Application of MaxEnt Modeling and HRM Analysis to Support the Conservation and Domestication of Gevuina avellana Mol. in Central Chile
Abstract
1. Introduction
2. Results
2.1. Study Area and Plant Materials
2.2. MaxEnt Model Projection of Potential Current and Future of G. avellana
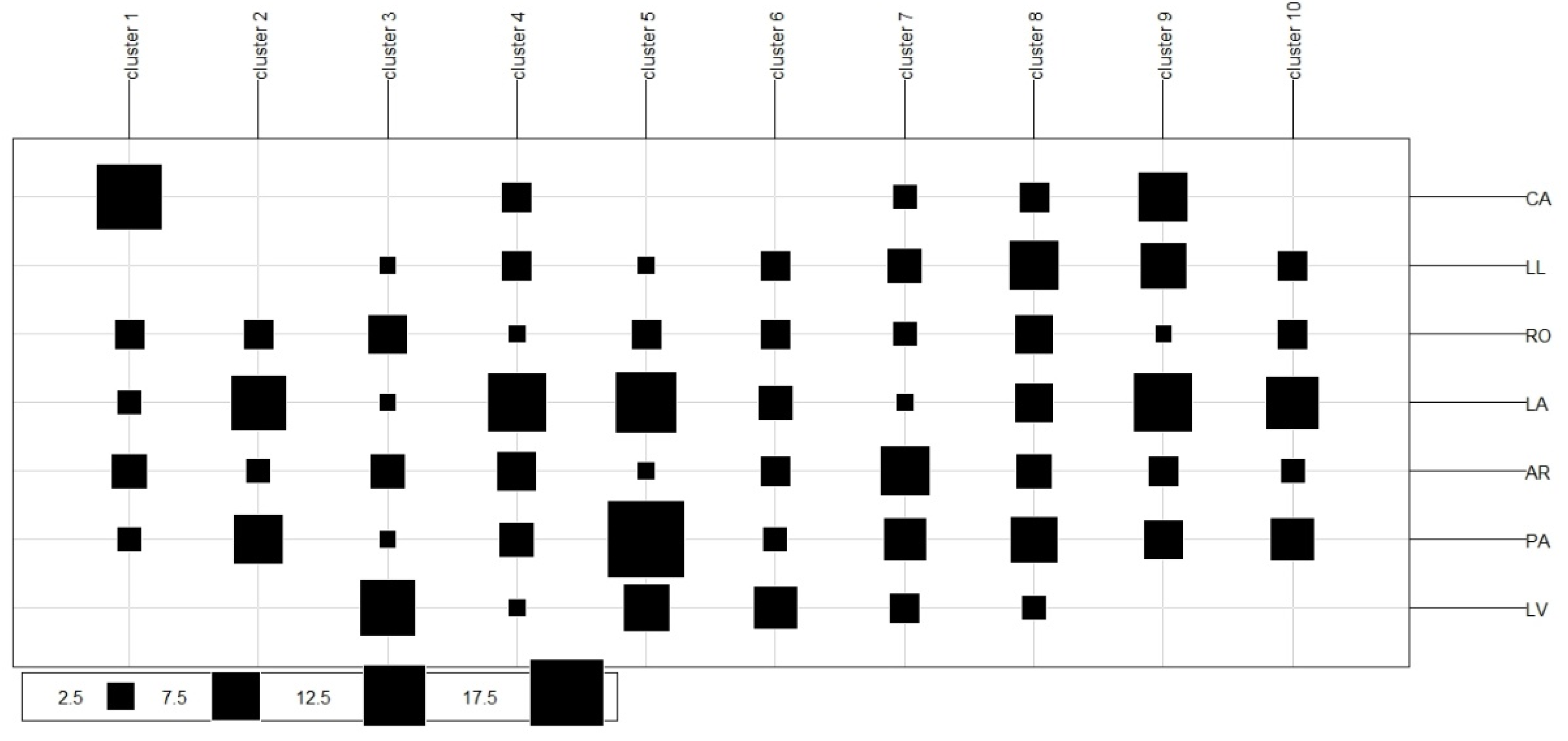
2.3. Genotyping with High-Resolution Melting Analysis
3. Discussion
4. Materials and Methods
4.1. Study Area and Plant Materials
4.2. MaxEnt Model Projection of Potential Current and Future of G. avellana
4.2.1. Data Sources
4.2.2. Variables Selection
4.2.3. Data Analysis
4.3. Genotyping with High-Resolution Melting Analysis
4.3.1. Nucleic Acid Preparation
4.3.2. Selection of Molecular Markers
4.3.3. Standardization and Selection of HRM Standard
4.3.4. Genotyping of Populations of G. avellana with High-Resolution Melting Analysis
4.3.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavín, A. Avellano Chileno (Gevuina avellana Mol.); Instituto de Investigaciones Agropecuarias (INIA Rahiun): Cauquenes, Chile, 2014; pp. 1–13. Available online: https://hdl.handle.net/20.500.14001/7850 (accessed on 14 July 2022).
