-Sancho, A.; Vargas-Chinchilla, M.; Cerdà-Subirachs, J.M.; Hernández-Pérez, R. Nuevo Bioestimulante de Floración y Maduración En Café (Coffea Arabica L.). Agron. Mesoam. 2021, 32, 983–990. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A Review of Biochar-Based Catalysts for Chemical Synthesis, Biofuel Production, and Pollution Control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Gibert, O.; Lefèvre, B.; Fernández, M.; Bernat, X.; Paraira, M.; Calderer, M.; Martínez-Lladó, X. Characterising Biofilm Development on Granular Activated Carbon Used for Drinking Water Production. Water Res. 2013, 47, 1101–1110. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.J.; Tafti, N.D.; Hollier, C.A.; Myers, G.; Wang, X. Effect of Alkali-Enhanced Biochar on Silicon Uptake and Suppression of Gray Leaf Spot Development in Perennial Ryegrass. Crop Prot. 2019, 119, 9–16. [Google Scholar] [CrossRef]
- Hou, J.; Pugazhendhi, A.; Phuong, T.N.; Thanh, N.C.; Brindhadevi, K.; Velu, G.; Lan Chi, N.T.; Yuan, D. Plant Resistance to Disease: Using Biochar to Inhibit Harmful Microbes and Absorb Nutrients. Environ. Res. 2022, 214, 113883. [Google Scholar] [CrossRef]
- Elad, Y.; Cytryn, E.; Harel, Y.M.; Lew, B.; Graber, E. The Biochar Effect: Plant Resistance to Biotic Stresses. Phytopathol. Mediterr. 2011, 50, 335–349. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar Mediates Systemic Response of Strawberry to Foliar Fungal Pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Ezra, D.; Harel, Y.M.; Kolton, M.; Elad, Y.; Rav-David, D.; Cytryn, E.; Borenstein, M.; Shulchani, R.; Graber, E.R. Induced Systemic Resistance in Strawberry (Fragaria× Ananassa) to Powdery Mildew Using Various Control Agents Induced Systemic Resistance in Strawberry (Fragaria × Ananassa) to Powdery Mildew Using Various Control Agents. Multitrophic Interact. Soil IOBC/Wprs Bull. 2011, 71, 47–51. [Google Scholar]
- Sehar, A.; Jabeen, K.; Iqbal, S.; Rasul, F.; Javad, S. Assessment of Biochar and Compost Antifungal Potential against Botryodiplodia Theobromae Pat. Int. J. Biol. Biotechnol. 2017, 14, 585–589. [Google Scholar]
- Draper, K.; Tomlinson, T. How Biochar Can Improve Sustainability for Coffee Cultivation and Processing. Available online: www.biochar-journal.org/en/ct/54 (accessed on 27 September 2022).
- Frenkel, O.; Jaiswal, A.K.; Elad, Y.; Lew, B.; Kammann, C.; Graber, E.R.; Nõ Lvak, H.; Truu, J.; Limane, B.; Truu, M.; et al. The Effect of Biochar on Plant Diseases: What Should We Learn While Designing Biochar Substrates? Vilnius Gedim. Tech. Univ. 2017, 25, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Anaya de la Rosa, R.K.; Cowie, A. Upcycling Biomass Residues: Learning from the Biochar for Sustainable Soils (B4SS) Project. In Proceedings of the International Conference & Expo on Recycling; Expert Opinion in Environmental Biology, Amsterdam, The Netherlands, 20 August 2018. [Google Scholar]
- Heikkinen, J.; Keskinen, R.; Soinne, H.; Hyväluoma, J.; Nikama, J.; Wikberg, H.; Källi, A.; Siipola, V.; Melkior, T.; Dupont, C.; et al. Possibilities to Improve Soil Aggregate Stability Using Biochars Derived from Various Biomasses through Slow Pyrolysis, Hydrothermal Carbonization, or Torrefaction. Geoderma 2019, 344, 40–49. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Zech, W. Black Carbon Contribution to Stable Humus in German Arable Soils. Geoderma 2007, 139, 220–228. [Google Scholar] [CrossRef]

| Coffea arabica Variety | Extract Plant | Biocontrol Mechanism | Reference |
|---|---|---|---|
| Caturra | Acetone and ethanol extracts of Ricinus communis, Datura ferox, Mansoa alliacea Tribulus terrestris, and Acacia farnesiana | Inhibition of the Hemileia vastatrix uredospore germination | [65] |
| Caturra | Alcoholic extract of chilca roots (aka Baccharis glutinosa) | Preventive effect and reduction of foliar damage in coffee trees if it is applied 24 h before exposure. | [66] |
