Drought Stress Priming Improved the Drought Tolerance of Soybean
Abstract
:1. Introduction
2. Results
2.1. Glycine Max C08 Demonstrated Drought Stress Memory
2.2. Priming for Drought Stress Resulted in Better Water Retention Abilities than in Unprimed Plants
2.3. Drought Stress Memory Reduced the Rates of Photosynthesis, Transpiration and Stomatal Conductance and Increased Water Usage Efficiency
2.4. Drought Stress Memory Did Not Affect Growth Performance beyond the Effects of Drought Stress Itself
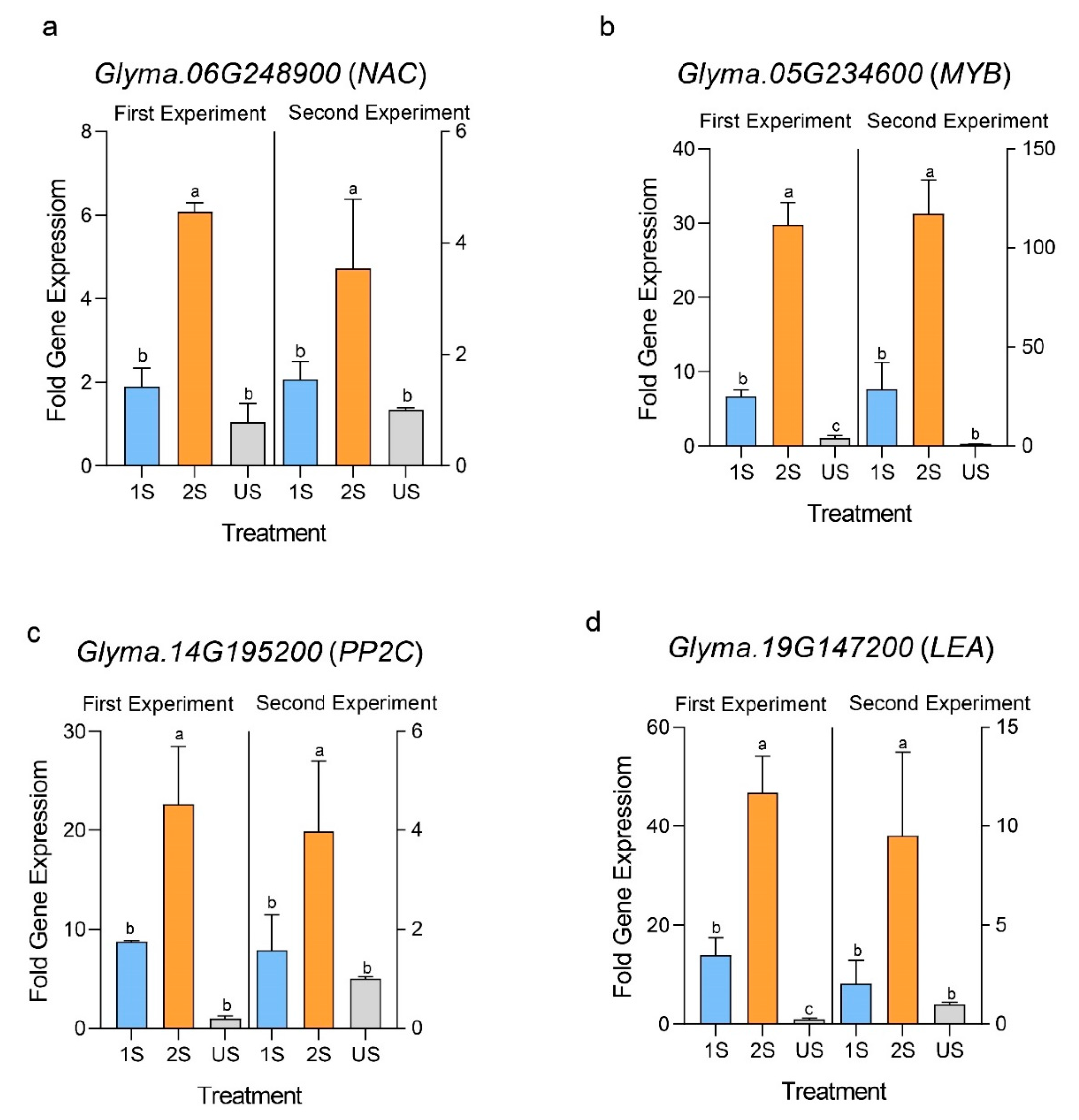
2.5. Drought Stress Memory Induced the Expressions of Selected Drought Priming-Responsive Genes
3. Materials and Methods
3.1. Plant Materials, Growth and Treatment Conditions
3.2. Determination of Drought Stress Index (DSI)
3.3. Determination of the Growth-Related Parameters
3.4. Determination of the Relative Water Content (RWC)
3.5. Calculation of the Shoot Water Content
3.6. Measurement of Photosynthesis-Related Parameters
3.7. Measurement of Rate of Water Loss (RWL) from Leaves
3.8. RT-qPCR Analyses
3.9. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. Soy scaffoldings poised to make cultured meat more affordable. JAMA 2020, 323, 1764. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef]
- Barnard, N.D.; Kahleova, H.; Holtz, D.N.; Del Aguila, F.; Neola, M.; Crosby, L.M.; Holubkov, R. The Women’s Study for the Alleviation of Vasomotor Symptoms (WAVS): A randomized, controlled trial of a plant-based diet and whole soybeans for postmenopausal women. Menopause 2021, 28, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Nutritional benefits of soy protein and soy fiber. J. Am. Diet. Assoc. 1991, 91, 816–819. [Google Scholar] [CrossRef]
- Bittencourt, G.A.; Vandenberghe, L.P.D.S.; Valladares-Diestra, K.; Herrmann, L.W.; Mello, A.F.M.D.; Vásquez, Z.S.; Karp, S.G.; Soccol, C.R. Soybean hulls as carbohydrate feedstock for medium to high-value biomolecule production in biorefineries: A review. Bioresour. Technol. 2021, 339, 125594. [Google Scholar] [CrossRef] [PubMed]
- Ayman, E.S.; Sorour, S.; Morsi, A.; Islam, M.S.; Saneoka, H. (PDF) Role of Osmoprotectants and compost application in improving water stress tolerance in soybean (Glycine max L.). Int. J. Curr. Res. 2016, 8, 25949–25954. [Google Scholar]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2012, 3, 52–58. [Google Scholar] [CrossRef]
- Leng, G.; Hall, J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci. Total Environ. 2019, 654, 811–821. [Google Scholar] [CrossRef]
