Antiviral Potentialities of Chemical Characterized Essential Oils of Acacia nilotica Bark and Fruits against Hepatitis A and Herpes Simplex Viruses: In Vitro, In Silico, and Molecular Dynamics Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. The Identification of the Chemical Constituents of Bark and Fruits of A. nilotica EOs
2.2. In Vitro Antiviral Activity
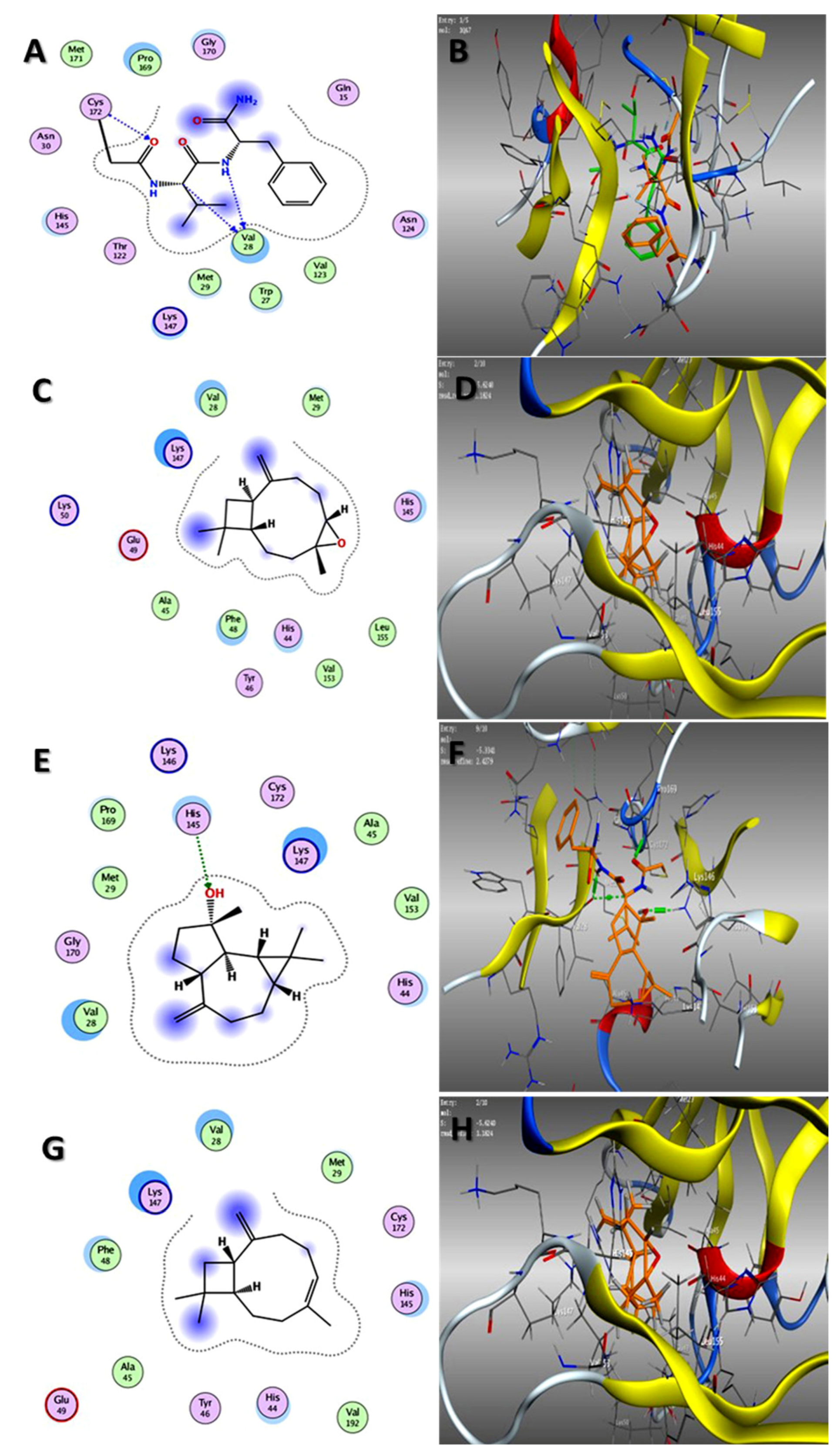
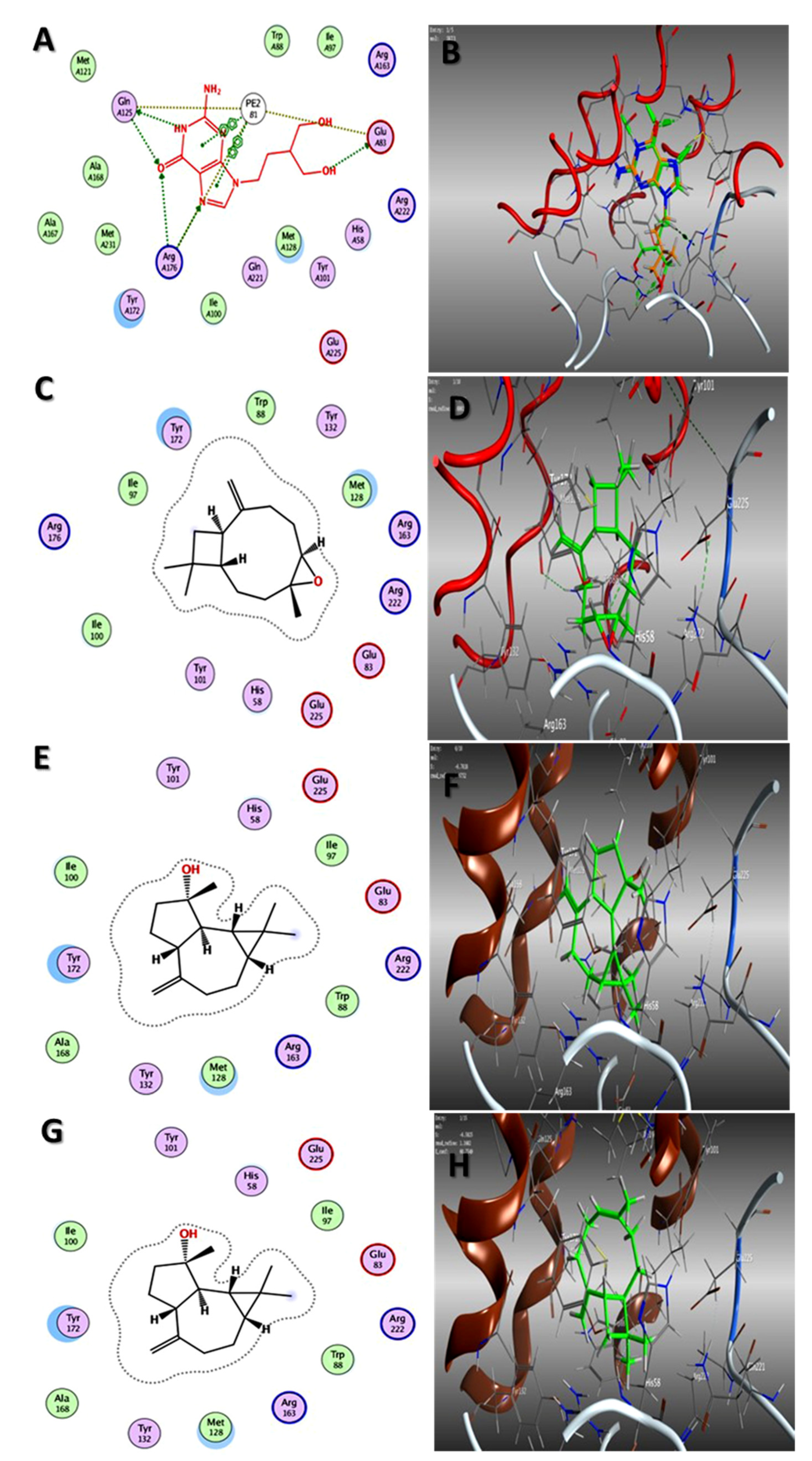
2.3. Molecular Docking Studies
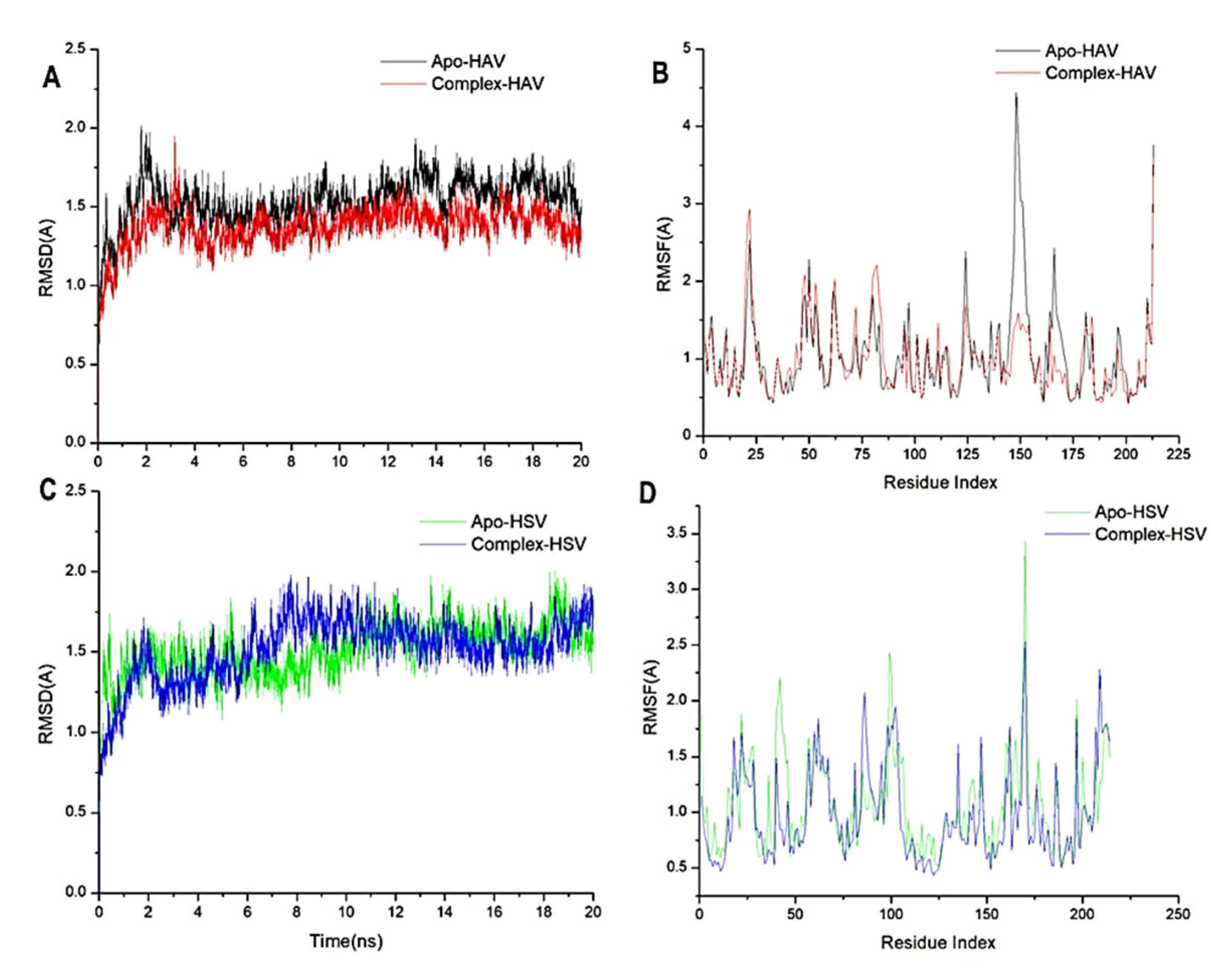
2.4. Molecular Dynamic and System Stability
Binding Interaction Mechanism Based on Binding Free Energy Calculation
3. Materials and Methods
3.1. Plant Materials and EOs Extraction
3.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.3. Antiviral Assays
3.3.1. Determination of the Maximum Non-Toxic Concentration (MNTC)
3.3.2. Antiviral Effect Percent Determination
3.4. Molecular Docking Studies
3.5. Molecular Dynamics Stimulation Section
3.5.1. System Preparation
3.5.2. Molecular Dynamic (MD) Simulations
3.5.3. Post-MD Analysis
3.5.4. Thermodynamic Calculation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, M.; Sharififar, F.; Arabzadeh, A.M.; Mehni, F.; Mirtadzadini, M.; Iranmanesh, Z.; Nikpour, N. In vitro evaluation of anti-herpes simplex-1 activity of three standardized medicinal plants from Lamiaceae. Anc. Sci. Life 2014, 34, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ubillas, R.; Jolad, S.; Bruening, R.; Kernan, M.; King, S.; Sesin, D.; Barrett, M.; Stoddart, C.; Flaster, T.; Kuo, J. SP-303, an antiviral oligomeric proanthocyanidin from the latex of Croton lechleri (Sangre de Drago). Phytomedicine 1994, 1, 77–106. [Google Scholar] [CrossRef]
- Schnitzler, P.; Nolkemper, S.; Stintzing, F.; Reichling, J. Comparative in vitro study on the anti-herpetic effect of phytochemically characterized aqueous and ethanolic extracts of Salvia officinalis grown at two different locations. Phytomedicine 2008, 15, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Berchová-Bímová, K.; Mazurakova, A.; Šamec, D.; Kubatka, P.; Hassan, S.T. Flavonoids target human herpesviruses that infect the nervous system: Mechanisms of action and therapeutic insights. Viruses 2022, 14, 592. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- De Logu, A.; Loy, G.; Pellerano, M.L.; Bonsignore, L.; Schivo, M.L. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antivir. Res. 2000, 48, 177–185. [Google Scholar] [CrossRef]
- Reichling, J. Plant-microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. In Functions Biotechnology of Plant Secondary Metabolites; Wink, M., Ed.; Sheffield Academic Press: Sheffield, UK, 1999; Volume 39, pp. 214–347. [Google Scholar]