- Orwa, B. Gevuina avellana Mol. Agroforestry Database 4.0. 2009, pp. 1–5. Available online: https://apps.worldagroforestry.org/treedb/AFTPDFS/Gevuina_avellana.PDF (accessed on 14 July 2022).
- Martínez, P. Aspectos Básicos del Avellano Chileno (Gevuina avellana Mol.); Instituto de Investigaciones Agropecuarias (INIA); Informativo N° 173; Ministerio de Agricultura (Minagri): Santiago, Chile, 2017; pp. 1–4. Available online: https://biblioteca.inia.cl/handle/20.500.14001/67235 (accessed on 10 July 2022).
- Halloy, S.; Grau, A.; McKenzie, B. Gevuina Nut (Gevuina avellana, Proteaceae), a Cool Climate Alternative to Macadamia. Econ. Bot. 1996, 50, 224–235. Available online: www.jstor.org/stable/4255833 (accessed on 10 July 2022). [CrossRef]
- Loewe, V.; Klein, F. Monografía de Avellano Chilenc (Gevuina avellana); Instituto Forestal (INFOR), Ministerio de Agricultura (Minagri): Santiago, Chile, 1997; pp. 1–49. Available online: https://bibliotecadigital.infor.cl/handle/20.500.12220/3994 (accessed on 4 August 2022).
- Álvarez, A. Manual de Buenas Prácticas Tradicionales de Recolección Sustentable de Frutos y Follajes de Avellano Chileno (Gevuina avellana Mol.); Instituto Forestal (Infor), Ministerio de Agricultura (Minagri): Santiago, Chile, 2019; pp. 1–66. Available online: https://bibliotecadigital.infor.cl/handle/20.500.12220/30337 (accessed on 4 August 2022).
- Ramirez, G.; Valenzuela, E.; Cristina San Martin, P. Nuevos antecedentes sobre desarrollo temprano, morfología y anatomía de las raíces proteiformes de Gevuina avellana. Agro Sur 2004, 32, 33–44. [Google Scholar] [CrossRef][Green Version]
- Loewe, V. Arboricultura Para Producción de Madera de Alto Valor. Una Nueva Alternativa Para el Sector Silvoagropecuario Chileno; Fundación para la Innovación Agraria (FIA), Ministerio de Agricultura: Santiago, Chile, 2003; pp. 1–56. Available online: http://bibliotecadigital.fia.cl/handle/20.500.11944/145454 (accessed on 10 July 2022).
- Donoso, C. Antecedentes sobre producción de avellanas. Bosque 1979, 3, 69–70. [Google Scholar] [CrossRef]
- Medel, F. Gevuina avellana Mol. Características y Mejoramiento Genético de un Frutal de Nuez Nativo para el Mercado Internacional. Rev. Frutícola 2000, 21, 1–10. Available online: https://www.researchgate.net/publication/271486793_Gevuina_avellana_Mol_Caracteristicas_y_mejoramiento_genetico_de_un_frutal_de_nuez_nativo_para_el_mercado_internacional (accessed on 10 July 2022).
- Medel, F. Clonal Selection in Gevuina avellana for Nutritional and Phytotherapy Purposes Clonal Selection in Gevuina avellana for Nutritional and Phytotherapy Purposes. Acta Hortic. 2005, 686, 625–630. [Google Scholar] [CrossRef]
- Medel, F. Potencial Productivo y Comercial del Avellano chileno (Gevuin). Seminario Internacional EXPONUT 2011, CHILENUT-PTI Hortofrutícola-PROCHILE, Chile. 2011, pp. 1–27. Available online: https://www.researchgate.net/publication/272166959_Potencial_Productivo_y_comercial_del_avellano_chileno_Gevuin (accessed on 10 July 2022).
- Medel, F. Producción, Características y Acción Fitoterapeutica del Complejo Lipídico de Gevuina Avellana Mol. 64o Congreso Agronómico de Chile, Viña Del Mar, Región de Valparaiso, Chile. 2013. Available online: https://doi.org/https://www.researchgate.net/publication/272179762 (accessed on 10 July 2022).
- Medel, F. El Gevuin, Avellano Chileno. Investigación y Desarrollo Frutícola para el Sur de Chile; Universidad Austral de Chile: Valdivia, Chile, 2012; pp. 1–6. Available online: https://www.researchgate.net/publication/271505359_El_Gevuin_avellano_chileno (accessed on 10 July 2022).