- Sacita, A.S.; June, T.; Impron, I. Soybean adaptation to water stress on vegetative and generative phases. ATJ 2018, 3, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Godwin, J.; Farrona, S. Plant epigenetic stress memory induced by drought: A physiological and molecular perspective. Methods Mol. Biol. 2020, 2093, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.-H.; Nam, H.G.; Park, Y.S. Stress memory in plants: A negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. Plant J. 2003, 36, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought “train” transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virlouvet, L.; Fromm, M. Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol. 2015, 205, 596–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Virlouvet, L.; Liu, N.; Riethoven, J.-J.; Fromm, M.; Avramova, Z. Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana. BMC Plant Biol. 2014, 14, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Vignjevic, M.; Jiang, D.; Jacobsen, S.; Wollenweber, B. Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 2014, 65, 6441–6456. [Google Scholar] [CrossRef] [Green Version]
- Auler, P.A.; Amaral, M.N.D.; Braga, E.J.B.; Maserti, B. Drought stress memory in rice guard cells: Proteome changes and genomic stability of DNA. Plant Physiol. Biochem. 2021, 169, 49–62. [Google Scholar] [CrossRef]
- Ellouzi, H.; Hamed, K.B.; Asensi-Fabado, M.A.; Müller, M.; Abdelly, C.; Munné-Bosch, S. Drought and cadmium may be as effective as salinity in conferring subsequent salt stress tolerance in Cakile maritima. Planta 2013, 237, 1311–1323. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Cotado, A.; Fleta-Soriano, E.; Munné-Bosch, S. Drought stress memory in the photosynthetic mechanisms of an invasive CAM species, Aptenia cordifolia. Photosyn. Res. 2017, 131, 241–253. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Pintó-Marijuan, M.; Munné-Bosch, S. Evidence of drought stress memory in the facultative cam, Aptenia cordifolia: Possible role of phytohormones. PLoS ONE 2015, 10, e01353912015. [Google Scholar] [CrossRef] [Green Version]
- Fleta-Soriano, E.; Munné-Bosch, S. Stress memory and the inevitable effects of drought: A physiological perspective. Front. Plant Sci. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, N.; Virlouvet, L.; Riethoven, J.-J.; Fromm, M.; Avramova, Z. Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, W.-S.; Wang, Q.; Huang, M.; Wong, F.-L.; Liu, A.; Ng, M.-S.; Li, K.-P.; Sze, C.-C.; Li, M.-W.; Lam, H.-M. Priming-induced alterations in histone modifications modulate transcriptional responses in soybean under salt stress. Plant J. 2022, 109, 1575–1590. [Google Scholar] [CrossRef]
- Nguyen, T.T.Q.; Trinh, L.T.H.; Pham, H.B.V.; Le, T.V.; Phung, T.K.H.; Lee, S.-H.; Cheong, J.-J. Evaluation of proline, soluble sugar and ABA content in soybean Glycine max (L.) under drought stress memory. AIMS Bioeng. 2020, 7, 114–123. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Chae, S.; Oh, N.-I.; Nguyen, N.H.; Cheong, J.-J. Recurrent drought conditions enhance the induction of drought stress memory genes in Glycine max L. Front. Genet. 2020, 11, 576086. [Google Scholar] [CrossRef] [PubMed]