- Jama-Kmiecik, A.; Sarowska, J.; Wojnicz, D.; Choroszy-Król, I.; Frej-Mądrzak, M. Natural products and their potential anti-HAV activity. Pathogens 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, J.A. Hepatitis A: Old and New. Clin. Microbiol. Rev. 2001, 14, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral active compounds derived from natural sources against herpes simplex viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef]
- Lyu, S.-Y.; Rhim, J.-Y.; Park, W.-B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharmacal. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Čulenová, M.; Sychrová, A.; Hassan, S.T.; Berchová-Bímová, K.; Svobodová, P.; Helclová, A.; Michnová, H.; Hošek, J.; Vasilev, H.; Suchý, P. Multiple In vitro biological effects of phenolic compounds from Morus alba root bark. J. Ethnopharmacol. 2020, 248, 112296. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Reichling, J.; Schneele, J.; Schnitzler, P. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomedicine 2008, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Gauss-Müller, V.; Cordes, S.; Tamura, R.; Okitsu, K.; Shuang, W.; Nakamoto, S.; Fujiwara, K.; Imazeki, F.; Yokosuka, O. Hepatitis A virus (HAV) proteinase 3C inhibits HAV IRES-dependent translation and cleaves the polypyrimidine tract-binding protein. J. Viral Hepat. 2010, 17, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S. Alpha-herpesvirus thymidine kinase genes mediate viral virulence and are potential therapeutic targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. Lipids Essent Oils Antimicrob. Agents 2011, 25, 203–238. [Google Scholar]
- Abdallah, H.M.; Ammar, N.M.; Abdelhameed, M.F.; Gendy, A.E.-N.G.E.; Ragab, T.I.; Abd-ElGawad, A.M.; Farag, M.A.; Alwahibi, M.S.; Elshamy, A.I. Protective mechanism of Acacia saligna butanol extract and its nano-formulations against ulcerative colitis in rats as revealed via biochemical and metabolomic assays. Biology 2020, 9, 195. [Google Scholar] [CrossRef]
- Rather, L.J.; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Malviya, S.; Rawat, S.; Kharia, A.; Verma, M. Medicinal attributes of Acacia nilotica Linn.—A comprehensive review on ethnopharmacological claims. Int. J. Pharm. Life Sci. 2011, 2, 830–837. [Google Scholar]
- Ogunbinu, A.; Okeniyi, S.; Flamini, G.; Cioni, P.; Ogunwande, I.; Babalola, I. Essential oil composition of Acacia nilotica Linn., and Acacia albida Delile (Leguminosae) from Nigeria. J. Essent. Oil Res. 2010, 22, 540–542. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Matsui, T.; Matsumoto, K.; Shimoda, M.; Kubmarawa, D. Constituents of the essential oil from the leaves of Acacia tortilis (Forsk.) Hayne. J. Essent. Oil Res. 2008, 20, 116–119. [Google Scholar] [CrossRef]