| Typica and Caturra | Extract of Cinnamomum verum, C. sinensis, Larrea tridentata, Eucalyptus globulus, Brassica nigra, and Piper nigrum | Preventive effect of reducing incidence and severity of coffee rust after the application of commercial products | [63] |
| Coffea arabica L. | Supercritical extract of Lippia graveolens | Antifungal effects on Hemileia vastatrix uredospores in vitro | [67] |
| Catucaií 2SL, Catuaií IAC 62 and Mundo Novo 379/19 | Essential oil of cinnamon, citronella, lemongrass, clove, tea tree, thyme, neem, and eucalyptus | Inhibition of the germination of urediniospores, antimicrobial agents of the terpene group, and guaiacol, with doses equivalent to 1000 µL L−1 | [42] |
| Coffea arabica L. | Botanical extracts of Cymbopogon citratus, Aloe barbadensis, Moringa oleifera, and Nicotiana tabacum | Inhibition of the germination of Hemileia vastatrix uredospores | [68] |
| Coffea arabica L. | Ethanolic extract from leaves of Piper aduncum L. | Uredospore mycelium germination inhibition in vitro | [69] |
| Coffea arabica L. | Aqueous extracts from leaves of Ardisia compressa, Eriobotrya japonica, Ocimun basilicum | Novel aqueous extract with antifungal activity | [70] |
| Coffea arabica L. | Oil of Eremanthus erythropappus leaves | Inhibition of the germination of Hemileia vastatrix uredospores | [71] |
| Coffea arabica L. | Extract from leaves of Allium sativum and Vernonia polysphaera | Inhibition of the germination of Hemileia vastatrix uredospores in vitro | [70] |
| Coffea arabica L. | Extracts from bulbs of Allium sativum, leaves of Vernonia polysphaera, and flower buds of Syzygium aromaticum | Inhibition of mycelial growth in vivo | [72] |
| Coffea arabica L. | Essential oil and extract of Cymbopogon nardus leaves | Inhibition of the germination of Hemileia vastatrix uredospores | [71] |
| Specie | Inoculum Concentration | Reduction of Lesions in Coffee Leaves (%) | Reduction of Uredospores Germination (%) | Biocontrol Mechanism | References |
|---|---|---|---|---|---|
| Bacteria Bacillus thuringiensis | NR 1 | 76–96 | NR 1 | They induce systemic resistance in coffee trees and the production of hydrolytic enzymes (β-1,3-glucanase and chitinase) in the tissues of the leaves. | [78] |
| Bacteria Bacillus lentimorbus | 1 × 108 CFU 2 | NR 1 | 50 | They produce hydrolytic enzymes (β-1,3-glucanase and chitinase) and fungicidal metabolites. | [66] |
| Bacteria Bacillus cereus | 1 × 108 CFU 2 | NR 1 | 50 | They produce hydrolytic enzymes (β-1,3-glucanase and chitinase) and fungicidal metabolites. | [85] |
| Bacteria Bacillus subtilis | From 1 to 4.3 × 108 CFU 2 | 87 | 100 | Natural antagonist, Induces systemic resistance in coffee trees, and production of metabolites with fungicidal activity | [86] |
| Bacteria Pseudomonas fluorescens | From 1 to 4.3 × 108 CFU 2 | 36 | 64 | Natural antagonist, Induces systemic resistance in coffee trees, and production of metabolites with fungicidal activity | [94] |
| Bacteria Salmonella enterica | 1 × 108 CFU 2 | 74 3 | NR 1 | Induction of systemic plant resistance and colonization of infection sites. | [78,95] |
| Fungus Lecanicillium spp. | 5 × 106 spores | NR 1 | 68 after five days of application | Hyperparasitism | [42] |
| Fungus Calcarisporium sp. | 5 × 106 spores | NR 1 | 51% after five days of application | Hyperparasitism | [39] |
| Fungus Simplicillium spp. | 5 × 106 spores | NR 1 | 89% after one day of application | Hyperparasitism | [39] |
| Bacteria Pectobacterium carotovorum | 1 × 108 CFU 2 | 55 3 | NR 1 | Bacterias induce systemic plant resistance. They also colonized the infection sites. | [39] |
| Bacteria Brevibacillus choshinensis | 1 × 108 CFU 2 | NR 1 | 9–28 | They induce systemic plant resistance. They also colonized the infection sites. | [39] |
| Chitosan oligomers from fungal classes of Basidiomycetes, Ascomycetes, Zygomycetes, and Deuteromycetes | NS 4 | NR 1 | 99% on coffee leaf discs | Antifungal activity, through inhibition of the germination of HV spores | [39] |
