Physiological Response, Oxidative Stress Assessment and Aquaporin Genes Expression of Cherry Tomato (Solanum lycopersicum L.) Exposed to Hyper-Harmonized Fullerene Water Complex
Abstract
:1. Introduction
2. Results
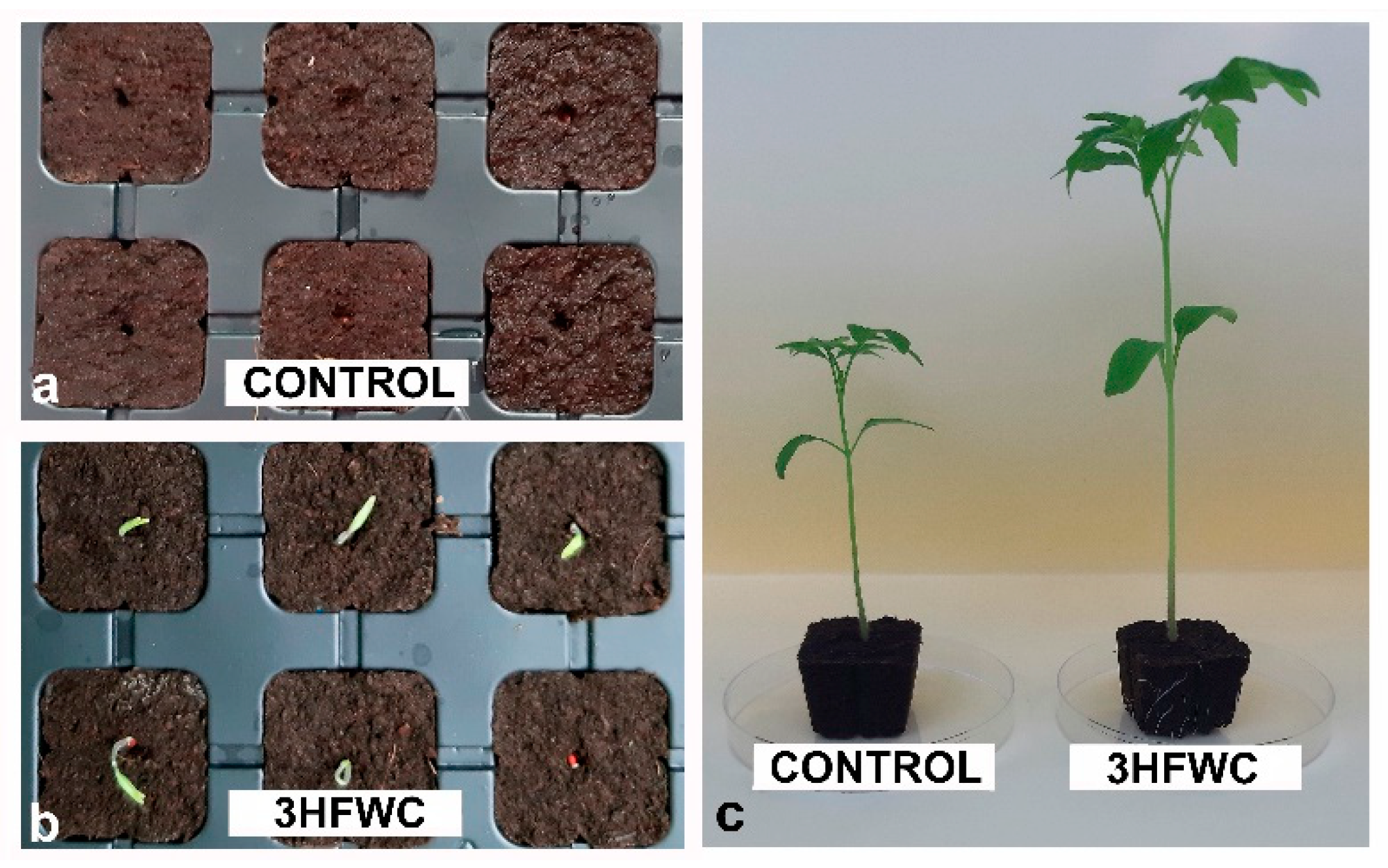
2.1. The Effect of 3HFWC Nanosubstance on Cherry Tomato Seed Germination, Seedlings Growth and Development
2.2. The Effects of Short and Long-Term Exposure to 3HFWC Nanosubstance on Cherry Tomato Physiological and Molecular Response
2.2.1. Photosynthetic Pigments Content
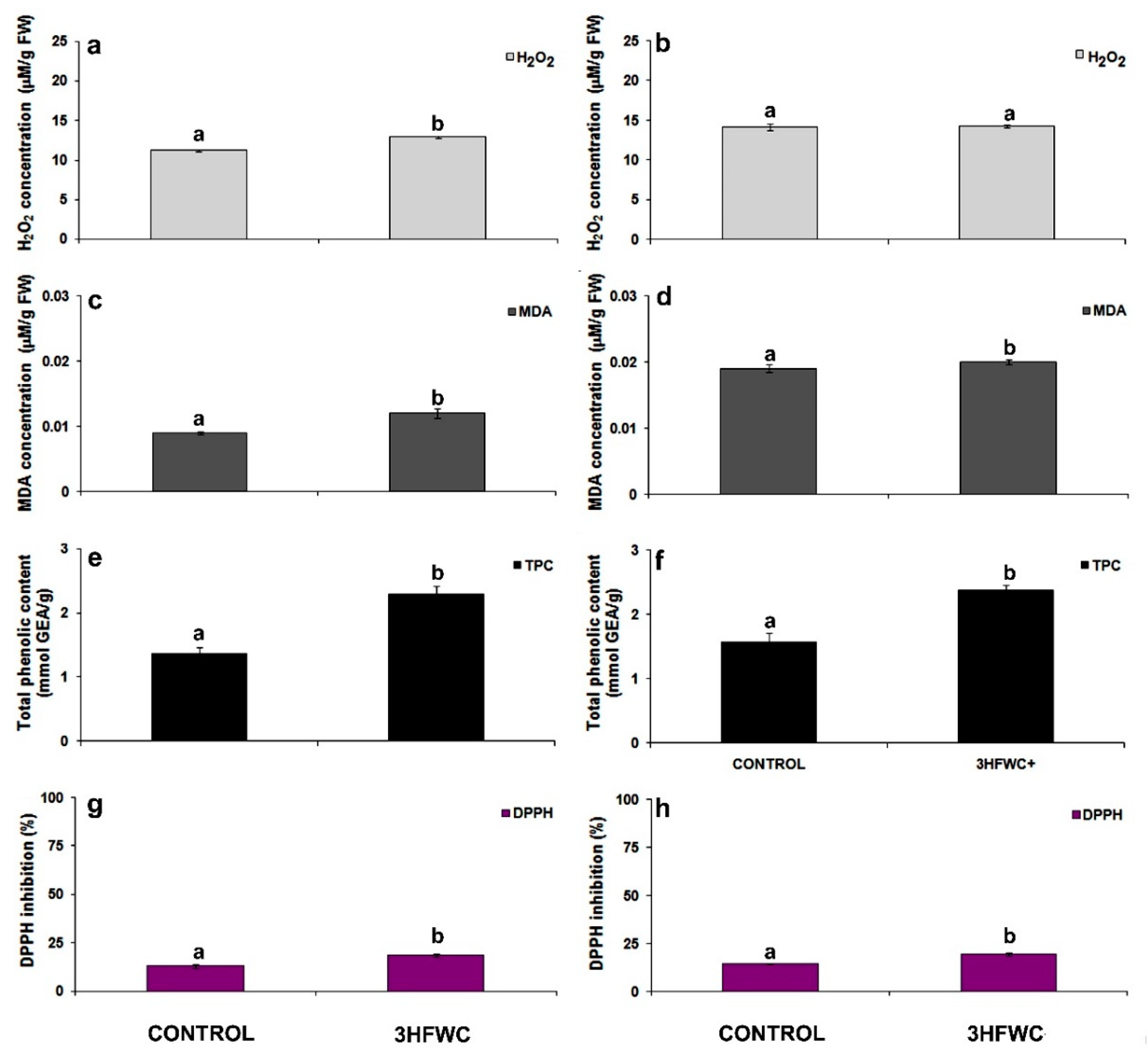
2.2.2. Oxidative Stress Assessment
2.2.3. Antioxidative Enzyme Activity
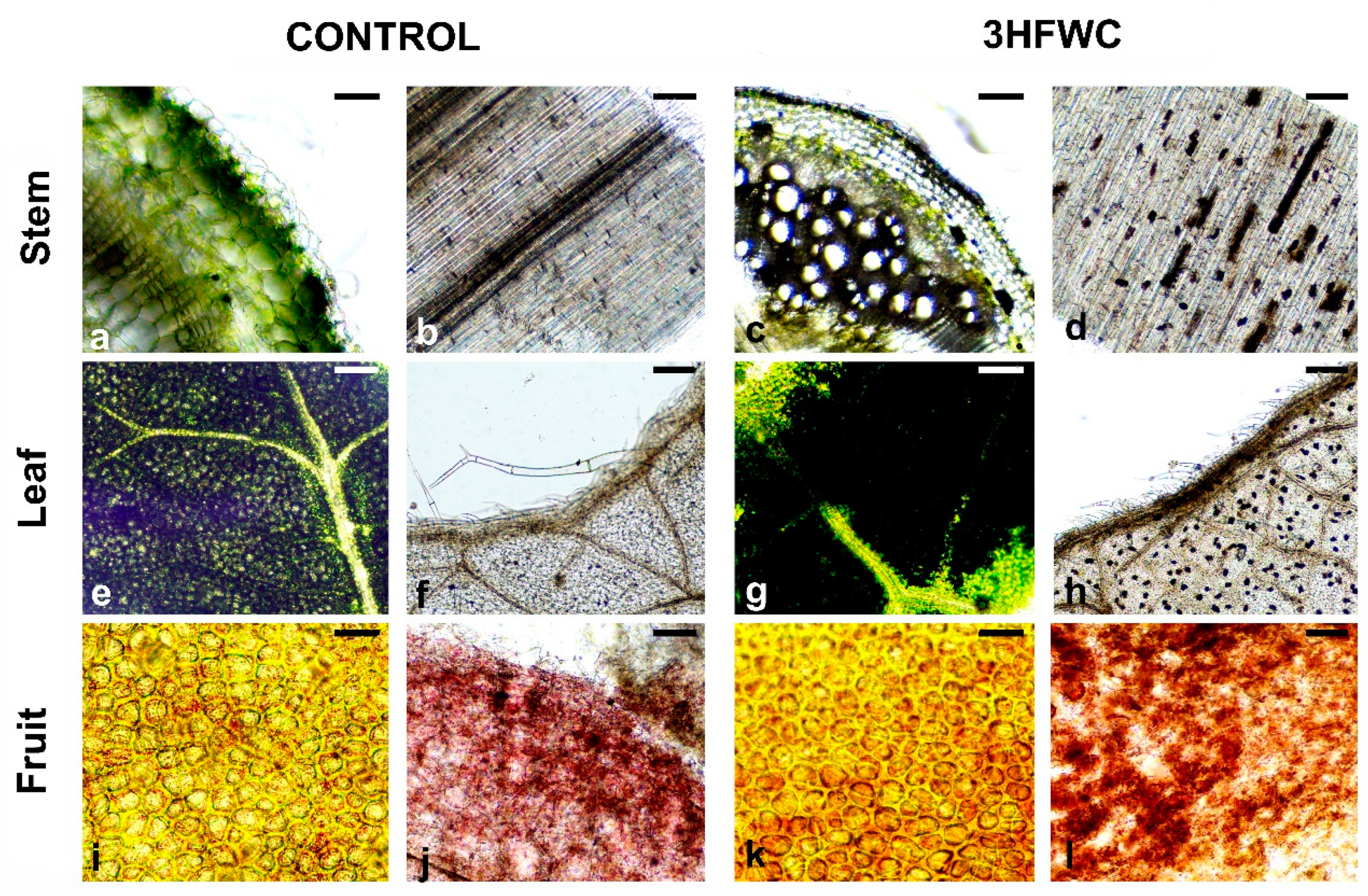
2.3. Histological Analysis of Cherry Tomato Plants after Long-Term Exposure to 3HFWC Nanosubstance
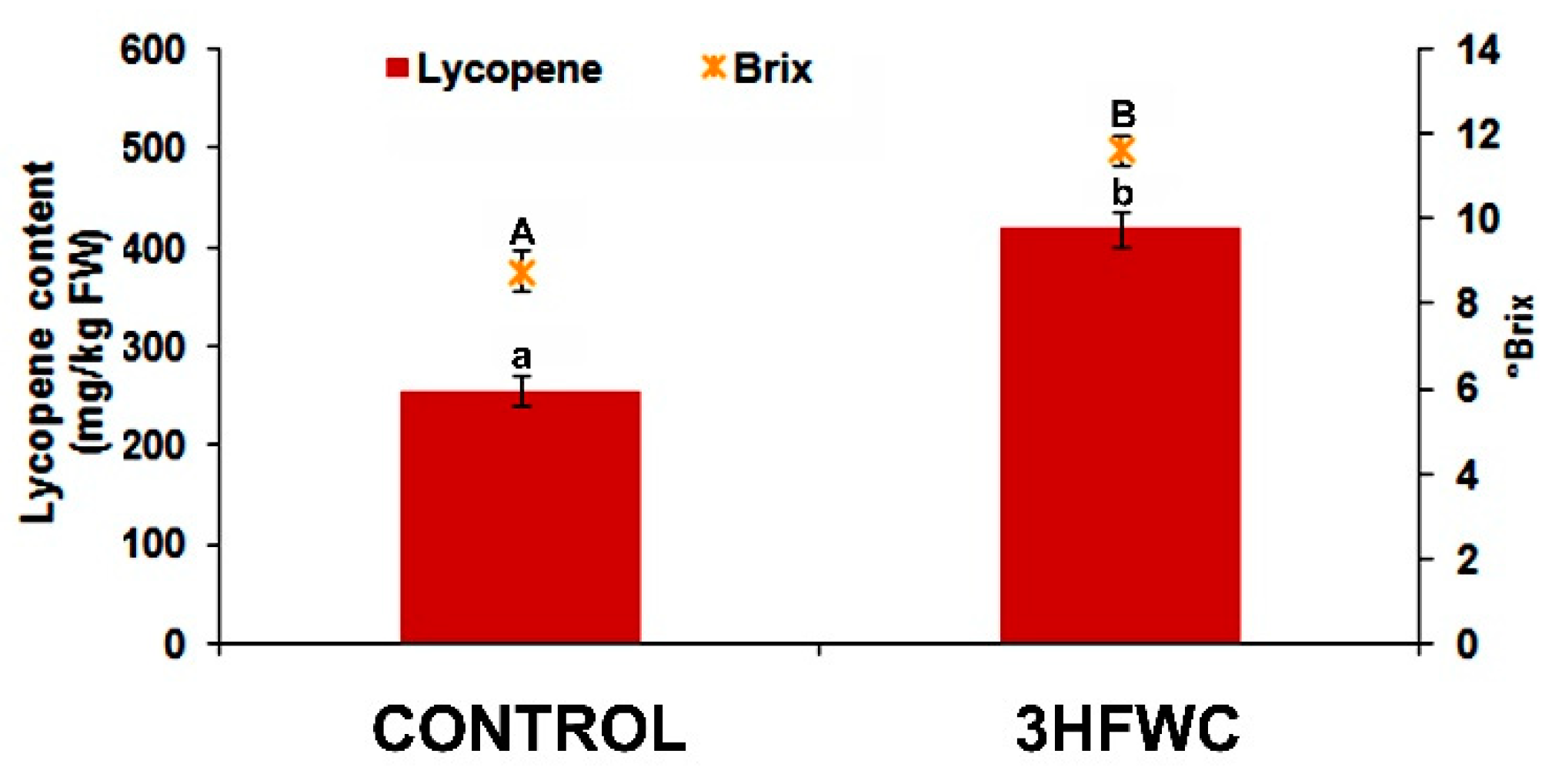
2.4. Fruit Quality of 3HFWC Nanosubstance Exposed Cherry Tomato Plants
2.5. The Effect of 3HFWC Nanosubstance Onaqaporine Gene Expresion
3. Discussion
3.1. The Effect of 3HFWC Nanosubstance on Cherry Tomato Seed Germination and Seedling Growth
3.2. The Effect of 3HFWC Nanosubstance on Photosynthetic Pigment Content
3.3. The Effect 3HFWC Nanosubstance on ROS Production and Antioxidant Properties
3.4. The Effect of 3HFWC Nanosubstance on Aquaporin Genes Expression
3.5. Effect of 3HFWC Nanosubstance on Fruit Size and Lycopene Production
4. Materials and Methods
4.1. Plant Material, 3HFWC Nanosubstance Treatment and Growth Conditions
4.2. Measurement of Morphological Parameters of Seedlings and Fruits
4.3. Histological Analysis
4.4. Photosynthetic Pigments Content
4.5. Oxidative Stress Assessment
4.5.1. Histochemical Localization of O2− and H2O2 Production
4.5.2. MDA and H2O2 Content Analysis
4.5.3. Antioxidant Enzyme Activity
4.5.4. Total Polyphenol Content
4.5.5. DPPH Radical Scavenging Capacity Assay
4.6. Aquaporin Genes Expression Analysis
4.6.1. RNA Isolation and Reverse Transcription PCR (RT-PCR)
4.6.2. Quantitative Real-Time PCR (qRT-PCR)
4.7. Determination of Lycopene Content and BRIX
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Rejeski, D.; Hull, M.S. Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Bijali, J.; Acharya, K. Current Trends in Nano-technological Interventions on Plant Growth and Development: A Review. IET Nanobiotechnol. 2020, 14, 113–119. [Google Scholar] [CrossRef]
- Li, C.; Yan, B. Opportunities and Challenges of Phyto-Nanotechnology. Environ. Sci. Nano 2020, 7, 2863–2874. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon Nanomaterials: Production, Impact on Plant Development, Agricultural and Environmental Applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Matija, L.; Tsenkova, R.; Munćan, J.; Miyazaki, M.; Banba, K.; Tomić, M.; Jeftić, B. Fullerene Based Nanomaterials for Biomedical Applications: Engineering, Functionalization and Characterization. AMR 2013, 633, 224–238. [Google Scholar] [CrossRef]
- Kojić, D.; Purać, J.; Čelić, T.V.; Jović, D.; Vukašinović, E.L.; Pihler, I.; Borišev, I.; Djordjevic, A. Effect of Fullerenol Nanoparticles on Oxidative Stress Induced by Paraquat in Honey Bees. Environ. Sci. Pollut. Res. 2020, 27, 6603–6612. [Google Scholar] [CrossRef]