- Medel, F.; Medel, G. Growth of Seedling Rootstocks of Gevuina avellana Mol. by Soil and Foliar Fertilization. Acta Hortic. 2002, 594, 179–183. [Google Scholar] [CrossRef]
- Medel, G.; Medel, F.; Mcconchie, C. Phenological Development and Growing Degree Days in Gevuina avellana Mol. Phenological Development and Growing Degree Days in Gevuina avellana Mol. In Proceedings of the VIII International Congress on Hazelnut, Temuco, Chile, 19–22 March 2012; pp. 355–362. [Google Scholar] [CrossRef]
- Doll, U.; San Martín, J.; Ravanal, C.; Cifuentes, S.; Muñoz, M. Evaluación de la producción potencial de frutos de Gevuina avellana, durante una temporada (1999–2000) en el secano costero de la VII Región. Bosque 2005, 26, 87–96. [Google Scholar] [CrossRef][Green Version]
- Muñoz, C. Análisis Comparativo de la Producción Frutícola en Racimos de Gevuina avellana Mol. en Tres Sitios de la Cordillera de la Costa de la VII Región de Chile; Ingeniería Forestal; Facultad de Ciencias Forestales, Universidad de Talca: Talca, Chile, 2002; pp. 1–118. Available online: http://dspace.utalca.cl/handle/1950/3685 (accessed on 10 July 2022).
- Bahamondes, J. Aplicación de Métodos de Inferencia Filogenética Para el Estudio de la Variabilidad y la Filogenia del Avellano Chileno, Gevuina avellana Molina; Ingeniería Agrícola, Universidad Austral de Chile: Valdivia, Chile, 2009; pp. 1–77. Available online: http://cybertesis.uach.cl/tesis/uach/2009/fab151a/doc/fab151a.pdf (accessed on 10 July 2022).
- Hardner, C.M.; Peace, C.; Lowe, A.J.; Neal, J.; Pisanu, P.; Powell, M.; Schmidt, A.; Spain, C.; Williams, K. Genetic Resources and Domestication of Macadamia. Hortic. Rev. 2009, 35, 1–125. [Google Scholar] [CrossRef]
- Flores, M.; Claudia, G.; Oscar, E.V.; Doria, F.; Guzman, L.; Perez, C. Chemical evaluation and thermal behavior of Chilean hazelnut oil (Gevuina avellana Mol.) a comparative study with extra virgin olive oil. Eur. Food Res. Technol. 2019, 245, 1021–1029. [Google Scholar] [CrossRef]
- Jil, P. Variación del Contenido de los Ácidos Grasos en el Aceite Extruído en frío de Nueve Clones de Gevuina avellana Mol. Licenciatura en Biología; Facultad de Ciencias Agrarias, Universidad Austral de Chile: Valdivia, Chile, 2006; pp. 1–83. Available online: http://cybertesis.uach.cl/tesis/uach/2006/faj.61v/doc/faj.61v.pdf (accessed on 10 July 2022).
- Leidy, L.; Ramos, P.; Jiménez-aspee, F.; Theoduloz, C.; Burgos-edwards, A.; Domínguez-perles, R.; Oger, C.; Durand, T.; Gil-izquierdo, Á.; Bustamante, L.; et al. Phenolic, oxylipin and fatty acid profiles of the Chilean hazelnut (Gevuina avellana): Antioxidant activity and inhibition of pro-inflammatory and metabolic syndrome-associated enzymes. Food Chem. 2019, 298, 1–13. [Google Scholar] [CrossRef]
- Romero, N.; Robert, P.; Masson, L.; Ortiz, J.; Pavez, J.; Garrido, C.; Foster, M.; Dobarganes, C. Effect of α -tocopherol and α -tocotrienol on the performance of Chilean hazelnut oil (Gevuina avellana Mol.) at high temperature. J. Sci. Food Agric. 2004, 948, 943–948. [Google Scholar] [CrossRef]
- Cáceres, J. Diseño de Estrategias de Comercialización de Productos Derivados de la Avellana Para Pequeños Propietarios Forestales de la Comuna de San Fabián de Alico, Región del Bío Bío; Magíster en Dirección de Empresas; Universidad del BíoBío: Chillán, Chile, 2015; Available online: http://repobib.ubiobio.cl/jspui/handle/123456789/1691 (accessed on 20 September 2022).
- Carrillo, D.; Villarroel, M.; Huiriqueo, C.; Hazbun, J. Desarrollo de una formulación optimizada de galletas para celiacos utilizando harina desgrasada de avellana chilena (Gevuina avellana Mol.) y harina de quinoa (Chenopodium quinoa Willd). Arch. Latinoam. Nutr. 2009, 59, 184–190. Available online: https://www.researchgate.net/publication/242165744%0A (accessed on 20 September 2022).
- Homazabal, M. Incorporación de Avellana Chilena (Gevuina avellana Mol.) a Manjar Amoldado Artesanal; Ingeniería en Agronomía, Facultad de Agronomía, Universidad de Concepción: Chillán, Chile, 2005; pp. 1–2. Available online: http://repositorio.udec.cl/xmlui/handle/11594/8097 (accessed on 20 September 2022).