| Fungus Fusarium spp. | 1 × 106 spores | 83–86 | 95–99 after 40 days of application | NR 1 | [96] |
| Fungus Penicillium spp. | 1 × 106 spores | 80–92 | 90–98 after 40 days of application | NR 1 | [97] |
| Fungus Acremonium sp. | 1 × 106 spores | 84 | 91 after 40 days of application | NR 1 | [97] |
| Fungus Cladosporium sp. | 1 × 106 spores | 89 | 96 after 40 days of application | NR 1 | [97] |
| Fungus Aspergillus sp. | 1 × 106 spores | 97 | 97 after 40 days of application | NR 1 | [97] |
| Unfavourable Effect | Biochar Impact |
|---|---|
| Reduced availability of soil water | Reduced moisture retention and water content, negative effects on crop yields |
| Soil erosion | Particulate matter emissions, acceleration of biochar degradation, loss of soil fertility |
| Low biodegradability | Low environmental sustainability due to the accumulation on the soil for decades |
| Rise in soil salinity | Plant growth inhibition, negative effects on crop yields and economic impact |
| Excessive increase in soil pH | Inhibited plant growth due to precipitation and availability of nutrients, extreme pH, changed mobility of PTEs. |
| Excessive sorption of nutrients | Nutrient immobilisation and reduced bioavailability for plants and microflora, plant growth inhibition, reduced yields |
| Formation of toxic PAHs | Toxicity to soil macro- and microbiota, increased human health risks in case of PAHs distribution in environment and their accumulation in the crop biomass/food chain |
| Formation of toxic VOCs | Plant growth inhibition, human health risk in case of VOCs distribution in environment and their accumulation in the crop biomass |
| Presence of PTEs | Decreased plant growth, inhibition, mortality, genotoxic effects, human health risk in case of PTEs distribution in environment and their accumulation in the crop biomass/food chain |
| Formation of toxic dioxins | Human health risks in case of dioxin distribution in the environment and their accumulation in the crop biomass/food chain |
| Changes in microbial communities | Shifts in the fungi-to-bacteria ratio, decreased microbial activity, N mineralisation, SOC sequestration |
| Adverse effects of biochar on soil invertebrates | Reproduction and growth inhibition, mortality, genotoxicity—decrease in biochar incorporation, soil enzyme activity and thus plant productivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, N.E.T.; Acosta, Y.A.; Parra-Arroyo, L.; Martínez-Prado, M.A.; Rivas-Galindo, V.M.; Iqbal, H.M.N.; Bonaccorso, A.D.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Towards an Eco-Friendly Coffee Rust Control: Compilation of Natural Alternatives from a Nutritional and Antifungal Perspective. Plants 2022, 11, 2745. https://doi.org/10.3390/plants11202745
Castillo NET, Acosta YA, Parra-Arroyo L, Martínez-Prado MA, Rivas-Galindo VM, Iqbal HMN, Bonaccorso AD, Melchor-Martínez EM, Parra-Saldívar R. Towards an Eco-Friendly Coffee Rust Control: Compilation of Natural Alternatives from a Nutritional and Antifungal Perspective. Plants. 2022; 11(20):2745. https://doi.org/10.3390/plants11202745
Chicago/Turabian StyleCastillo, Nora E. Torres, Yovanina Aguilera Acosta, Lizeth Parra-Arroyo, María Adriana Martínez-Prado, Verónica M. Rivas-Galindo, Hafiz M. N. Iqbal, A. Damiano Bonaccorso, Elda M. Melchor-Martínez, and Roberto Parra-Saldívar. 2022. "Towards an Eco-Friendly Coffee Rust Control: Compilation of Natural Alternatives from a Nutritional and Antifungal Perspective" Plants 11, no. 20: 2745. https://doi.org/10.3390/plants11202745
APA StyleCastillo, N. E. T., Acosta, Y. A., Parra-Arroyo, L., Martínez-Prado, M. A., Rivas-Galindo, V. M., Iqbal, H. M. N., Bonaccorso, A. D., Melchor-Martínez, E. M., & Parra-Saldívar, R. (2022). Towards an Eco-Friendly Coffee Rust Control: Compilation of Natural Alternatives from a Nutritional and Antifungal Perspective. Plants, 11(20), 2745. https://doi.org/10.3390/plants11202745