- Koruga, D.J.; Matija, L.; Mišic, N.; Rakin, P. Fullerene C60: Properties and Possible Applications. MSF 1996, 214, 49–56. [Google Scholar] [CrossRef]
- Rašović, I. Water-Soluble Fullerenes for Medical Applications. Mater. Sci. Technol. 2017, 33, 777–794. [Google Scholar] [CrossRef] [Green Version]
- Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Protective Effects of Fullerenol C60(OH)24 against Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity in Rats with Colorectal Cancer. Biomaterials 2009, 30, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, S.; Jeftic, B.; Sarac, D.; Matovic, V.; Slavkovic, M.; Koruga, D. Influence of Hyper-harmonized Fullerene Water Complex on Collagen Quality and Skin Function. J. Cosmet. Dermatol. 2020, 19, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Sachkova, A.S.; Kovel, E.S.; Churilov, G.N.; Guseynov, O.A.; Bondar, A.A.; Dubinina, I.A.; Kudryasheva, N.S. On Mechanism of Antioxidant Effect of Fullerenols. Biochem. Biophys. Rep. 2017, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Cowling, W.; Pareek, A.; Gupta, K.J.; Singla-Pareek, S.L.; Foyer, C.H. Gaining Acceptance of Novel Plant Breeding Technologies. Trends Plant Sci. 2021, 26, 575–587. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the Service of Biotechnology. Plant Cell Tiss. Organ Cult. 2015, 120, 881–902. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.M.; Deng, X.J.; He, Z.P.; Wang, J. Extraction of Functional Capsanthin. AMR 2012, 441, 517–521. [Google Scholar] [CrossRef]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the Health-Promoting Effects of Tomato Fruit for Biofortified Food. Mediat. Inflamm. 2014, 2014, 139873. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene—A Bioactive Carotenoid Offering Multiple Health Benefits: A Review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Raffo, A.; Leonardi, C.; Fogliano, V.; Ambrosino, P.; Salucci, M.; Gennaro, L.; Bugianesi, R.; Giuffrida, F.; Quaglia, G. Nutritional Value of Cherry Tomatoes (Lycopersicon esculentum Cv. Naomi F1) Harvested at Different Ripening Stages. J. Agric. Food Chem. 2002, 50, 6550–6556. [Google Scholar] [CrossRef] [PubMed]
- Lenucci, M.S.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant Composition in Cherry and High-Pigment Tomato Cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Cañas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Ambikapathi, R.; Lee, E.H.; Olszyk, D. Effects of Functionalized and Nonfunctionalized Single-Walled Carbon Nanotubes on Root Elongation of Select Crop Species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex Genetic, Photothermal, and Photoacoustic Analysis of Nanoparticle-Plant Interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alimohammadi, M.; Xu, Y.; Wang, D.; Biris, A.S.; Khodakovskaya, M.V. Physiological Responses Induced in Tomato Plants by a Two-Component Nanostructural System Composed of Carbon Nanotubes Conjugated with Quantum Dots and Its In Vivo Multimodal Detection. Nanotechnology 2011, 22, 295101. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Teixeira da Silva, J.A. The Effect of Carbon Nanotubes on the Seed Germination and Seedling Growth of Four Vegetable Species. J. Crop Sci. Biotechnol. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Awasthi, K.; Tripathi, D.K.; Srivastava, R. Functionalization of multi-walled carbon nanotubes and its impact on growth of tomato plant (Solanum lycopersicum L.). JEBAS 2020, 8, 469–478. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; de Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Surface Chemistry of Carbon Nanotubes Impacts the Growth and Expression of Water Channel Protein in Tomato Plants. Small 2012, 8, 2328–2334. [Google Scholar] [CrossRef]
- Ratnikova, T.A.; Podila, R.; Rao, A.M.; Taylor, A.G. Tomato Seed Coat Permeability to Selected Carbon Nanomaterials and Enhancement of Germination and Seedling Growth. Sci. World J. 2015, 2015, 419215. [Google Scholar] [CrossRef] [Green Version]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of Carbon Nanohorns with Plants: Uptake and Biological Effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef]
- Zhang, Z.; Skjeseth, G.; Elameen, A.; Haugslien, S.; Sivertsen, A.; Clarke, J.L.; Wang, Q.-C.; Blystad, D.-R. Field Performance Evaluation and Genetic Integrity Assessment in Argyranthemum ‘Yellow Empire’ Plants Recovered from Cryopreserved Shoot Tips. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 505–513. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, J.; Wang, R.; Zhang, H.; Xing, B.; Naeem, M.; Yao, T.; Li, R.; Xu, R.; Zhang, Z.; et al. Effects of Graphene Oxide on Tomato Growth in Different Stages. Plant Physiol. Biochem. 2021, 162, 447–455. [Google Scholar] [CrossRef] [PubMed]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Cabrera, R.I.; Juárez-Maldonado, A. Carbon Nanotubes Decrease the Negative Impact of AlternariaSolani in Tomato Crop. Nanomaterials 2021, 11, 1080. [Google Scholar] [CrossRef] [PubMed]
- McGehee, D.L.; Lahiani, M.H.; Irin, F.; Green, M.J.; Khodakovskaya, M.V. Multiwalled Carbon Nanotubes Dramatically Affect the Fruit Metabolome of Exposed Tomato Plants. ACS Appl. Mater. Interfaces 2017, 9, 32430–32435. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.; Ikhtiari, R.; Fugetsu, B.; Matsuoka, M.; Akasaka, T.; Watari, F. Phytotoxicity of Multi-Walled Carbon Nanotubes Assessed by Selected Plant Species in the Seedling Stage. Appl. Surf. Sci. 2012, 262, 120–124. [Google Scholar] [CrossRef]
- Feng, P.; Geng, B.; Cheng, Z.; Liao, X.; Pan, D.; Huang, J. Graphene Quantum Dots-Induced Physiological and Biochemical Responses in Mung Bean and Tomato Seedlings. Braz. J. Bot. 2019, 42, 29–41. [Google Scholar] [CrossRef]