- Neira, M. Desarrollo de Una Mezcla de “Miel Crema” de Abeja (Apis Mellífera) con Avellana Chilena (Gevuina avellana Mol.) Para Consumo Humano; Ingeniería en Alimentos, Facultad de Ciencias Agrarias, Universidad Austral de Chile: Valdivia, Chile, 2003; pp. 1–140. Available online: http://cybertesis.uach.cl/tesis/uach/2003/fap222d/pdf/fap222d.pdf (accessed on 20 September 2022).
- Manquilán, T. Estudio del valor Funcional y Nutricional de Productos Regionales; Gobierno Regional Región de Los Ríos, Ministerio de Agricultura (Minagri): Valdivia, Chile; Universidad Austral de Chile: Valdivia, Chile, 2017; pp. 1–154. [Google Scholar]
- Moure, A.; Rua, M.; Sineuro, J.; Dominguez, H. Aqueous Extraction and Membrane Isolation of Protein from Defatted Gevuina avellana. J. Food Sci. 2002, 67, 688–696. [Google Scholar] [CrossRef]
- Moure, Â.; Franco, D.; Sineiro, J.; Domõânguez, H.; Jose, M. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res. Int. 2001, 34, 103–109. [Google Scholar] [CrossRef]
- Schmeda-hirschmann, G.; De Andrade, J.P.; Jiménez-aspee, F.; Mieres-castro, D. OPEN A cyclic dipeptide from the Chilean hazelnut cotyledons (Gevuina avellana Mol., Proteaceae). Sci. Rep. 2020, 1–5. [Google Scholar] [CrossRef]
- Uquiche, E.; Jeréz, M.; Ortíz, J. Effect of pretreatment with microwaves on mechanical extraction yield and quality of vegetable oil from Chilean hazelnuts (Gevuina avellana Mol.). Innov. Food Sci. Emerg. Technol. 2008, 9, 495–500. [Google Scholar] [CrossRef]
- Montenegro, G.; Gómez, M.; Díaz-forestier, J.; Pizarro, R. Aplicación de la Norma Chilena O fi cial de denominación de origen botánico de la miel para la caracterización de la producción apícola. Cienc. Investig. Agrar. 2008, 35, 181–190. Available online: https://www.academia.edu/7859883/Aplicación_de_la_Norma_Chilena_Oficial_de_denominación_de_origen_botánico_de_la_miel_para_la_caracterización_de_la_producción_apícola (accessed on 20 September 2022).
- Montenegro, G.; Gómez, M.; Pizarro, R.; Casaubon, G. Implementación de un panel sensorial para mieles chilenas. Cienc. E Investig. Agrar. 2008, 35, 51–58. Available online: https://www.academia.edu/7859884/Implementación_de_un_panel_sensorial_para_mieles_chilenas (accessed on 20 September 2022).
- Baughman, O.W.; Kulpa, S.M.; Sheley, R.L. Four paths toward realizing the full potential of using native plants during ecosystem restoration in the Intermountain West. Rangelands 2022, 44, 218–226. [Google Scholar] [CrossRef]
- Sharrock, S. Plant conservation report 2020: A review of progress in implementation of the Global Strategy for Plant Conserva- 780 tion 2011–2020. Secretariat of the Convention on Biological Diversity. CBD Tech. Ser. 2020, 81, 1–70. Available online: https://www.cbd.int/doc/publications/cbd-ts-95-en.pdf (accessed on 10 July 2022).
