The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses
Abstract
1. Introduction
2. Results
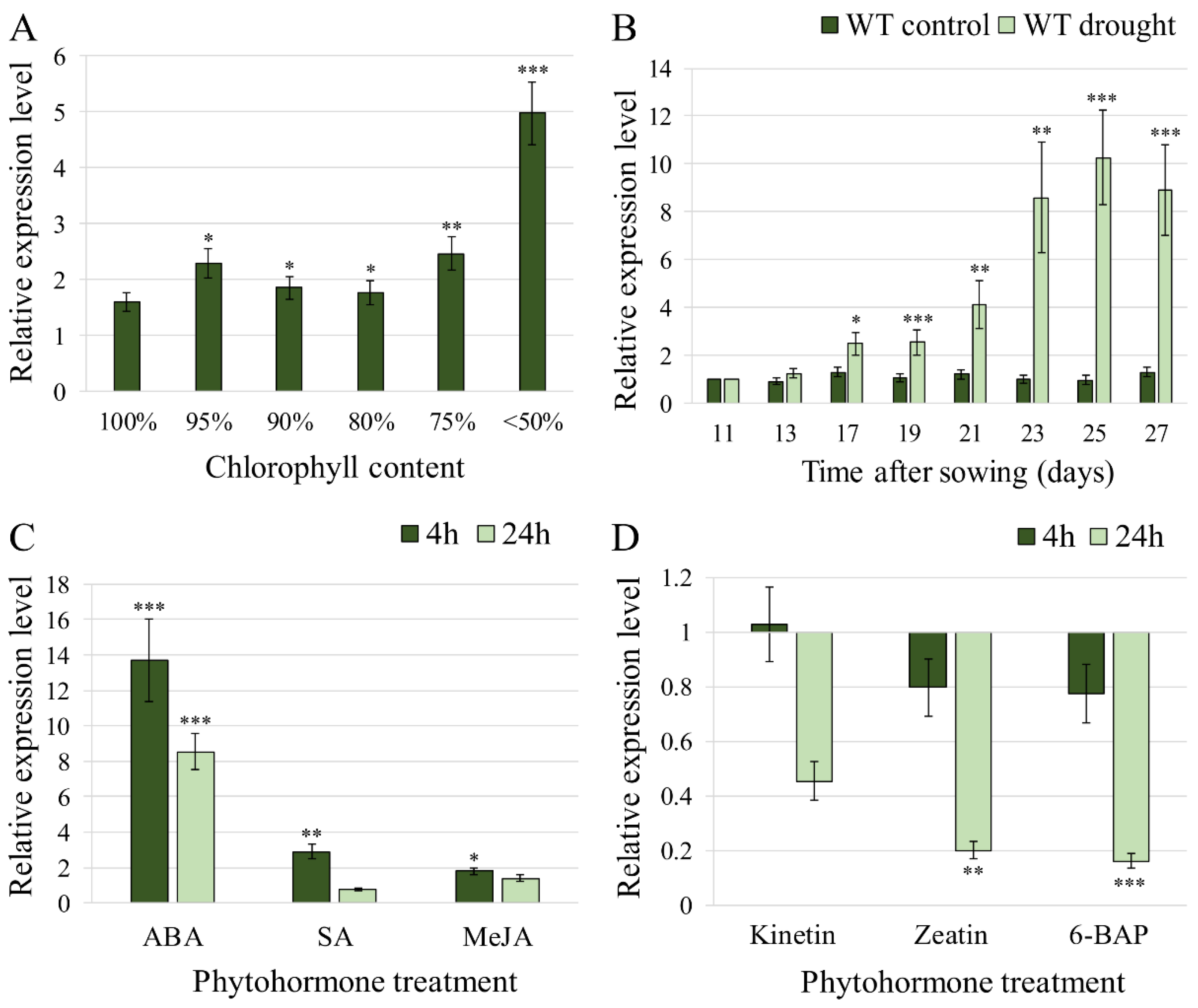
2.1. HvFP1 Was Expressed during the Developmental Leaf Senescence and in Response to Drought Stress and ABA Treatment
2.2. Overexpression of HvFP1 Affected the Time Course of the Developmental Leaf Senescence, but Not of the Drought-Induced Premature Senescence
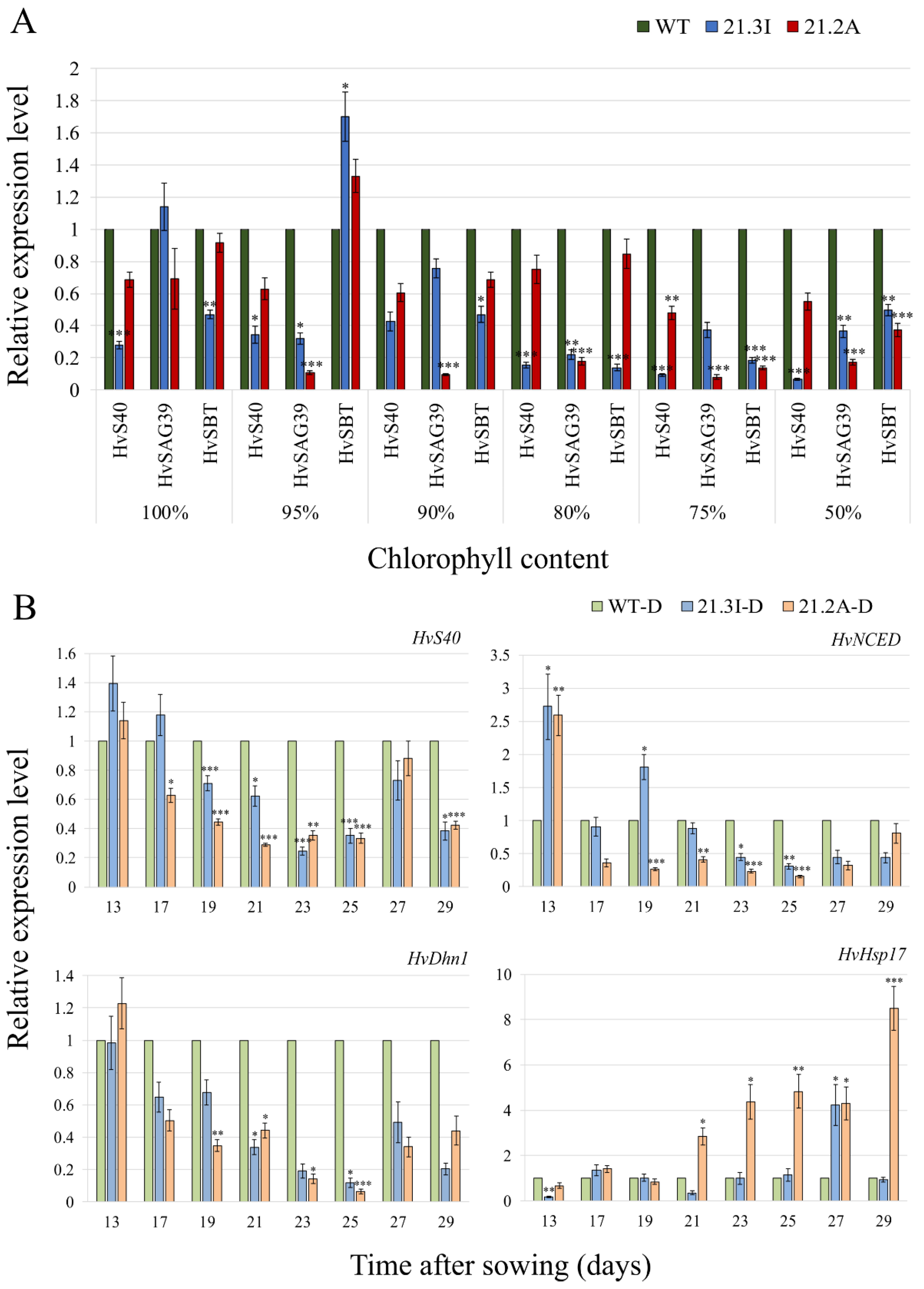
2.3. Overexpression of HvFP1 Delayed the Expression of the Senescence Associated Genes, but Also of the Drought Stress-Related Genes
2.4. Comparative Transcriptomic Analyses of the WT and HvFP1 OE Barley Primary Leaves
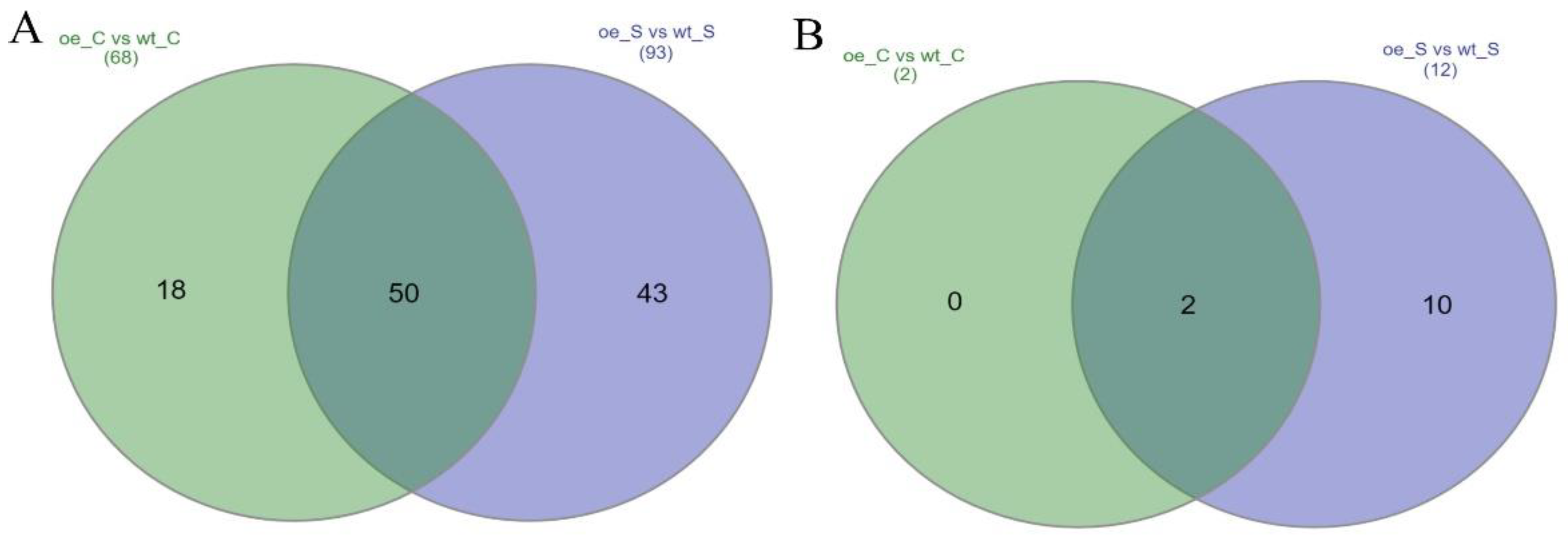
2.4.1. Differentially Expressed Gene Analysis
2.4.2. Functional Enrichment Analysis
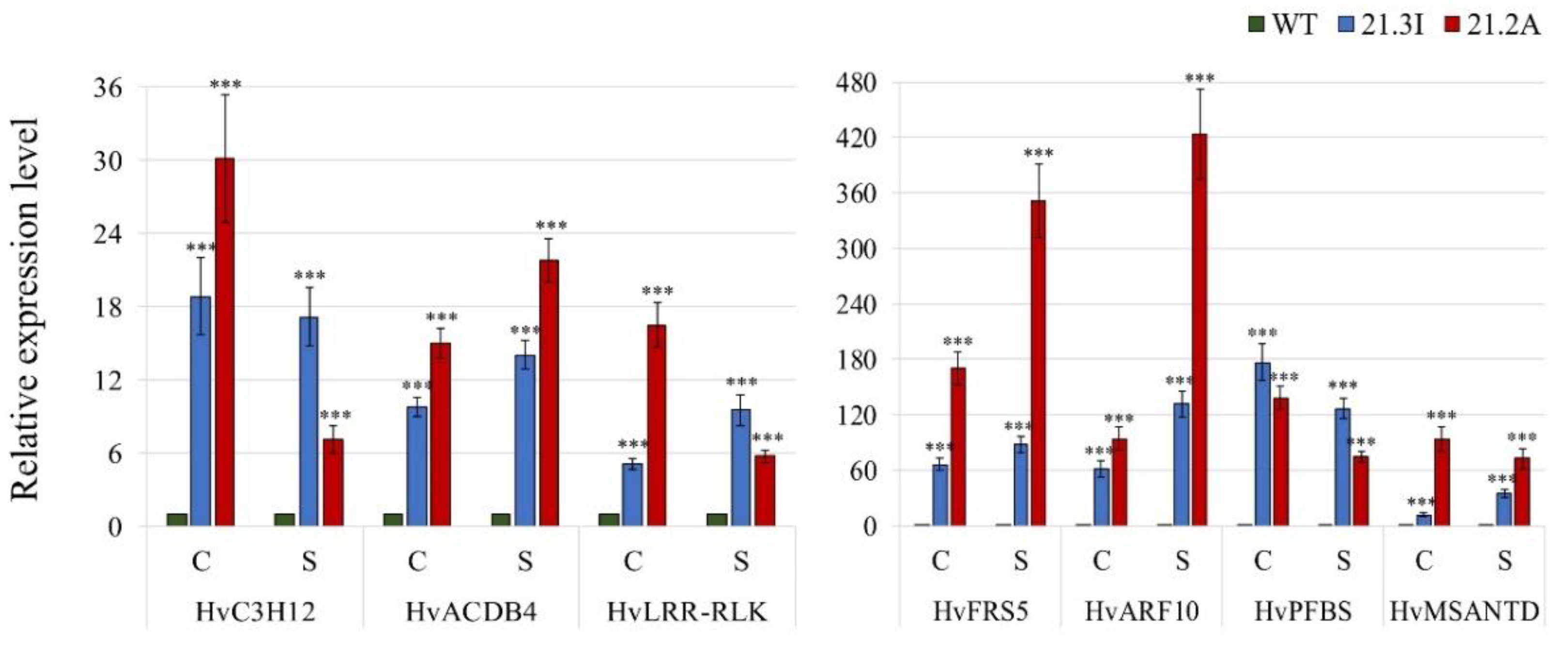
2.4.3. Validation of the RNA Seq Results
3. Discussion
3.1. HvFP1 Is Induced during the Developmental and Drought Induced Leaf Senescence
3.2. Regulation of HvFP1 by the Various Phytohormones
3.3. Overexpression of HvFP1 Causes Distinct Changes in the ABA- and Stress-Related Gene Expression and in the Course of Leaf Senescence
3.4. Identification of the Target Genes of the HvFP1 Regulatory Pathways
4. Materials and Methods
4.1. Plant Cultivation
4.2. Drought Stress
4.3. Developmental Senescence
4.4. Phytohormone Treatments
4.5. Photosystem II Efficiency
4.6. Chlorophyll Content
4.7. Isolation of Total RNA
4.8. Synthesis of Complementary DNA (cDNA)
4.9. Quantitative Real-Time PCR (qRT-PCR)
4.10. Sample Preparation for the RNA Sequencing
4.11. Mapping to Reference the Genome and Quantification
4.12. Differential Expression Analysis
4.13. Functional Analysis
4.14. Promoter Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitra, A.; Kataki, S.; Singh, A.N.; Gaur, A.; Razafindrabe, B.H.N.; Kumar, P.; Chatterjee, S.; Gupta, D.K. Plant Stress, Acclimation, and Adaptation: A Review. In Plant Growth and Stress Physiology; Gupta, D.K., Palma, J.M., Eds.; Plant in Challenging Environments; Springer International Publishing: Cham, Switzerland, 2021; Volume 3, ISBN 978-3-030-78419-5. [Google Scholar]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing Trade-Offs between Biotic and Abiotic Stress Responses through