- Avoseh, O.N.; Oyedeji, O.-o.O.; Aremu, K.; Nkeh-Chungag, B.N.; Songca, S.P.; Oluwafemi, S.O.; Oyedeji, A.O. Chemical composition and anti-inflammatory activities of the essential oils from Acacia mearnsii De Wild. Nat. Prod. Res. 2015, 29, 1184–1188. [Google Scholar] [CrossRef]
- El Ayeb-Zakhama, A.; Sakka-Rouis, L.; Bergaoui, A.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and allelopathic potential of essential oils obtained from Acacia cyanophylla Lindl. cultivated in Tunisia. Chem. Biodivers. 2015, 12, 615–626. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Sarma, B.; Singh, H. Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chem. -Biol. Interact. 2009, 181, 20–28. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.; Singh, R.; Prakash, D.; Sarma, B.; Singh, H. Antioxidant and anti-quorum sensing activities of green pod of Acacia nilotica L. Fooda Chem. Toxicol. 2009, 47, 778–786. [Google Scholar] [CrossRef]
- Ahmad, M.; Zaman, F.; Sharif, T.; Ch, M.Z. Antidiabetic and hypolipidemic effects of aqueous methanolic extract of Acacia nilotica pods in alloxan-induced diabetic rabbits. Scand. J. Lab. Anim. Sci. 2008, 35, 29–34. [Google Scholar]
- Fatima, F.; Khalid, A.; Nazar, N.; Abdalla, M.; Mohomed, H.; Toum, A.M.; Magzoub, M.; Ali, M. In vitro assessment of anti-cutaneous leishmaniasis activity of some Sudanese plants. Turk. Parazitol. Derg. 2005, 29, 3–6. [Google Scholar]
- El-Tahir, A.; Satti, G.M.; Khalid, S.A. Antiplasmodial activity of selected Sudanese medicinal plants with emphasis on Acacia nilotica. Phytother. Res. 1999, 13, 474–478. [Google Scholar] [CrossRef]
- Essa, A.F.; El-Hawary, S.S.; Abd-El Gawad, A.M.; Kubacy, T.M.; AM El-Khrisy, E.E.D.; Elshamy, A.I.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Nasser El Gendy, A.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Jun, Y.; Lee, S.M.; Ju, H.K.; Lee, H.J.; Choi, H.-K.; Jo, G.S.; Kim, Y.-S. Comparison of the profile and composition of volatiles in coniferous needles according to extraction methods. Molecules 2016, 21, 363. [Google Scholar] [CrossRef] [PubMed]
- Block, S.; Flamini, G.; Brkic, D.; Morelli, I.; Quetin-Leclercq, J. Analysis of the essential oil from leaves of Croton zambesicus Muell. Arg. growing in Benin. Flavour Fragr. J. 2006, 21, 222–224. [Google Scholar] [CrossRef]
- Vernin, G.; Metzger, J.; Mondon, J.-P.; Pieribattesti, J.-C. GC/MS analysis of the leaf oil of Cryptomeria japonica D. Don from Reunion Island. J. Essent. Oil Res. 1991, 3, 197–207. [Google Scholar] [CrossRef]