- Hu, J.; Zhou, Q.; Cao, Q.; Hu, J. Effects of ecological restoration measures on vegetation and soil properties in semi-humid sandy land on the southeast Qinghai-Tibetan Plateau, China. Glob. Ecol. Conserv. 2022, 33, 1–11. [Google Scholar] [CrossRef]
- Nunes, S.; Gastauer, M.; Cavalcante, R.B.L.; Ramos, S.J.; Caldeira, C.F.; Silva, D.; Rodrigues, R.R.; Salomão, R.; Oliveira, M.; Souza-Filho, P.W.M.; et al. Challenges and opportunities for large-scale reforestation in the Eastern Amazon using native species. For. Ecol. Manag. 2020, 466, 1–15. [Google Scholar] [CrossRef]
- Crow, T.M.; Hufford, K.M.; Burney, O.T. Plant performance predicted by genetic variation and environmental distance in important restoration shrub species Cercocarpus montanus. Restor. Ecol. 2022, 30, 1–29. [Google Scholar] [CrossRef]
- Poupon, V.; Chakraborty, D.; Stejskal, J.; Konrad, H.; Schueler, S.; Lstibůrek, M. Accelerating Adaptation of Forest Trees to Climate Change Using Individual Tree Response Functions. Front. Plant Sci. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Wang, T.; O’Neill, G.A.; Aitken, S.N. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 2010, 20, 153–163. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 1–14. [Google Scholar] [CrossRef]
- Fernando, D.R. Plant–Metal Interactions in the Context of Climate Change. Stresses 2022, 2, 79–89. [Google Scholar] [CrossRef]
- Dai, X.; Wu, W.; Ji, L.; Tian, S.; Yang, B.; Guan, B.; Wu, D. MaxEnt model-based prediction of potential distributions of Parnassia wightiana (Celastraceae) in China. Biodivers. Data J. 2022, 10, 1–16. [Google Scholar] [CrossRef]
- Fick, S.E. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 1-14. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2018, 50, 68–75. [Google Scholar] [CrossRef]
- Boral, D.; Moktan, S. Predictive distribution modeling of Swertia bimaculata in Darjeeling-Sikkim Eastern Himalaya using MaxEnt: Current and future scenarios. Ecol. Process. 2021, 10, 1–17. [Google Scholar] [CrossRef]
- Khan, A.M.; Li, Q.; Saqib, Z.; Khan, N.; Habib, T.; Khalid, N.; Majeed, M.; Tariq, A. MaxEnt Modelling and Impact of Climate Change on Habitat Suitability Variations of Economically Important Chilgoza Pine (Pinus gerardiana Wall.) in South Asia. Forests 2022, 13, 715. [Google Scholar] [CrossRef]
- Purohit, S.; Rawat, N. MaxEnt modeling to predict the current and future distribution of Clerodendrum infortunatum L. under climate change scenarios in Dehradun district, India. Model. Earth Syst. Environ. 2022, 8, 2051–2063. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Riahi, K. Shared Socioeconomic Pathways: An Overview. Glob. Environ. Chang. 2016; forthcoming. Available online: https://unfccc.int/sites/default/files/part1_iiasa_rogelj_ssp_poster.pdf (accessed on 12 July 2022).
- UNFCCC. CGE Training Materials for Vulnerability and Adaptation Assessment: Chapter 3 Baseline socioeconomic scenarios. In Project Report; UNFCCC: New York, NY, USA, 2012; pp. 1–28. Available online: https://unfccc.int/sites/default/files/resource/Chapter_3_updated_2021.pdf (accessed on 10 July 2022).
- Skidmore, A.K.; Coops, N.C.; Neinavaz, E.; Ali, A.; Schaepman, M.E.; Paganini, M.; Kissling, W.D.; Vihervaara, P.; Darvishzadeh, R.; Feilhauer, H.; et al. Priority list of biodiversity metrics to observe from space. Nat. Ecol. Evol. 2021, 5, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Galuszynski, N.C.; Potts, A.J. Application of High Resolution Melt analysis (HRM) for screening haplotype variation in a non-model plant genus: Cyclopia (honeybush). PeerJ 2020, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; de Munhoz, C.F. Microsatellite markers: What they mean and why they are so useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef]
- MacKay, J.F.; Wright, C.D.; Bonfiglioli, R.G. A new approach to varietal identification in plants by microsatellite high resolution melting analysis: Application to the verification of grapevine and olive cultivars. Plant Methods 2008, 4, 1–5. [Google Scholar] [CrossRef]
- Desai, H.; Hamid, R.; Ghorbanzadeh, Z.; Bhut, N.; Padhiyar, S.M.; Kheni, J.; Tomar, R.S. Genic microsatellite marker characterization and development in little millet (Panicum sumatrense) using transcriptome sequencing. Sci. Rep. 2021, 11, 20620. [Google Scholar] [CrossRef]
- Kalia, R.K.; Rai, M.K.; Kalia, S.; Singh, R.; Dhawan, A.K. Microsatellite markers: An overview of the recent progress in plants. Euphytica 2011, 177, 309–334. [Google Scholar] [CrossRef]