Leaf Age-Dependent Variation in Stress Hormone Cross-Talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Jibran, R.; Hunter, D.A.; Dijkwel, P.P. Hormonal Regulation of Leaf Senescence through Integration of Developmental and Stress Signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic Acid: A Key Regulator of Abiotic Stress Tolerance in Plants. Plant Gene 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress Induced and Nuclear Localized HIPP26 from Arabidopsis Thaliana Interacts via Its Heavy Metal Associated Domain with the Drought Stress Related Zinc Finger Transcription Factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
- Crowell, D.N.; Huizinga, D.H. Protein Isoprenylation: The Fat of the Matter. Trends Plant Sci. 2009, 14, 163–170. [Google Scholar] [CrossRef]
- Barth, O.; Zschiesche, W.; Siersleben, S.; Humbeck, K. Isolation of a Novel Barley CDNA Encoding a Nuclear Protein Involved in Stress Response and Leaf Senescence. Physiol. Plant 2004, 121, 282–293. [Google Scholar] [CrossRef]
- Tran, L.-S.P.; Nakashima, K.; Sakuma, Y.; Osakabe, Y.; Qin, F.; Simpson, S.D.; Maruyama, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Co-expression of the Stress-inducible Zinc Finger Homeodomain ZFHD1 and NAC Transcription Factors Enhances Expression of the ERD1 Gene in Arabidopsis. Plant J. 2006, 18, 46–63. [Google Scholar] [CrossRef]
- Zschiesche, W.; Barth, O.; Daniel, K.; Böhme, S.; Rausche, J.; Humbeck, K. The Zinc-binding Nuclear Protein HIPP 3 Acts as an Upstream Regulator of the Salicylate-dependent Plant Immunity Pathway and of Flowering Time in Arabidopsis Thaliana. New Phytol. 2015, 207, 1084–1096. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H. Multifaceted Roles of FHY3 and FAR1 in Light Signaling and Beyond. Trends Plant Sci. 2015, 20, 453–461. [Google Scholar] [CrossRef]
- Ai, Q.; Pan, W.; Zeng, Y.; Li, Y.; Cui, L. CCCH Zinc Finger Genes in Barley: Genome-Wide Identification, Evolution, Expression and Haplotype Analysis. BMC Plant Biol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Li, H.-Y.; Xiao, S.; Chye, M.-L. Ethylene- and Pathogen-Inducible Arabidopsis Acyl-CoA-Binding Protein 4 Interacts with an Ethylene-Responsive Element Binding Protein. J. Exp. Bot. 2008, 59, 3997–4006. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative Expression Software Tool (REST©) for Group-Wise Comparison and Statistical Analysis of Relative Expression Results in Real-Time PCR. Nucleic Acids Res. 2002, 30, 10. [Google Scholar] [CrossRef]