- Jordan, J.T.; Oates, R.P.; Subbiah, S.; Payton, P.R.; Singh, K.P.; Shah, S.A.; Green, M.J.; Klein, D.M.; Cañas-Carrell, J.E. Carbon Nanotubes Affect Early Growth, Flowering Time and Phytohormones in Tomato. Chemosphere 2020, 256, 127042. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, Q.; Ma, X.; Hamdi, H.; White, J.C. Multiwalled Carbon Nanotubes and C60 Fullerenes Differentially Impact the Accumulation of Weathered Pesticides in Four Agricultural Plants. Environ. Sci. Technol. 2013, 47, 12539–12547. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, C.; Ma, X.; White, J.C. Fullerene-Enhanced Accumulation of p,p′-DDE in Agricultural Crop Species. Environ. Sci. Technol. 2012, 46, 9315–9323. [Google Scholar] [CrossRef]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.C.; Rao, A.M.; Marcus, R.K. Nanobiotechnology Can Boost Crop Production and Quality: First Evidence from Increased Plant Biomass, Fruit Yield and Phytomedicine Content in Bitter Melon (Momordica charantia). BMC Biotechnol. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borišev, M.; Borišev, I.; Župunski, M.; Arsenov, D.; Pajević, S.; Ćurčić, Ž.; Vasin, J.; Djordjevic, A. Drought Impact Is Alleviated in Sugar Beets (Beta vulgaris L.) by Foliar Application of Fullerenol Nanoparticles. PLoS ONE 2016, 11, e0166248. [Google Scholar] [CrossRef] [PubMed]
- Panova, G.G.; Kanash, E.V.; Semenov, K.N.; Charykov, N.A.; Khomyakov, Y.V.; Anikina, L.M.; Artem’eva, A.M.; Kornyukhin, D.L.; Vertebnyi, V.E.; Sinyavina, N.G.; et al. Fullerene derivatives influence production process, growth and resistance to oxidative stress in barley and wheat plants. Sel’skokhozyaĭstvennaya Biol. 2018, 53, 38–49. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Folta, K.M.; Krishna, V.; Bai, W.; Indeglia, P.; Georgieva, A.; Nakamura, H.; Koopman, B.; Moudgil, B. Polyhydroxy Fullerenes (Fullerols or Fullerenols): Beneficial Effects on Growth and Lifespan in Diverse Biological Models. PLoS ONE 2011, 6, e19976. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Fullerenol Regulates Oxidative Stress and Tissue Ionic Homeostasis in Spring Wheat to Improve Net-Primary Productivity under Salt-Stress. Ecotoxicol. Environ. Saf. 2021, 211, 111901. [Google Scholar] [CrossRef]
- Xiong, J.-L.; Li, J.; Wang, H.-C.; Zhang, C.-L.; Naeem, M.S. Fullerol Improves Seed Germination, Biomass Accumulation, Photosynthesis and Antioxidant System in Brassica napus L. under Water Stress. Plant Physiol. Biochem. 2018, 129, 130–140. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Lahiani, M.H. Nanoparticles and Plants: From Toxicity to Activation of Growth. In Book Handbook of Nanotoxicology, Nanomedicine and Stem Cell Use in Toxicology; Saura, C.S., Daniel, A.C., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 121–130. [Google Scholar]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Markelić, M.; Drača, D.; Krajnović, T.; Jović, Z.; Vuksanović, M.; Koruga, D.; Mijatović, S.; Maksimović-Ivanić, D. Combined Action of Hyper-Harmonized Hydroxylated Fullerene Water Complex and Hyperpolarized Light Leads to Melanoma Cell Reprogramming In Vitro. Nanomaterials 2022, 12, 1331. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of Carbon Nanomaterials in the Plant System: A Perspective View on the Pros and Cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Raza, S.H.; Akram, N.A.; Ashraf, M. Fullerenol [60] Nano-Cages for Protection of Crops Against Oxidative Stress: A Critical Review. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.S.; Ke, P.C. Differential Uptake of Carbon Nanoparticles by Plant and Mammalian Cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiong, F.; Fan, Y.; Li, J.; Wang, H.; Xing, G.; Yan, F.; Tai, F.; He, R. Facile and Scalable Fabrication Engineering of Fullerenol Nanoparticles by Improved Alkaline-Oxidation Approach and Its Antioxidant Potential in Maize. J. Nanopart. Res. 2016, 18, 338. [Google Scholar] [CrossRef]
- Panova, G.G.; Ktitorova, I.N.; Skobeleva, O.V.; Sinjavina, N.G.; Charykov, N.A.; Semenov, K.N. Impact of Polyhydroxy Fullerene (Fullerol or Fullerenol) on Growth and Biophysical Characteristics of Barley Seedlings in Favourable and Stressful Conditions. Plant Growth Regul. 2016, 79, 309–317. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Seed Pre-treatment with Polyhydroxy Fullerene Nanoparticles confer Salt Tolerance in Wheat Through Upregulation and Phosphorus Uptake. J. Soil. Plant Nutr. 2019, 19, 734–742. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N.; Panova, G.G. Fullerenol Can Ameliorate Iron Deficiency in Cucumber Grown Hydroponically. J. Plant Growth Regul. 2021, 40, 1017–1031. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Ruan, L.; Chen, L.; Li, H.; Chang, X.-L.; Zhang, X.; Yang, S.-T. Bioaccumulation of 13C-Fullerenol Nanomaterials in Wheat. Environ. Sci. Nano 2016, 3, 799–805. [Google Scholar] [CrossRef]
- Shukla, P.K.; Mirsa, P.; Kole, C. Uptake, Tralskocation, Accumulation, Transformation, and Generational Transmission of Nanoparticles in Plants. Plant Nanotechnol. 2016, 183–218. [Google Scholar] [CrossRef]
- Bhati, A.; Gunture, G.; Tripathi, K.M.; Singh, A.; Sarkar, S.; Sonkar, S.K. Exploration of Nano Carbons in Relevance to Plant Systems. New J. Chem. 2018, 42, 16411–16427. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K. Carbon and Fullerene Nanomaterials in Plant System. J. Nanobiotechnol. 2014, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2018, 66, 6654–6662. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS Scavenging and Antioxidant Signalling by Redox Metallic and Fullerene Nanomaterials: Potential Implications in ROS Associated Degenerative Disorders. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.Y.; Lu, F.-J.; Lin, J.-T. Free Radical Scavenging Activity of Water-Soluble Fullerenols. J. Chem. Soc. Chem. Commun. 1995, 12, 1283. [Google Scholar] [CrossRef]