- Lateef, D.D. DNA Marker Technologies in Plants and Applications for Crop Improvements. J. Biosci. Med. 2015, 3, 7–18. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.; Wang, Y.; Zong, J.; Chen, J.; Li, D.; Li, L.; Wang, J.; Liu, J. High-throughput SSR marker development and its application in a centipedegrass (Eremochloa ophiuroides (Munro) Hack.) genetic diversity analysis. PLoS ONE 2018, 13, e0202605. [Google Scholar] [CrossRef] [PubMed]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Islam, N.K.; Latif, M.A. A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. Int. J. Mol. Sci. 2013, 14, 22499–22528. [Google Scholar] [CrossRef]
- Xanthopoulou, A.; Ganopoulos, I.; Tsaballa, A.; Nianiou-Obeidat, I.; Kalivas, A.; Tsaftaris, A.; Madesis, P. Summer Squash Identification by High-Resolution-Melting (HRM) Analysis Using Gene-Based EST-SSR Molecular Markers. Plant Mol. Biol. Report. 2014, 32, 395–405. [Google Scholar] [CrossRef]
- Varshney, R.K.; Graner, A.; Sorrells, M.E. Genic microsatellite markers in plants: Features and applications. Trends Biotechnol. 2005, 23, 48–55. [Google Scholar] [CrossRef]
- Barut, M.; Nadeem, M.A.; Akgür, Ö.; Tansi, L.S.; Aasim, M.; Altaf, M.T.; Baloch, F.S. Medicinal and aromatic plants in the omics era: Application of plant breeding and biotechnology for plant secondary metabolite production. Turk. J. Agric. For. 2022, 46, 182–203. [Google Scholar] [CrossRef]
- Liuming, L.; Yunyao, Y.; Hewen, Z.; Pingsheng, L.; Zenghui, H. Scientia Horticulturae Development of EST-SSR markers and association analysis of floral scent in tree peony. Sci. Hortic. 2021, 289, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, W.; Zhao, Y.; Zhang, J.; Wang, N.; Ntakirutimana, F.; Yan, J.; Wang, Y. EST-SSR marker development based on RNA-sequencing of E. sibiricus and its application for phylogenetic relationships analysis of seventeen Elymus species. BMC Plan Biol. 2019, 19, 235. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, S.; Zhang, J.; Liu, Y.; Yu, X.; Yang, M. Primer development and functional classification of EST-SSR markers in Ulmus species. Genet. Genome 2020, 32, 3437–3446. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Ganopoulos, I.; Moraitou-Daponta, E.; Lioliopoulou, F.; Ntantali, O.; Panagiotaki, P.; Vellios, E.K. High-Resolution Melting (HRM) Analysis Reveals Genotypic Differentiation of Venturia inaequalis Populations in Greece. Front. Ecol. Evol. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Zambounis, A.; Stefanidou, E.; Madesis, P.; Hrustić, J.; Mihajlović, M.; Tanović, B. Genotypic differentiation of Monilinia spp. populations in Serbia using a high-resolution melting (HRM) analysis. Plant Prot. Sci. 2021, 57, 38–46. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Sawongta, N.; Madesis, P. Bar-HRM for Species Confirmation of Native Plants Used in Forest Restoration in Northern Thailand. Forest 2022, 13, 997. [Google Scholar] [CrossRef]
- Thongkhao, K.; Tungphatthong, C.; Phadungcharoen, T.; Sukrong, S. The use of plant DNA barcoding coupled with HRM analysis to differentiate edible vegetables from poisonous plants for food safety. Food Control 2020, 109, 1–18. [Google Scholar] [CrossRef]
- Esfanjani, J.; Ghorbani, A.; Zare Chahouki, M.A. Maxent modeling for predicting impacts of environmental factors on the potential distribution of artemisia aucheri and bromus tomentellus-Festuca ovina in Iran. Pol. J. Environ. Stud. 2018, 27, 1041–1047. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; & Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Cobos, M.E.; Townsend Peterson, A.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 2, 1–15. [Google Scholar] [CrossRef]
- Bornet, B.; Branchard, M. Nonanchored Inter Simple Sequence Repeat (ISSR) Markers: Reproducible and Specific Tools for Genome Fingerprinting. Plant Mol. Biol. Report. 2001, 19, 209–215. [Google Scholar] [CrossRef]
- Dos Santos, L.F.; De Oliveira, E.J.; Dos Santos Silva, A.; De Carvalho, F.M.; Costa, J.L.; Pádua, J.G. ISSR markers as a tool for the assessment of genetic diversity in Passiflora. Biochem. Genet. 2011, 49, 540–554. [Google Scholar] [CrossRef]
- Ng, W.L.; Tan, S.G. Inter-Simple Sequence Repeat (ISSR) markers: Are we doing it right? ASM Sci. J. 2015, 9, 30–39. [Google Scholar]
- Díaz, L.M. Determinación de la Diversidad y Estructura Genética en Poblaciones de Avellano Gevuina Avellana Mol. Mediante el Uso de Marcadores Moleculares Tipos Microsatélites de Secuencias Expresadas (EST-SSR); Universidad Austral de Chile: Valdivia, Chile, 2010; pp. 1–113. Available online: https://kipdf.com/universidad-austral-de-chile_5ae454e37f8b9a9f7d8b4626.html (accessed on 10 July 2022).
- Ferrada, P.V. Determinación de Polimorfismo e Identificación de Haplotipos en Gevuina Avellana y Embothrium Coccineum (Magnoliópsida: Proteaceae), Especies Nativas de Chile, Utilizando Marcadores Moleculares en cpDNA. Bioquímico; Universidad Austral de Chile: Valdivia, Chile, 2011; pp. 1–124. Available online: http://cybertesis.uach.cl/tesis/uach/2011/fcf368d/doc/fcf368d.pdf (accessed on 10 July 2022).