- Mascher, M. Source Code of the TRITEX Assembly Pipeline. 2019. Available online: https://search.datacite.org/works/10.5447/ipk/2019/19 (accessed on 19 September 2022).
- Dykema, P.E.; Sipes, P.R.; Marie, A.; Biermann, B.J.; Crowell, D.N.; Randall, S.K. A New Class of Proteins Capable of Binding Transition Metals. Plant Mol. Biol. 1999, 41, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, H.; Li, X. UBIQUITIN-SPECIFIC PROTEASE16 Interacts with a HEAVY METAL ASSOCIATED ISOPRENYLATED PLANT PROTEIN27 and Modulates Cadmium Tolerance. Plant Signal. Behav. 2013, 8, e25680. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Rono, J.K.; Zhang, B.Q.; Liu, X.S.; Wang, M.Q.; Wang, L.L.; Wu, X.C.; Chen, X.; Wei Cao, H.; Yang, Z.M. Identification of Novel Rice (Oryza Sativa) HPP and HIPP Genes Tolerant to Heavy Metal Toxicity. Ecotoxicol. Environ. Saf. 2019, 175, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Rono, J.K.; Liu, X.S.; Feng, S.J.; Li, H.; Chen, X.; Yang, Z.M. Functional Characterization of a New Metallochaperone for Reducing Cadmium Concentration in Rice Crop. J. Clean. Prod. 2020, 11, 123152. [Google Scholar] [CrossRef]
- Manara, A.; Fasani, E.; Molesini, B.; DalCorso, G.; Pennisi, F.; Pandolfini, T.; Furini, A. The Tomato Metallocarboxypeptidase Inhibitor I, Which Interacts with a Heavy Metal-Associated Isoprenylated Protein, Is Implicated in Plant Response to Cadmium. Molecules 2020, 17, 700. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Liu, X.S.; Feng, S.J.; Zhao, Y.N.; Wang, L.L.; Rono, J.K.; Li, H.; Yang, Z.M. Developing a Cadmium Resistant Rice Genotype with OsHIPP29 Locus for Limiting Cadmium Accumulation in the Paddy Crop. Chemosphere 2020, 247, 125958. [Google Scholar] [CrossRef]