- Yin, J.-J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The Scavenging of Reactive Oxygen Species and the Potential for Cell Protection by Functionalized Fullerene Materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, X.; Zhao, Y. Mechanisms of Antioxidant Activities of Fullerenols from First-Principles Calculation. J. Phys. Chem. A 2018, 122, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, Biochemistry of Nanoparticles, and Their Role in Antioxidant Defense System in Plants. In Nanotechnology and Plant Sciences; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–17. ISBN 978-3-319-14501-3. [Google Scholar]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of Synthesis and Antioxidant Potential of Fullerenol Nanoparticles. J. Nanomater. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Sook Kim-Han, J.; Erlanger, B.F.; Huang, T.; Epstein, C.J.; Dugan, L.L. A Biologically Effective Fullerene (C60) Derivative with Superoxide Dismutase Mimetic Properties. Free. Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Aoshima, H.; Saitoh, Y.; Miwa, N. Highly Hydroxylated or γ-Cyclodextrin-Bicapped Water-Soluble Derivative of Fullerene: The Antioxidant Ability Assessed by Electron Spin Resonance Method and β-Carotene Bleaching Assay. Bioorgan. Med. Chem. Lett. 2009, 19, 5293–5296. [Google Scholar] [CrossRef]
- Grebowski, J.; Krokosz, A.; Konarska, A.; Wolszczak, M.; Puchala, M. Rate Constants of Highly Hydroxylated Fullerene C60 Interacting with Hydroxyl Radicals and Hydrated Electrons. Pulse Radiolysis Study. Radiat. Phys. Chem. 2014, 103, 146–152. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.; Sun, Y.; Mukhtar, M. The Roles of Aquaporins in Plant Stress Responses. JDB 2016, 4, 9. [Google Scholar] [CrossRef]
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in Plants. Physiol. Rev. 2015, 95, 38. [Google Scholar] [CrossRef] [PubMed]
- Yaneff, A.; Vitali, V.; Amodeo, G. PIP1 Aquaporins: Intrinsic Water Channels or PIP2 Aquaporin Modulators? FEBS Lett. 2015, 589, 3508–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzpatrick, K.L.; Reid, R.J. The Involvement of Aquaglyceroporins in Transport of Boron in Barley Roots. Plant Cell Environ. 2009, 32, 1357–1365. [Google Scholar] [CrossRef]
- Vajpai, M.; Mukherjee, M.; Sankararamakrishnan, R. Cooperativity in Plant Plasma Membrane Intrinsic Proteins (PIPs): Mechanism of Increased Water Transport in Maize PIP1 Channels in Hetero-Tetramers. Sci. Rep. 2018, 8, 12055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, M.D.; Diehn, T.A.; Richet, N.; Chaumont, F.; Bienert, G.P. Heterotetramerization of Plant PIP1 and PIP2 Aquaporins Is an Evolutionary Ancient Feature to Guide PIP1 Plasma Membrane Localization and Function. Front. Plant Sci. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Becerra, H.; Vazquez-Hernandez, M.C.; Saenz de la O, D.; Alvarado-Mariana, M.; Guevara-Gonzalez, R.G.; Garcia-Trejo, J.F.; Feregrino-Perez, A.A. Role of Stress and Defense in Plant Secondary Metabolites Production. In Bioactive Natural Products for Pharmaceutical Applications; Pal, D., Nayak, A.K., Eds.; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2021; Volume 140, ISBN 978-3-030-54026-5. [Google Scholar] [CrossRef]