- Salasiah, M.; Alona, C.; Meekiong, K. Essential oil components in selected species of Alpinieae (zingiberaceae) from Sarawak and its taxonomic correlation. J. Trop. For. Sci. 2022, 34, 221–235. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El Gendy, A.E.N.G.; Assaeed, A.M. Chemical characterization of Euphorbia heterophylla L. essential oils and their antioxidant activity and allelopathic potential on Cenchrus echinatus L. Chem. Biodivers. 2019, 16, e1900051. [Google Scholar] [CrossRef] [PubMed]
- Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Assaeed, A.M.; Elgamal, A.M.; Gendy, A.E.-N.G.E.; Mohamed, T.A.; Dar, B.A.; Mohamed, T.K.; Elshamy, A.I. Essential oil of Calotropis procera: Comparative chemical profiles, antimicrobial activity, and allelopathic potential on weeds. Molecules 2020, 25, 5203. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.I.; Yassine, Y.M.; Elshamy, A.I.; El-Beih, A.A.; El-Shazly, M.; Singab, A.N.B. Essential oil and antimicrobial activity of aerial parts of Cyperus leavigatus L.(Family: Cyperaceae). J. Essent. Oil Bear. Plants 2015, 18, 416–422. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.E.N.G.; Al-Rowaily, S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Assaeed, A.M.; Al-Rowaily, S.L. Interspecific variations in the habitats of Reichardia tingitana (L.) Roth leading to changes in its bioactive constituents and allelopathic activity. Saudi J. Biol. Sci. 2020, 27, 489–499. [Google Scholar] [CrossRef]
- Wardana, A.P.; Aminah, N.S.; Rosyda, M.; Abdjan, M.I.; Kristanti, A.N.; Tun, K.N.W.; Choudhary, M.I.; Takaya, Y. Potential of diterpene compounds as antivirals, a review. Heliyon 2021, 7, e07777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-J.; Li, Y.-H.; Jiang, J.-D.; Yu, S.-S.; Wang, X.-J.; Zhuang, P.-Y.; Zhang, Y.; Qu, J.; Ma, S.-G.; Li, Y. Diterpenes and sesquiterpenes with anti-Coxsackie virus B3 activity from the stems of Illicium jiadifengpi. Tetrahedron 2014, 70, 4494–4499. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for Antiviral Activities of Isolated Compounds from Essential Oils. Evid. -Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakamura, S.; Hosokawa, K.; Ishimaru, H.; Saito, N.; Ryu, K.; Fujimuro, M.; Nakashima, S.; Matsuda, H. New diterpenes from Nigella damascena seeds and their antiviral activities against herpes simplex virus type-1. J. Nat. Med. 2018, 72, 439–447. [Google Scholar] [CrossRef]
- Vallim, M.A.; Barbosa, J.E.; Cavalcanti, D.N.; De-Paula, J.C.; Silva, V.; Teixeira, V.L.; Paixão, I. In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. J. Med. Plants Res. 2010, 4, 2379–2382. [Google Scholar]