- Liu, J.; Liao, S.; Li, M.; Du, X. Arabis Paniculata ApHIPP3 Increases Cd Tolerance by Interacting with ApCHC1. J. Genet. 2022, 101, 21. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Feng, C.; Xu, H.; Huang, X.; Wang, Q.; Duan, X.; Wang, X.; Wei, G.; Huang, L.; et al. Isolation and Characterisation of CDNA Encoding a Wheat Heavy Metal-Associated Isoprenylated Protein Involved in Stress Responses. Plant Biol. 2015, 11, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Radakovic, Z.S.; Anjam, M.S.; Escobar, E.; Chopra, D.; Cabrera, J.; Silva, A.C.; Escobar, C.; Sobczak, M.; Grundler, F.M.W.; Siddique, S. Arabidopsis HIPP27 Is a Host Susceptibility Gene for the Beet Cyst Nematode Heterodera Schachtii: Host Susceptibility Gene for Cyst Nematode. Mol. Plant Path. 2018, 19, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Cowan, G.H.; Roberts, A.G.; Jones, S.; Kumar, P.; Kalyandurg, P.B.; Gil, J.F.; Savenkov, E.I.; Hemsley, P.A.; Torrance, L. Potato Mop-Top Virus Co-Opts the Stress Sensor HIPP26 for Long-Distance Movement. Plant Physiol. 2018, 176, 2052–2070. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Interactions between Cytokinin Signalling and Abiotic Stress Responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [PubMed]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of ABA and MAPK Signaling Pathways in Plant Abiotic Stress Responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic Acid and Its Function in Plant ImmunityF: Defense Signal Molecule Salicylic Acid. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Holub, J.; Hanuš, J.; Hanke, D.E.; Strnad, M. Biological Activity of Cytokinins Derived from Ortho- and Meta-Hydroxybenzyladenine. Plant Growth Regul. 1998, 26, 109–115. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; van Staden, J. The Involvement of Cytokinins in Plant Responses to Environmental Stress. Plant Growth Regul. 1997, 25, 79–103. [Google Scholar]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, H.; Xiong, L. Endogenous Auxin and Jasmonic Acid Levels Are Differentially Modulated by Abiotic Stresses in Rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of Drought Tolerance by Gene Manipulation of 9-Cis-Epoxycarotenoid Dioxygenase, a Key Enzyme in Abscisic Acid Biosynthesis in Arabidopsis: Regulation of Drought Tolerance by AtNCED3. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, L.; Svensson, J.T.; Rodriguez, E.M.; Wahid, A.; Malatrasi, M.; Kato, K.; Wanamaker, S.; Resnik, J.; Close, T.J. Dehydrin Gene Expression Provides an Indicator of Low Temperature and Drought Stress: Transcriptome-Based Analysis of Barley (Hordeum Vulgare L.). Funct. Integr. Genom. 2008, 8, 387–405. [Google Scholar] [CrossRef]