- Ali, S.A.; Al-Tohamy, R.; Koutra, E.; Moawad, M.M.; Kornaros, M.; Mustafa, A.M.; Mahmoud, Y.A.G.; Badr, A.; Osman, M.E.H.; Elsamahy, T.; et al. Nanobiotechnological Advancements in Agriculture and Food Industry: Applications, Nanotoxicity, and Future Perspectives. Sci. Total Environ. 2021, 792, 148359. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Khare, S.; Cerniglia, C.E.; Boy, R.; Ivanov, I.N.; Khodakovskaya, M. The Impact of Tomato Fruits Containing Multi-Walled Carbon Nanotube Residues on Human Intestinal Epithelial Cell Barrier Function and Intestinal Microbiome Composition. Nanoscale 2019, 11, 3639–3655. [Google Scholar] [CrossRef]
- Sarkar, P.; Ghosal, K.; Chakraborty, D.; Sarkar, K. Biocompatibility and Biomedical Applications of Various Carbon-Based Materials. In Handbook of Carbon-Based Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 829–875. ISBN 978-0-12-821996-6. [Google Scholar] [CrossRef]
- Miljkovic, S.; Jeftic, B.; Stankovic, I.; Stojiljkovic, N.; Koruga, D. Mechanisms of Skin Moisturization with Hyperharmonized Hydroxyl Modified Fullerene Substance. J. Cosmet. Dermatol. 2021, 20, 3018–3025. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 978-0-12-182048-0. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 291–297. ISBN 978-1-60761-701-3. [Google Scholar]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of Photo-Oxidative Stress Responses in Leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 978-0-12-182005-3. [Google Scholar] [CrossRef]
- Kukavica, B.; Jovanovic, S.V. Senescence-Related Changes in the Antioxidant Status of Ginkgo and Birch Leaves during Autumn Yellowing. Physiol. Plant 2004, 122, 321–327. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kim, J.K.; Noh, J.H.; Lee, S.E.; Choi, J.S.; Suh, H.S.; Chung, H.Y.; Song, Y.O.; Choi, W.C. The First Total Synthesis of 2,3,6-Tribromo-4,5-Dihydroxybenzyl Methyl Ether (TDB) and Its Antioxidant Activity. Bull. Korean Chem. Soc. 2002, 23, 661–662. [Google Scholar] [CrossRef]
- Gašić, K.; Hernandez, A.; Korban, S.S. RNA Extraction from Different Apple Tissues Rich in Polyphenols and Polysaccharides for CDNA Library Construction. Plant Mol. Biol. Rep. 2004, 22, 437–438. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon Enhances Water Stress Tolerance by Improving Root Hydraulic Conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Q.-T.; Lei, Q.; Feng, C.; Zheng, X.; Zhou, F.; Li, L.; Liu, X.; Wang, Z.; Kong, J. Ectopically Expressing MdPIP1;3, an Aquaporin Gene, Increased Fruit Size and Enhanced Drought Tolerance of Transgenic Tomatoes. BMC Plant Biol. 2017, 17, 246. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Type | Concentration | Application | Effect | References |
|---|---|---|---|---|
| Nanotubes (single walled) SWCN | 104, 315, 1750 mg/L | In vitro | Root elongation inhibition | [23] |
| 50 μg/mL | In vitro | Biomass increase | [24] | |
| 50 μg/mL | In vitro | Biomass increase | [25] | |
| 0–40 mg/L | In vitro/ex vitro | Seed germination and growth improved in vitro/shoot growth retardation ex vitro | [26] | |
| 1 or 10 mg/kg | Soil | Delay of growth and flowering | [37] | |
| SWCD-QD | 50 μg/mL each | In vitro | Increased leaf senescence, root growth inhibition | [25] |
| SWCN-OH | 1 or 10 mg/kg | Soil | Delay of growth and flowering | [37] |