- Dunkić, V.; Vuko, E.; Bezić, N.; Kremer, D.; Ruščić, M. Composition and antiviral activity of the essential oils of Eryngium alpinum and E. amethystinum. Chem. Biodivers. 2013, 10, 1894–1902. [Google Scholar]
- Farag, R.S.; Shalaby, A.S.; El-Baroty, G.A.; Ibrahim, N.A.; Ali, M.A.; Hassan, E.M. Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytother. Res. 2004, 18, 30–35. [Google Scholar] [CrossRef]
- Venturi, C.R.; Danielli, L.J.; Klein, F.; Apel, M.A.; Montanha, J.A.; Bordignon, S.A.; Roehe, P.M.; Fuentefria, A.M.; Henriques, A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulata and Glechon marifolia. Pharm. Biol. 2015, 53, 682–688. [Google Scholar] [CrossRef]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral activity of some plant oils against herpes simplex virus type 1 in Vero cell culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P. Antiviral activity of selected Lamiaceae essential oils and their monoterpenes against SARS-CoV-2. Front. Pharmacol. 2022, 13, 893634. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Eisvand, F.; Hadizadeh, F.; Mosaffa, F.; Ghasemi, A.; Ghodsi, R. Design, synthesis and biological evaluation of novel 5, 6, 7-trimethoxy-N-aryl-2-styrylquinolin-4-amines as potential anticancer agents and tubulin polymerization inhibitors. Bioorganic Chem. 2020, 98, 103711. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; El-Rashedy, A.A.; Kamel, S. Synthesis of novel heterocyclic compounds based on dialdehyde cellulose: Characterization, antimicrobial, antitumor activity, molecular dynamics simulation and target identification. Cellulose 2021, 28, 8355–8374. [Google Scholar] [CrossRef]
- Bergmann, E.M.; Cherney, M.M.; Mckendrick, J.; Frormann, S.; Luo, C.; Malcolm, B.A.; Vederas, J.C.; James, M.N. Crystal structure of an inhibitor complex of the 3C proteinase from hepatitis A virus (HAV) and implications for the polyprotein processing in HAV. Virology 1999, 265, 153–163. [Google Scholar] [CrossRef]
- Champness, J.N.; Bennett, M.S.; Wien, F.; Visse, R.; Summers, W.C.; Herdewijn, P.; De Clercq, E.; Ostrowski, T.; Jarvest, R.L.; Sanderson, M.R. Exploring the active site of herpes simplex virus type-1 thymidine kinase by X-ray crystallography of complexes with aciclovir and other ligands. Proteins Struct. Funct. Bioinform. 1998, 32, 350–361. [Google Scholar] [CrossRef]