- Krupinska, K.; Dähnhardt, D.; Fischer-Kilbienski, I.; Kucharewicz, W.; Scharrenberg, C.; Trösch, M.; Buck, F. Identification of WHIRLY1 as a Factor Binding to the Promoter of the Stress- and Senescence-Associated Gene HvS40. J. Plant Growth Regul. 2014, 33, 91–105. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-Cis-Epoxycarotenoid Dioxygenase 3 Regulates Plant Growth and Enhances Multi-Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef]
- Suprunova, T.; Krugman, T.; Fahima, T.; Chen, G.; Shams, I.; Korol, A.; Nevo, E. Differential Expression of Dehydrin Genes in Wild Barley, Hordeum Spontaneum, Associated with Resistance to Water Deficit. Plant Cell Environ. 2004, 27, 1297–1308. [Google Scholar] [CrossRef]
- Zou, J.; Liu, A.; Chen, X.; Zhou, X.; Gao, G.; Wang, W.; Zhang, X. Expression Analysis of Nine Rice Heat Shock Protein Genes under Abiotic Stresses and ABA Treatment. J. Plant Physiol. 2009, 166, 851–861. [Google Scholar] [CrossRef]
- Sun, X.; Sun, C.; Li, Z.; Hu, Q.; Han, L.; Luo, H. AsHSP17, a Creeping Bentgrass Small Heat Shock Protein Modulates Plant Photosynthesis and ABA-dependent and Independent Signalling to Attenuate Plant Response to Abiotic Stress. Plant Cell Environ. 2016, 39, 1320–1337. [Google Scholar] [CrossRef]
- Janack, B.; Sosoi, P.; Krupinska, K.; Humbeck, K. Knockdown of WHIRLY1 Affects Drought Stress-Induced Leaf Senescence and Histone Modifications of the Senescence-Associated Gene HvS40. Plants 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Krupinska, K.; Haussühl, K.; Schäfer, A.; van der Kooij, T.A.W.; Leckband, G.; Lörz, H.; Falk, J. A Novel Nucleus-Targeted Protein Is Expressed in Barley Leaves during Senescence and Pathogen Infection. Plant Physiol. 2002, 130, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Kilbienski, I.; Miao, Y.; Roitsch, T.; Zschiesche, W.; Humbeck, K.; Krupinska, K. Nuclear Targeted AtS40 Modulates Senescence Associated Gene Expression in Arabidopsis Thaliana during Natural Development and in Darkness. Plant Mol. Biol. 2010, 73, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Szczerba, M.W.; Li, X.; Lin, Y. Identification and Application of a Rice Senescence-Associated Promoter. Plant Physiol. 2010, 153, 1239–1249. [Google Scholar] [CrossRef]
