Decreased Salinity Offsets the Stimulation of Elevated pCO2 on Photosynthesis and Synergistically Inhibits the Growth of Juvenile Sporophyte of Saccharina japonica (Laminariaceae, Phaeophyta)
Abstract
1. Introduction
2. Results
2.1. Carbonate Parameters
2.2. Isolated and Interactive Effects of pCO2 and Salinity on Growth and Physiological Traits of S. japonica
2.2.1. Relative Growth Rate
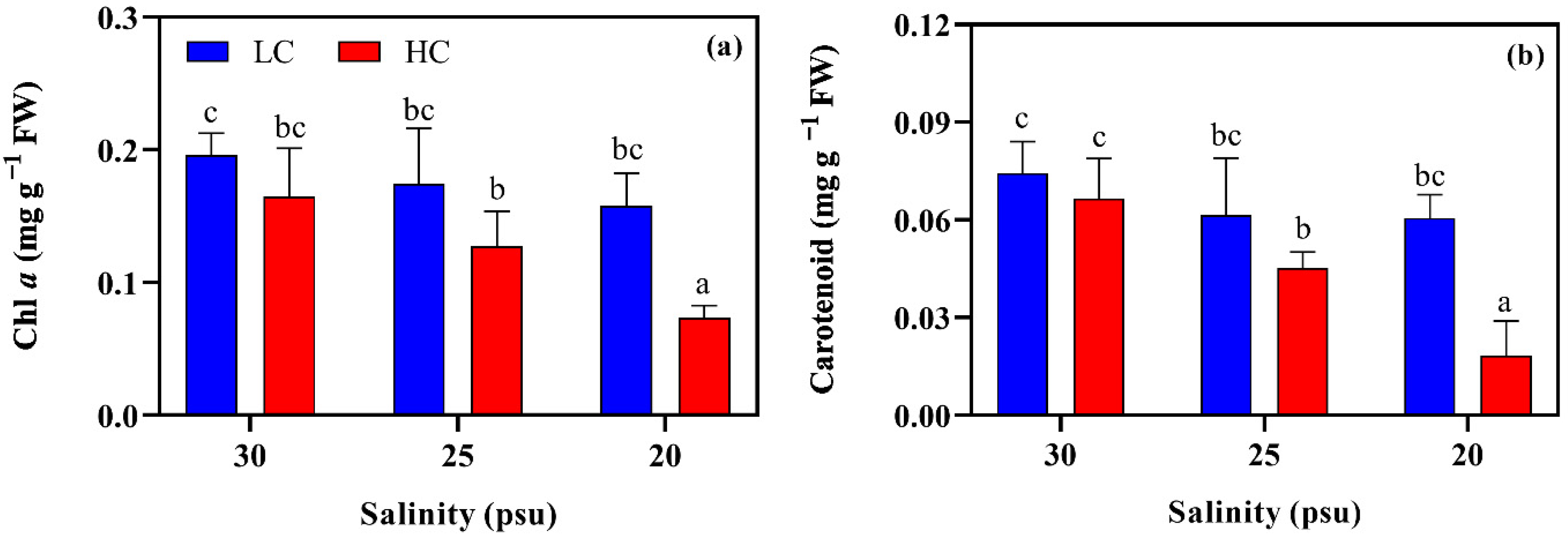
2.2.2. Pigment Contents
2.2.3. Photosynthetic Performance
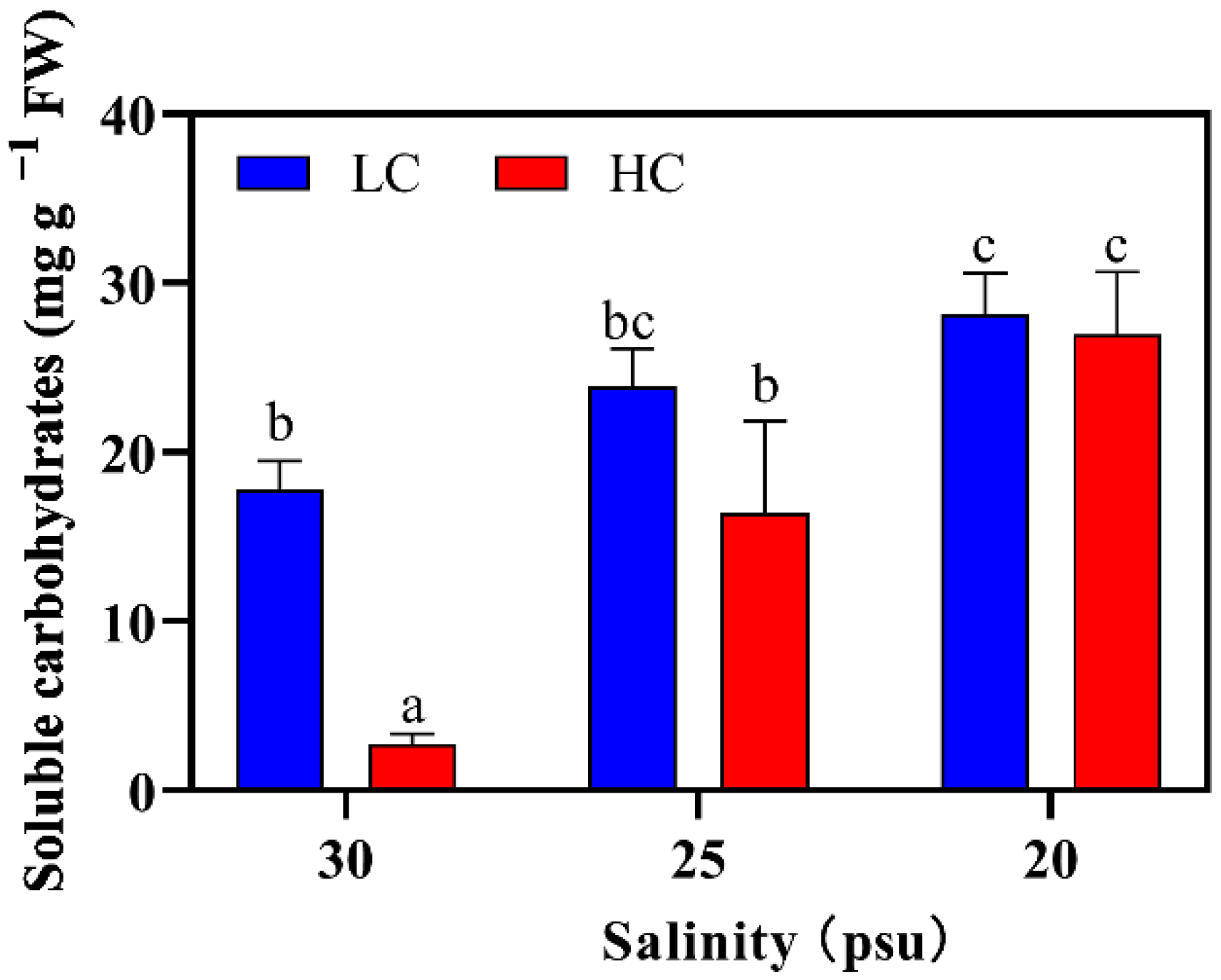
2.2.4. Soluble Carbohydrates Content
3. Discussion
4. Materials and Methods
4.1. Samples Collection and Maintenance
4.2. Experimental Design
4.3. Growth Rates
4.4. Chlorophyll Fluorescence
4.5. Biochemical Compositions
4.5.1. Pigments
4.5.2. Soluble Carbohydrates
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feely, R.; Doney, S.; Cooley, S. Ocean Acidification: Present Conditions and Future Changes in a High-CO2 World. Oceanography 2009, 22, 36–47. [Google Scholar] [CrossRef]
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, H.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide; The Royal Society: London, UK, 2005; ISBN 978-0-85403-617-2. [Google Scholar]
- Das, S.; Mangwani, N. Ocean Acidification and Marine Microorganisms: Responses and Consequences. Oceanologia 2015, 57, 349–361. [Google Scholar] [CrossRef]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Kinnby, A.; White, J.C.B.; Toth, G.B.; Pavia, H. Ocean Acidification Decreases Grazing Pressure but Alters Morphological Structure in a Dominant Coastal Seaweed. PLoS ONE 2021, 16, e0245017. [Google Scholar] [CrossRef]