- Essa, A.F.; El-Hawary, S.S.; Emam, S.E.; Kubacy, T.M.; El-Khrisy, E.E.-D.A.; Younis, I.Y.; Elshamy, A.I. Characterization of undescribed melanoma inhibitors from Euphorbia mauritanica L. cultivated in Egypt targeting BRAFV600E and MEK 1 kinases via in-silico study and ADME prediction. Phytochemistry 2022, 198, 113154. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Hospital, A.; Goñi, J.R.; Orozco, M.; Gelpí, J.L. Molecular dynamics simulations: Advances and applications. Adv. Appl. Bioinform. Chem. 2015, 8, 37–47. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.v.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Ylilauri, M.; Pentikäinen, O.T. MMGBSA as a tool to understand the binding affinities of filamin–peptide interactions. J. Chem. Inf. Model. 2013, 53, 2626–2633. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
- Greenidge, P.A.; Kramer, C.; Mozziconacci, J.-C.; Wolf, R.M. MM/GBSA binding energy prediction on the PDBbind data set: Successes, failures, and directions for further improvement. J. Chem. Inf. Model. 2013, 53, 201–209. [Google Scholar] [CrossRef]
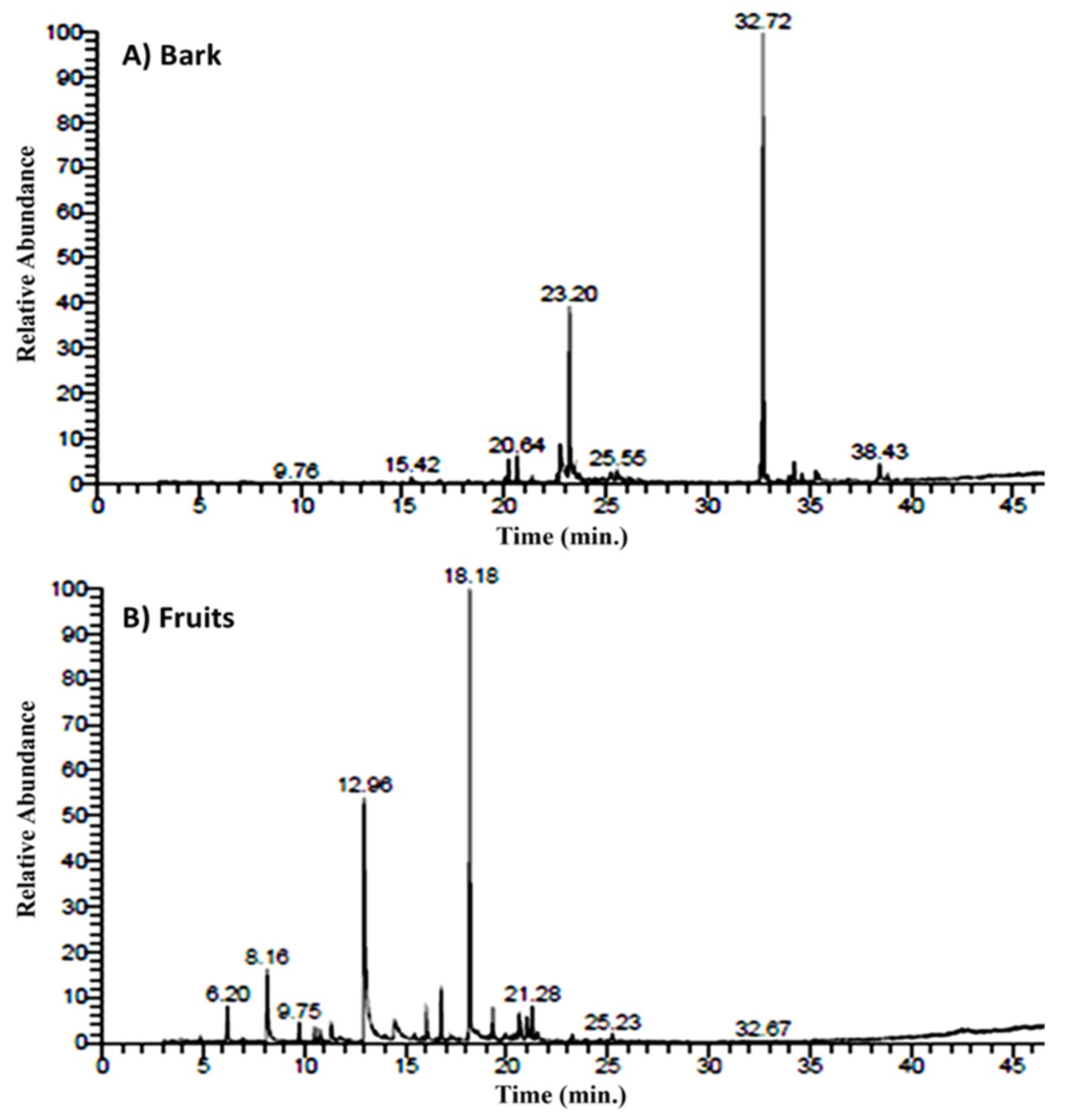
| No | Rt 1 | Compound | Type | Relative Concentration (%) | KI | ||
|---|---|---|---|---|---|---|---|
| Bark | Fruit | Lit. 2 | Exp. 3 | ||||
| 1 | 3.89 | α-Pinene | MH | - | 0.11 ± 0.01 | 932 | 935 |
| 2 | 4.85 | β-Pinene | MH | - | 0.35 ± 0.02 | 974 | 979 |
| 3 | 6.20 | 1,8-Cineole | OM | - | 3.52 ± 0.06 | 1026 | 1020 |
| 4 | 6.41 | γ-Terpinene | MH | - | 7.35 ± 0.08 | 1054 | 1059 |
| 5 | 6.63 | α-Linalool | OM | - | 1.82 ± 0.04 | 1095 | 1091 |
| 6 | 6.94 | Camphor | OM | - | 0.36 ± 0.02 | 1146 | 1144 |
| 7 | 9.75 | Borneol | OM | - | 2.31 ± 0.04 | 1169 | 1165 |
| 8 | 10.50 | 4-Terpineol | OM | - | 1.15 ± 0.03 | 1177 | 1175 |
| 9 | 10.77 | α-Terpineol | OM | - | 1.16 ± 0.05 | 1186 | 1185 |
| 10 | 11.74 | Cumin aldehyde | OM | - | 0.49 ± 0.02 | 1238 | 1235 |
| 11 | 12.96 | Z-Anethole | OM | - | 22.87 ± 0.23 | 1249 | 1245 |
| 12 | 13.99 | Bornyl acetate | OM | - | 0.23 ± 0.02 | 1285 | 1283 |
| 13 | 14.47 | 2-Caren-10-al | OM | - | 3.51 ± 0.05 | 1289 | 1287 |
| 14 | 15.45 | α-Terpinyl acetate | OM | - | 0.47 ± 0.02 | 1316 | 1314 |
| 15 | 15.82 | Myrtenyl acetate | OM | 0.84 ± 0.03 | - | 1324 | 1322 |
| 16 | 16.04 | α-Elemene | SH | - | 4.69 ± 0.06 | 1335 | 1339 |
| 17 | 16.13 | α-Cubebene | SH | - | 0.32 ± 0.01 | 1351 | 1346 |
| 18 | 16.54 | α-Copaene | SH | 0.35 ± 0.02 | 0.23 ± 0.01 | 1374 | 1370 |
| 19 | 16.67 | β-Elemene | SH | - | 3.72 ± 0.08 | 1389 | 1392 |
| 20 | 16.84 | Methyl eugenol | OM | - | 0.54 ± 0.02 | 1403 | 1401 |
| 21 | 18.18 | trans-Caryophyllene | SH | 0.24 ± 0.01 | 36.95 ± 0.18 | 1407 | 1405 |
| 22 | 18.35 | Longifolene | SH | - | 0.41 ± 0.01 | 1408 | 1413 |
| 23 | 18.58 | Aromadendrene | SH | - | 0.36 ± 0.02 | 1439 | 1433 |
| 24 | 19.40 | α-Humulene | SH | 0.27 ± 0.01 | 4.05 ± 0.06 | 1452 | 1455 |
| 25 | 19.85 | γ-Muurolene | SH | - | 0.26 ± 0.01 | 1478 | 1475 |
| 26 | 19.96 | Germacrene-D | SH | 0.14 ± 0.01 | 0.46 ± 0.02 | 1481 | 1484 |