- Wijewardana, C.; Reddy, K.R.; Krutz, L.J.; Gao, W.; Bellaloui, N. Drought stress has transgenerational effects on soybean seed germination and seedling vigor. PLoS ONE 2019, 14, e0214977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarah, N.H.; Mullen, R.E.; Cianzio, S.R.; Scott, P. Dehydrin-like proteins in soybean seeds in response to drought stress during seed filling. Crop Sci. 2006, 46, 2141–2150. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Liu, X.; Qi, X.; Lam, H.-M.; Zhang, J. Differences between soybean genotypes in physiological response to sequential soil drying and rewetting. Crop J. 2014, 2, 366–380. [Google Scholar] [CrossRef] [Green Version]
- Lam, H.M.; Xu, X.; Liu, X.; Chen, W.; Yang, G.; Wong, F.L.; Li, M.W.; He, W.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 12, 1053–1059. [Google Scholar] [CrossRef]
- Hogg, R.V.; Tanis, E.A.; Zimmerman, D.L. Distributions of Functions of Random Variables. In Probability and Statistical Inference, 9th ed.; Lynch, D., Cummings, C., Lepre, C., Ashraf, S., Eds.; Pearson Education Limited: Harlow, UK, 2015; p. 202. [Google Scholar]
- Engelbrecht, B.; Tyree, M.; Kursar, T. Visual assessment of wilting as a measure of leaf water potential and seedling drought survival. J. Trop. Ecol. 2007, 23, 497–500. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Leaf and shoot water content and leaf dry matter content of mediterranean woody species with different post-fire regenerative strategies. Ann. Bot. 2007, 99, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Neves-Borges, A.C.; Guimarães-Dias, F.; Cruz, F.; Mesquita, R.O.; Nepomuceno, A.L.; Romano, E.; Loureiro, M.E.; de Fátima Grossi-de-Sá, M.; Alves-Ferreira, M. Expression pattern of drought stress marker genes in soybean roots under two water deficit systems. Genet. Mol. Biol. 2012, 35, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Yim, A.K.-Y.; Wong, J.W.-H.; Ku, Y.-S.; Qin, H.; Chan, T.-F.; Lam, H.-M. Using RNA-seq data to evaluate reference genes suitable for gene expression studies in soybean. PLoS ONE 2015, 10, e01363432015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, W.J.; Zhang, J. Root signals and the regulation of growth and development of plants in drying soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Zhou, Q.; Wang, X.; Song, S.; Dong, S. Physiological response of soybean plants to water deficit. Front. Plant Sci. 2021, 12, 809692. [Google Scholar] [CrossRef]
- Banik, P.; Zeng, W.; Tai, H.; Bizimungu, B.; Tanino, K. Effects of drought acclimation on drought stress resistance in potato (Solanum tuberosum L.) genotypes. Environ. Exp. Bot. 2016, 126, 76–89. [Google Scholar] [CrossRef]