| SWCNT-COOH | 1 mg/10 mL | Overnight dip | Increased biomass production | [27] |
| Nanotubes (multi walled) MWCN | 50 μg/mL | In vitro | Fresh biomass increase, | [47] |
| 40 μg/L | In vitro | Increased germination rate and biomass | [28] | |
| 500–5000 mg/kg | Vermiculite | Unaffected to fresh biomass | [38] | |
| 50–200 μg/L | Soil | Significant increase in plant height, flower and fruit formation | [39] | |
| 50 mg/L (0–60 min) | In vitro | Seed germination and seedlings length increased | [29] | |
| 50 mg/L | Hydroponic system | Increased fruit weight and number | [34] | |
| 1 or 10 g/kg | Soil | Delay of growth and flowering | [37] | |
| 100 mg/L | Foliar application | Increasing fruit yield& decreasing pathogen | [33] | |
| Nanohorns | 25, 50, 100 μg/mL | In vitro | Increased seed germination | [30] |
| Graphene | 500–2000 mg/L | Hydroponic culture | Inhibited plant growth and biomass | [35] |
| 40 μg/L | Seed pretreatment | Better seed germination, longer steam but less biomass | [31] | |
| Graphene oxide | 20, 50 mg/L | In vitro | Improve biomass and root system | [32] |
| Graphene-QD | 0–1500 mg/L | In vitro/hydroponic | Growth inhibition on higher concentrations | [36] |
| Fullerene | 40 mg/L | Vermiculite | Unaffected on fresh biomass | [40] |
| 500–5000 mg/kg | Vermiculite | Unaffected to fresh biomass and pesticide accumulation | [39] | |
| Fullerol C60(OH)20 | 50 mg/L (0–60 min) | In vitro | No effect on seed germination and plant growth | [29] |
| Treatment | Seedlings | ||
|---|---|---|---|
| Height (cm) | Weight (g) | Average Leaf Number | |
| Control | 7.37 ± 0.13 a * | 0.24 ± 0.08 a | 5.00 ± 0.10 a |
| 3HFWC | 12.65 ± 0.81 b | 0.83 ± 0.03 b | 5.70 ± 0.33 b |
| Plants | Fruit | ||
|---|---|---|---|
| Weight (g) | Width (mm) | Length (mm) | |
| Control | 6.85 ± 0.20 a * | 12.13 ± 0.10 a | 19.41 ± 0.36 a |
| 3HFWC | 9.07 ± 0.30 b | 13.20 ± 0.33 b | 21.91 ± 0.24 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subotić, A.; Jevremović, S.; Milošević, S.; Trifunović-Momčilov, M.; Đurić, M.; Koruga, Đ. Physiological Response, Oxidative Stress Assessment and Aquaporin Genes Expression of Cherry Tomato (Solanum lycopersicum L.) Exposed to Hyper-Harmonized Fullerene Water Complex. Plants 2022, 11, 2810. https://doi.org/10.3390/plants11212810
Subotić A, Jevremović S, Milošević S, Trifunović-Momčilov M, Đurić M, Koruga Đ. Physiological Response, Oxidative Stress Assessment and Aquaporin Genes Expression of Cherry Tomato (Solanum lycopersicum L.) Exposed to Hyper-Harmonized Fullerene Water Complex. Plants. 2022; 11(21):2810. https://doi.org/10.3390/plants11212810
Chicago/Turabian StyleSubotić, Angelina, Slađana Jevremović, Snežana Milošević, Milana Trifunović-Momčilov, Marija Đurić, and Đuro Koruga. 2022. "Physiological Response, Oxidative Stress Assessment and Aquaporin Genes Expression of Cherry Tomato (Solanum lycopersicum L.) Exposed to Hyper-Harmonized Fullerene Water Complex" Plants 11, no. 21: 2810. https://doi.org/10.3390/plants11212810