| 27 | 20.02 | α-Amorphene | SH | 0.51 ± 0.02 | - | 1483 | 1486 |
| 28 | 20.12 | α-Selinene | SH | - | 0.54 ± 0.01 | 1498 | 1495 |
| 29 | 20.21 | α-Muurolene | SH | 2.42 ± 0.04 | - | 1500 | 1498 |
| 30 | 20.64 | Bicyclogermacrene | SH | 2.41 ± 0.06 | - | 1501 | 1504 |
| 31 | 21.35 | δ-Cadinene | SH | 0.75 ± 0.02 | - | 1522 | 1525 |
| 32 | 22.58 | α-Calacorene | SH | 1.18 ± 0.05 | - | 1545 | 1543 |
| 33 | 22.19 | E-Nerolidol | OS | 0.16 ± 0.01 | - | 1531 | 1535 |
| 34 | 22.76 | Spathulenol | OS | 4.74 ± 0.05 | - | 1577 | 1574 |
| 35 | 23.20 | Caryophyllene oxide | OS | 19.11 ± 0.09 | - | 1582 | 1585 |
| 36 | 23.45 | Globulol | OS | 1.01 ± 0.02 | - | 1590 | 1593 |
| 37 | 23.70 | Veridiflorol | OS | 0.83 ± 0.01 | - | 1596 | 1598 |
| 38 | 24.45 | Neoclovenoxid | OS | 0.55 ± 0.01 | - | 1608 | 1605 |
| 39 | 24.68 | Isospathulenol | OS | 0.14 ± 0.00 | - | 1630 | 1632 |
| 40 | 24.85 | tau-Cadinol | OS | 0.51 ± 0.02 | - | 1640 | 1642 |
| 42 | 25.22 | Cubenol | OS | 0.75 ± 0.03 | - | 1645 | 1644 |
| 43 | 25.28 | Torreyol | OS | 0.68 ± 0.03 | - | 1646 | 1648 |
| 44 | 25.55 | α-Cadinol | OS | 1.35 ± 0.06 | - | 1654 | 1657 |
| 45 | 25.68 | Khusinol | OS | 0.44 ± 0.01 | - | 1658 | 1659 |
| 46 | 32.58 | Cryptomeridiol | OS | 1.27 ± 0.07 | - | 1813 | 1816 |
| 47 | 32.72 | Stachene | DH | 48.34 ± 0.25 | - | 1931 | 1934 |
| 48 | 34.26 | Trachyloban | DH | 2.25 ± 0.07 | - | 1965 | 1968 |
| 49 | 34.62 | Isokaurene | DH | 1.01 ± 0.04 | - | 1997 | 1999 |
| 50 | 35.31 | Kaur-16-ene | DH | 1.94 ± 0.06 | - | 2017 | 2015 |
| 51 | 34.02 | Phytol | OD | 0.76 ± 0.02 | - | 1942 | 1940 |
| 52 | 36.42 | Sclareol | OD | 0.14 ± 0.00 | - | 2223 | 2221 |
| 53 | 37.11 | 4,8,13-Duvatriene-1,3-diol | OD | 0.16 ± 0.00 | - | 2400 | 2403 |
| 54 | 38.43 | n-Nonacosane | Others | 2.51 ± 0.06 | - | 2900 | 2900 |
| 56 | 45.21 | n-Dotriacontane | Others | 0.98 ± 0.03 | - | 3200 | 3200 |
| Monoterpene Hydrocarbons (MH) | 0 | 7.81 | |||||
| Oxygenated Monoterpenes (OM) | 0.84 | 55.51 | |||||
| Sesquiterpene Hydrocarbons (SH) | 8.27 | 34.91 | |||||
| Oxygenated Sesquiterpenes (OS) | 31.54 | 3.52 | |||||
| Diterpene Hydrocarbons (DH) | 53.54 | 0 | |||||
| Oxygenated Diterpenes (OD) | 1.06 | 0 | |||||
| Others | 3.49 | 0 | |||||
| Total | 98.74 | 98.23 | |||||
| Acacia nilotica | MNTC (µg/mL) a | Antiviral Effect % | Selectivity Index (SI) | ||||
|---|---|---|---|---|---|---|---|
| HAV | HSV1 | HSV2 | HAV | HSV1 | HSV2 | ||
| Bark EO | 500 ± 6.2 | 47.26 ± 2.05 | 35.98 ± 1.31 | 9.07 ± 0.36 | 2.3 | 1.6 | ND |
| Fruits EO | 1000 ± 11.4 | 9.42 ± 0.62 | 14.26 ± 0.54 | 3.99 ± 0.15 | 3.8 | 5.7 | 1.6 |
| Acyclovir | >387.63 | 12.24 | |||||