- Ristic, Z.; Jenks, M.A. Leaf cuticle and water loss in maize lines differing in dehydration avoidance. J. Plant Physiol. 2002, 159, 645–651. [Google Scholar] [CrossRef]
- Bacher, H.; Sharaby, Y.; Walia, H.; Peleg, Z. Modifying root-to-shoot ratio improves root water influxes in wheat under drought stress. J. Exp. Bot. 2022, 73, 1643–1654. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Mwenye, O.J.; Rensburg, L.V.; Biljon, A.V.; Merwe, R.V.D. Seedling Shoot and Root Growth Responses among Soybean (Glycine max) Genotypes to Drought Stress. In Soybean-Biomass, Yield and Productivity; Kasai, M., Ed.; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-78985-373-5. [Google Scholar]
- Liu, X.; Challabathula, D.; Quan, W.; Bartels, D. Transcriptional and metabolic changes in the desiccation tolerant plant Craterostigma plantagineum during recurrent exposures to dehydration. Planta 2019, 249, 1017–1035. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kang, K.; Gan, L.; Ning, S.; Xiong, J.; Song, S.; Xi, L.; Lai, S.; Yin, Y.; Gu, J.; et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol. J. 2019, 17, 2123–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Wang, X.; Tian, Y.; Zhang, D.; Zhang, L. The functional analysis of a wheat group 3 late embryogenesis abundant protein in Escherichia coli and Arabidopsis under abiotic stresses. Plant Signal. Behav. 2019, 14, 1667207. [Google Scholar] [CrossRef]
- Samtani, H.; Sharma, A.; Khurana, P. Overexpression of HVA1 Enhances Drought and Heat Stress Tolerance in Triticum aestivum Doubled Haploid Plants. Cells 2022, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.-Q.; Safdar, M.E.; Brestic, M.; Aziz, A.; Hayyat, M.S.; Asghar, M.A.; Wang, X.C.; et al. Drought Tolerance of Soybean (Glycine max L. Merr.) by Improved Photosynthetic Characteristics and an Efficient Antioxidant Enzyme Activities under a Split-Root System. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Andersen, M.N.; Jacobsen, S.-E.; Jensen, C.R. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ. Exp. Bot. 2005, 54, 33–40. [Google Scholar] [CrossRef]
- Jung, C.; Nguyen, N.H.; Cheong, J.-J. Transcriptional regulation of protein phosphatase 2C genes to modulate abscisic acid signaling. Int. J. Mol. Sci. 2020, 21, 9517. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhao, S.; Guo, Y. The PYR-PP2C-CKL2 module regulates ABA-mediated actin reorganization during stomatal closure. New Phytol. 2022, 233, 2168–2184. [Google Scholar] [CrossRef]