- Roberts, I.N.; Veliz, C.G.; Criado, M.V.; Signorini, A.; Simonetti, E.; Caputo, C. Identification and Expression Analysis of 11 Subtilase Genes during Natural and Induced Senescence of Barley Plants. J. Plant Physiol. 2017, 211, 70–80. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Q.; Guo, Y.; Yang, J.; Wang, M.; Duan, X.; Niu, J.; Liu, S.; Zhang, J.; Lu, Y.; et al. Arabidopsis Subtilase SASP Is Involved in the Regulation of ABA Signaling and Drought Tolerance by Interacting with OPEN STOMATA 1. J. Exp. Bot. 2018, 69, 4403–4417. [Google Scholar] [CrossRef]
- Miersch, I.; Heise, J.; Zelmer, I.; Humbeck, K. Differential Degradation of the Photosynthetic Apparatus During Leaf Senescence in Barley (Hordeum Vulgare L.). Plant Biol. 2000, 2, 618–623. [Google Scholar] [CrossRef]
- Kotakis, C.; Kyzeridou, A.; Manetas, Y. Photosynthetic Electron Flow during Leaf Senescence: Evidence for a Preferential Maintenance of Photosystem I Activity and Increased Cyclic Electron Flow. Photosynthetica 2014, 52, 413–420. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and Let Live: Leaf Senescence Contributes to Plant Survival under Drought Stress. Funct. Plant Biol. 2004, 31, 203. [Google Scholar] [CrossRef]
- Ma, L.; Li, G. FAR1-RELATED SEQUENCE (FRS) and FRS-RELATED FACTOR (FRF) Family Proteins in Arabidopsis Growth and Development. Front. Plant Sci. 2018, 9, 692. [Google Scholar] [CrossRef]
- Kou, X.; Zhao, X.; Wu, B.; Wang, C.; Wu, C.; Yang, S.; Zhou, J.; Xue, Z. Auxin Response Factors Are Ubiquitous in Plant Growth and Development, and Involved in Crosstalk between Plant Hormones: A Review. Appl. Sci. 2022, 12, 1360. [Google Scholar] [CrossRef]
- Gavassi, M.A.; Monteiro, C.C.; Campos, M.L.; Melo, H.C.; Carvalho, R.F. Phytochromes Are Key Regulators of Abiotic Stress Responses in Tomato. Sci. Hortic. 2017, 222, 126–135. [Google Scholar] [CrossRef]
- Junior, C.A.S.; D’Amico-Damião, V.; Carvalho, R.F. Phytochrome Type B Family: The Abiotic Stress Responses Signaller in Plants. Ann. Appl. Biol. 2021, 178, 135–148. [Google Scholar] [CrossRef]