| Amantadine | 51.62 | ||||||
| Plant Part EO | Name of Phytoligands | ΔG * (kcal/mol) | |
|---|---|---|---|
| HAV 3C Protease | HSV TK | ||
| Bark | Spathulenol | −5.23 | −6.83 |
| Caryophyllene oxide | −5.43 | −6.96 | |
| Stachene | - | - | |
| Fruits | γ-Terpinene | −4.85 | −5.80 |
| Z-Anethole | −4.66 | −5.20 | |
| Trans-caryophyllene | −5.26 | −6.79 | |
| Co-crystallized inhibitor of HAV 3C protease | −6.61 | ND | |
| Co-crystallized inhibitor of HSV TK 1 | ND | −7.85 | |
| Energy Components (kcal/mol) | |||||
|---|---|---|---|---|---|
| Complex | ΔEvdW | ΔEelec | ΔGgas | ΔGsolv | ΔGbind |
| Caryophyllene oxide -HAV | −20.44 ± 0.27 | −1.52 ± 0.12 | −12.96 ± 0.33 | 3.60 ± 0.11 | −19.35 ± 0.24 |
| Caryophyllene oxide -HSV | −34.86 ± 0.081 | −2.58 ± 0.07 | −37.45 ± 0.11 | 5.40 ± 0.05 | −32.04 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Gendy, A.E.-N.G.; Essa, A.F.; El-Rashedy, A.A.; Elgamal, A.M.; Khalaf, D.D.; Hassan, E.M.; Abd-ElGawad, A.M.; Elgorban, A.M.; Zaghloul, N.S.; Alamery, S.F.; et al. Antiviral Potentialities of Chemical Characterized Essential Oils of Acacia nilotica Bark and Fruits against Hepatitis A and Herpes Simplex Viruses: In Vitro, In Silico, and Molecular Dynamics Studies. Plants 2022, 11, 2889. https://doi.org/10.3390/plants11212889
El Gendy AE-NG, Essa AF, El-Rashedy AA, Elgamal AM, Khalaf DD, Hassan EM, Abd-ElGawad AM, Elgorban AM, Zaghloul NS, Alamery SF, et al. Antiviral Potentialities of Chemical Characterized Essential Oils of Acacia nilotica Bark and Fruits against Hepatitis A and Herpes Simplex Viruses: In Vitro, In Silico, and Molecular Dynamics Studies. Plants. 2022; 11(21):2889. https://doi.org/10.3390/plants11212889
Chicago/Turabian StyleEl Gendy, Abd El-Nasser G., Ahmed F. Essa, Ahmed A. El-Rashedy, Abdelbaset M. Elgamal, Doaa D. Khalaf, Emad M. Hassan, Ahmed M. Abd-ElGawad, Abdallah M. Elgorban, Nouf S. Zaghloul, Salman F. Alamery, and et al. 2022. "Antiviral Potentialities of Chemical Characterized Essential Oils of Acacia nilotica Bark and Fruits against Hepatitis A and Herpes Simplex Viruses: In Vitro, In Silico, and Molecular Dynamics Studies" Plants 11, no. 21: 2889. https://doi.org/10.3390/plants11212889
APA StyleEl Gendy, A. E.-N. G., Essa, A. F., El-Rashedy, A. A., Elgamal, A. M., Khalaf, D. D., Hassan, E. M., Abd-ElGawad, A. M., Elgorban, A. M., Zaghloul, N. S., Alamery, S. F., & Elshamy, A. I. (2022). Antiviral Potentialities of Chemical Characterized Essential Oils of Acacia nilotica Bark and Fruits against Hepatitis A and Herpes Simplex Viruses: In Vitro, In Silico, and Molecular Dynamics Studies. Plants, 11(21), 2889. https://doi.org/10.3390/plants11212889