- Molinari, M.D.C.; Fuganti-Pagliarini, R.; Marin, S.R.R.; Ferreira, L.C.; de Barbosa, D.A.; Marcolino-Gomes, J.; Oliveira, M.C.N.D.; Mertz-Henning, L.M.; Kanamori, N.; Takasaki, H.; et al. Overexpression of AtNCED3 gene improved drought tolerance in soybean in greenhouse and field conditions. Genet. Mol. Biol. 2020, 43, e201902922020. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, W.; Qin, M.; Li, S.; Qiao, M.; Liu, Z.; Xiang, F. Drought Tolerance Conferred in Soybean (Glycine max. L.) by GmMYB84, a Novel R2R3-MYB Transcription Factor. Plant Cell Physiol. 2017, 58, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Soulages, J.L.; Kim, K.; Arrese, E.L.; Walters, C.; Cushman, J.C. Conformation of a group 2 late embryogenesis abundant protein from soybean. Evidence of poly (L-proline)-type II structure. Plant Physiol. 2003, 131, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.O.; Melo, B.P.; Lourenço-Tessutti, I.T.; Arraes, F.B.M.; Amorim, R.M.; Lisei-de-Sá, M.E.; Costa, J.A.; Leite, A.G.B.; Faheem, M.; Ferreira, M.A.; et al. Identification and characterization of the GmRD26 soybean promoter in response to abiotic stresses: Potential tool for biotechnological application. BMC Biotechnol. 2019, 19, 79. [Google Scholar] [CrossRef] [Green Version]
- Fraga, O.T.; Melo, B.P.D.; Quadros, I.P.S.; Reis, P.A.B.; Fontes, E.P.B. Senescence-Associated Glycine max (Gm)NAC Genes: Integration of Natural and Stress-Induced Leaf Senescence. Int. J. Mol. Sci. 2021, 22, 8287. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Ali, M.; Feng, X.; Li, X. The essence of NAC gene family to the cultivation of drought-resistant soybean (Glycine max L. Merr.) cultivars. BMC Plant Biol. 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]





| Exp. | Group | Shoot Weight | Root Weight | Shoot Length (SL) | Root Length (RL) | Node Number | RL to SL Ratio # |
|---|---|---|---|---|---|---|---|
| 1S | 0.246 ± 0.12 b | 0.074 ± 0.06 b | 47.5 ± 18.8 b | 13.1 ± 2.9 ab | 5.3 ± 0.9 b | 0.3 ± 0.2 b | |
| 1 | 2S | 0.236 ± 0.11 b | 0.094 ± 0.06 ab | 41.5 ± 18.7 b | 15.3 ± 4.9 a | 5.6 ± 0.8 b | 0.4 ± 0.2 a |
| US | 0.554 ± 0.29 a | 0.170 ± 0.14 a | 68.5 ± 27.9 a | 11.8 ± 2.1 b | 6.4 ± 1.5 a | 0.2 ± 0.1 c | |
| 1S | 0.310 ± 0.09 b | 0.090 ± 0.06 b | 53.7 ± 16.4 b | 14.2 ± 3.3 b | 5.9 ± 0.7 b | 0.3 ± 0.1 b | |
| 2 | 2S | 0.272 ± 0.12 b | 0.108 ± 0.06 b | 48.8 ± 15.7 b | 16.9 ± 3.2 a | 6.0 ± 0.9 b | 0.4 ± 0.2 a |
| US | 0.580 ± 0.13 a | 0.169 ± 0.08 a | 76.3 ± 14.3 a | 12.6 ± 2.0 c | 6.9 ± 0.5 a | 0.2 ± 0.03 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sintaha, M.; Man, C.-K.; Yung, W.-S.; Duan, S.; Li, M.-W.; Lam, H.-M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants 2022, 11, 2954. https://doi.org/10.3390/plants11212954
Sintaha M, Man C-K, Yung W-S, Duan S, Li M-W, Lam H-M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants. 2022; 11(21):2954. https://doi.org/10.3390/plants11212954
Chicago/Turabian StyleSintaha, Mariz, Chun-Kuen Man, Wai-Shing Yung, Shaowei Duan, Man-Wah Li, and Hon-Ming Lam. 2022. "Drought Stress Priming Improved the Drought Tolerance of Soybean" Plants 11, no. 21: 2954. https://doi.org/10.3390/plants11212954