- Sun, W.; Wei, J.; Wu, G.; Xu, H.; Chen, Y.; Yao, M.; Zhan, J.; Yan, J.; Wu, N.; Chen, H.; et al. CqZF-HD14 Enhances Drought Tolerance in Quinoa Seedlings through Interaction with CqHIPP34 and CqNAC79. Plant Sci. 2022, 15, 111406. [Google Scholar] [CrossRef]
- Ghimire, S.; Tang, X.; Zhang, N.; Liu, W.; Si, H. SUMO and SUMOylation in Plant Abiotic Stress. Plant Growth Regul. 2020, 91, 317–325. [Google Scholar] [CrossRef]
- Miura, K.; Lee, J.; Jin, J.B.; Yoo, C.Y.; Miura, T.; Hasegawa, P.M. Sumoylation of ABI5 by the Arabidopsis SUMO E3 Ligase SIZ1 Negatively Regulates Abscisic Acid Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 5418–5423. [Google Scholar] [CrossRef]
- Xiang, J.; Lin, J.; Tang, D.; Zhou, B.; Guo, M.; He, R.; Zhao, X.; Liu, X. A DHHC-Type Zinc Finger Protein Gene Regulates Shoot Branching in Arabidopsis. Afr. J. Biotechnol. 2010, 8, 7759–7766. [Google Scholar]
- Zhou, B.; Lin, J.Z.; Peng, D.; Yang, Y.Z.; Guo, M.; Tang, D.Y.; Tan, X.; Liu, X.M. Plant Architecture and Grain Yield Are Regulated by the Novel DHHC-Type Zinc Finger Protein Genes in Rice (Oryza Sativa L.). Plant Sci. 2017, 254, 12–21. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, S.; Li, H.; Tsao, S.; Chye, M. Arabidopsis Thaliana Acyl-CoA-binding Protein ACBP2 Interacts with Heavy-metal-binding Farnesylated Protein AtFP6. New Phytol. 2009, 181, 89–102. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chye, M.-L. Arabidopsis Acyl-CoA-Binding Protein ACBP2 Interacts With an Ethylene-Responsive Element-Binding Protein, AtEBP, via Its Ankyrin Repeats. Plant Mol. Biol. 2004, 54, 233–243. [Google Scholar] [CrossRef]
- Lai, S.-H.; Chye, M.-L. Plant Acyl-CoA-Binding Proteins—Their Lipid and Protein Interactors in Abiotic and Biotic Stresses. Cells 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Chen, M.-X.; Chen, Q.-F.; Xiao, S.; Chye, M.-L. Overexpression of Arabidopsis Acyl-CoA-Binding Protein ACBP2 Enhances Drought Tolerance: Arabidopsis ACBP2 Is ABA Responsive. Plant Cell Environ. 2013, 36, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, M.; Su, L.; Ge, K.; Li, L.; Li, X.; Liu, X.; Li, L. Negative Feedback Regulation of ABA Biosynthesis in Peanut (Arachis Hypogaea): A Transcription Factor Complex Inhibits AhNCED1 Expression during Water Stress. Sci. Rep. 2016, 6, 37943. [Google Scholar] [CrossRef] [PubMed]