- Fuentes, G.; Cisternas, A.; Valencia, G.; Mihoc, M.; Ruiz, E.; Hasbún, R.; Baeza, C.M. Diversidad taxonómica y genética del sitio prioritario Península de Hualpén, Región del Bío-Bío, Chile. Implicancias para la Conservación. Gayana. Bot. 2017, 74, 94–110. [Google Scholar] [CrossRef]
- Thibaut, J.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically struc- 739 tured populations. BMC Genet. 2009, 11, 1–15. [Google Scholar] [CrossRef]
- Beckwith, B.R.; Johansson, E.M.; Huff, V.J. Connecting people, plants and place: A native plant society’s journey towards a community of practice. People Nat. 2021, 1–12. [Google Scholar] [CrossRef]
- Hanson, C.; Buckingham, K.; Dewitt, S.; Laestadius, L. The Restoration Diagnostic; World Resources Institute: Washington, DC, USA, 2015; pp. 1–96. Available online: https://www.wri.org/research/restoration-diagnostic (accessed on 10 July 2022).
- Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 2002, 418, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Monaco, T.A. A role for assisted evolution in designing native plant materials for domesticated landscapes. Front. Ecol. Environ. 2009, 7, 541–547. [Google Scholar] [CrossRef]
- Cruzat, R. Producción de Avellana Chilena. Serie Experiencias de Innovación para el Emprendimiento Agrario; Fundación para la Innovación Agraria (FIA), Ministerio de Agricultura (Minagri): Santiago, Chile, 2009; pp. 1–30. Available online: http://bibliotecadigital.fia.cl/handle/20.500.11944/1936 (accessed on 10 July 2022).
- Medel, F. Plantas de calidad para la producción comercial de huertos de Gevuin (Avellano chileno). In Proceedings of the Seminario Internacional “Actualidad en la Producción y Comercialización de Frutos de Nuez”, EXPONUT, Santiago, Chile, 8 May 2014; Volume 8, pp. 1–2. Available online: https://www.researchgate.net/publication/272179948%0APlantas (accessed on 10 July 2022).
- Loewe, V.; Delard, C. Plantaciones de avellana CHILENO (Gevuina avellana Mol.) una Alternativa Para Producir Madera de alto Valor en Chile; Instituto Forestal (INFOR), Ministerio de Agricultura (Minagri): Santiago, Chile, 2007; pp. 49–68. Available online: https://www.infor.cl/?option=com_infor&task=GetRevista&format=raw&id=54 (accessed on 10 July 2022).
- Aguilera, M. Avances en Métodos y Técnicas de Manejo y Recolección Sustentable de Frutos de Avellano (Gevuina avellana) en Formaciones Boscosas Naturales de Chile; Instituto Forestal (INFOR), Ministerio de Agricultura (Minagri): San Pedro de la Paz, Chile, 2020; pp. 1–69. Available online: https://opia.fia.cl/601/w3-article-94348.html (accessed on 10 July 2022).
- Tacón, A. Avellano (Gevuina avellana Mol.). Cuaderno Para Recolectoras y Recolectores. Buenas Prácticas de Recolección Sustentable Para Productos Forestales No Madereros; Fundación para la Innovación Agraraia (FIA), Ministerio de Agricultura (Minagri): Santiago, Chile, 2017; pp. 1–40. Available online: http://bibliotecadigital.fia.cl/handle/20.500.11944/146015 (accessed on 10 July 2022).
- Temesgen, B. Global role of plant breeding in tackling climate change. Int. J. Agric. Sci. Food Technol. 2021, 7, 223–229. [Google Scholar] [CrossRef]
- Seeda, M.; El-nour, E.; Yassen, A. Climatic changes and their effects on physiological and nutritional status of crop plants: Importance and Strategies. A review. Middle East J. Agric. Res. 2021, 10, 6–72. [Google Scholar] [CrossRef]
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective—A review article. Agric. Food Secur. 2021, 10, 1–25. [Google Scholar] [CrossRef]
- Das, T.; Majumdar, M.H.D.; Devi, R.T.; Rajesh, T. Climate change impacts on plant diseases. SAARC J. Agric. 2017, 14, 200–209. [Google Scholar] [CrossRef]
- Acevedo, M.; Álvarez-Maldini, C.; Dumroese, R.K.; Bannister, J.R.; Cartes, E.; González, M. Native plant production in Chile. Is it possible to achieve restoration goals by 2035? Land 2021, 10, 71. [Google Scholar] [CrossRef]
- ODEPA. Oficina de Estudios y Políticas Agrarias (ODEPA); Ministerio de Agricultura (Minagri): Santiago, Chile, 2022; Available online: https://www.odepa.gob.cl/estadisticas-del-sector/catastros-fruticolas (accessed on 10 July 2022).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.4). Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 10 June 2022).