- Jamsheer K, M.; Jindal, S.; Sharma, M.; Awasthi, P.; S, S.; Sharma, M.; Mannully, C.T.; Laxmi, A. A Negative Feedback Loop of TOR Signaling Balances Growth and Stress-Response Trade-Offs in Plants. Cell Rep. 2022, 39, 110631. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Machey, K. Short Technical Reports. Modification of the TRI Reagent Procedure for Isolation of RNA from Polysaccharide- and Proteoglycan-Rich Sources. Biotechniques 1995, 19, 942–945. [Google Scholar] [PubMed]
- Chen, Y.; Hu, B.; Tan, Z.; Liu, J.; Yang, Z.; Li, Z.; Huang, B. Selection of Reference Genes for Quantitative Real-Time PCR Normalization in Creeping Bentgrass Involved in Four Abiotic Stresses. Plant Cell Rep. 2015, 34, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar Reddy, P.; Srinivas Reddy, D.; Sivasakthi, K.; Bhatnagar-Mathur, P.; Vadez, V.; Sharma, K.K. Evaluation of Sorghum [Sorghum Bicolor (L.)] Reference Genes in Various Tissues and under Abiotic Stress Conditions for Quantitative Real-Time PCR Data Normalization. Front. Plant Sci. 2016, 7, 529. [Google Scholar] [CrossRef]
- Gines, M.; Baldwin, T.; Rashid, A.; Bregitzer, P.; Maughan, P.J.; Jellen, E.N.; Klos, K.E. Selection of Expression Reference Genes with Demonstrated Stability in Barley among a Diverse Set of Tissues and Cultivars. Crop Sci. 2018, 58, 332–341. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasyri, A.; Barth, O.; Zschiesche, W.; Humbeck, K. The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses. Plants 2022, 11, 2851. https://doi.org/10.3390/plants11212851
Parasyri A, Barth O, Zschiesche W, Humbeck K. The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses. Plants. 2022; 11(21):2851. https://doi.org/10.3390/plants11212851
Chicago/Turabian StyleParasyri, Athina, Olaf Barth, Wiebke Zschiesche, and Klaus Humbeck. 2022. "The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses" Plants 11, no. 21: 2851. https://doi.org/10.3390/plants11212851
APA StyleParasyri, A., Barth, O., Zschiesche, W., & Humbeck, K. (2022). The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses. Plants, 11(21), 2851. https://doi.org/10.3390/plants11212851