- Sahu, S.K.; Thangaraj, M.; Kathiresan, K. DNA Extraction Protocol for Plants with High Levels of Secondary Metabolites and Polysaccharides without Using Liquid Nitrogen and Phenol. ISRN Mol. Biol. 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Słomka, M.; Sobalska-kwapis, M.; Wachulec, M.; Bartosz, G.; Strapagiel, D. High Resolution Melting (HRM) for High-Throughput Genotyping—Limitations and Caveats in Practical Case Studies. Int. J. Mol. Sci. 2017, 18, 2316. [Google Scholar] [CrossRef] [PubMed]













| Populations Code | Mountain | Coordinates | Elevation (masl) | |
|---|---|---|---|---|
| W | S | |||
| LV | Andes | −35.3828573 | −71.1113449 | 900–980 |
| LL | Andes | −35.4730431 | −71.1769904 | 850–890 |
| LA | Andes | −35.5629722 | −71.1587165 | 1200–1290 |
| PA | Andes | −35.604411 | −71.074437 | 1301–1420 |
| AR | Andes | −35.7034586 | −71.1104523 | 520–613 |
| RO | Andes | −35.8602489 | −71.271781 | 599–614 |
| CA | Costa | −35.9813956 | −72.649387 | 240–320 |
| Code | Environmental Variables | Units | Percent Contribution |
|---|---|---|---|
| Bio1 | Annual Mean Temperature | °C | 43.7 |
| Water Steam | Water vapor pressure | kPa | 23.4 |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | Percent | 7 |
| Bio17 | Precipitation of Driest Quarter | mm | 5.3 |
| Bio13 | Precipitation of Wettest Month | mm | 5.2 |
| Bio3 | Isothermality (BIO2/BIO7) (×100) | Percent | 4.6 |
| Wind | Wind speed | m s−1 | 4.3 |
| Bio16 | Precipitation of Wettest Quarter | mm | 2.7 |
| Radiation | Solar radiation | Kj m−2 day−1 | 1.4 |
| Bio6 | Min Temperature of Coldest Month | °C | 1.3 |
| Bio4 | Temperature Seasonality (standard deviation ×100) | °C or Percent | 1 |
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp − min temp)) | °C | 0.1 |
| Population | N | Pa | Ne | GST | %P | HRM Profile |
|---|---|---|---|---|---|---|
| La Veguilla | 29 | 49 | 1.45 | 0.0368 | 45.79 | 4 |
| Las Lomas | 30 | 34 | 1.31 | 0.0344 | 31.78 | 4 |
| Los Avellanos | 66 | 53 | 1.49 | 0.0361 | 49.53 | 5 |
| Punta de Águila | 60 | 59 | 1.55 | 0.0361 | 55.14 | 5 |
| Armerillo | 36 | 41 | 1.38 | 0.0358 | 38.32 | 4 |
| Roblería | 29 | 46 | 1.42 | 0.0361 | 42.99 | 3 |
| Canelillo | 30 | 20 | 1.18 | 0.0339 | 18.69 | 3 |
| Total | 280 | 9.78 | 0.0431 | 28 | ||
| Average | 43 | 1.40 | 0.0356 | 40.32 | 4 | |
| Source of Variation | df | SSD | CV | % Total | Fixation Index |
|---|---|---|---|---|---|
| Among populations | 6 | 65.716 | 10.953 | 5 | Fst = 0.052 ** |
| Within population | 273 | 945.030 | 3.462 | 95 | |
| Total | 279 | 1010.746 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya-Moraga, M.R.; Pérez-Ruíz, C. Application of MaxEnt Modeling and HRM Analysis to Support the Conservation and Domestication of Gevuina avellana Mol. in Central Chile. Plants 2022, 11, 2803. https://doi.org/10.3390/plants11202803
Moya-Moraga MR, Pérez-Ruíz C. Application of MaxEnt Modeling and HRM Analysis to Support the Conservation and Domestication of Gevuina avellana Mol. in Central Chile. Plants. 2022; 11(20):2803. https://doi.org/10.3390/plants11202803
Chicago/Turabian StyleMoya-Moraga, Mario René, and César Pérez-Ruíz. 2022. "Application of MaxEnt Modeling and HRM Analysis to Support the Conservation and Domestication of Gevuina avellana Mol. in Central Chile" Plants 11, no. 20: 2803. https://doi.org/10.3390/plants11202803
APA StyleMoya-Moraga, M. R., & Pérez-Ruíz, C. (2022). Application of MaxEnt Modeling and HRM Analysis to Support the Conservation and Domestication of Gevuina avellana Mol. in Central Chile. Plants, 11(20), 2803. https://doi.org/10.3390/plants11202803