- Evans, R.D.; Wilson, S.K.; Field, S.N.; Moore, J.A.Y. Importance of Macroalgal Fields as Coral Reef Fish Nursery Habitat in North-west Australia. Mar. Biol. 2014, 161, 599–607. [Google Scholar] [CrossRef]
- Fulton, C.J.; Berkström, C.; Wilson, S.K.; Abesamis, R.A.; Bradley, M.; Åkerlund, C.; Barrett, L.T.; Bucol, A.A.; Chacin, D.H.; Chong-Seng, K.M.; et al. Macroalgal Meadow Habitats Support Fish and Fisheries in Diverse Tropical Seascapes. Fish Fish. 2020, 21, 700–717. [Google Scholar] [CrossRef]
- Xiao, X.; Agustí, S.; Yu, Y.; Huang, Y.; Chen, W.; Hu, J.; Li, C.; Li, K.; Wei, F.; Lu, Y.; et al. Seaweed Farms Provide Refugia from Ocean Acidification. Sci. Total Environ. 2021, 776, 145192. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Y.; Li, J.; Gong, Q. Effects of Elevated PCO2 and Nutrient Enrichment on the Growth, Photosynthesis, and Biochemical Compositions of the Brown Alga Saccharina Japonica (Laminariaceae, Phaeophyta). PeerJ 2019, 7, e8040. [Google Scholar] [CrossRef]
- Xu, D.; Wang, D.; Li, B.; Fan, X.; Zhang, X.W.; Ye, N.H.; Wang, Y.; Mou, S.; Zhuang, Z. Effects of CO2 and Seawater Acidification on the Early Stages of Saccharina Japonica Development. Environ. Sci. Technol. 2015, 49, 3548–3556. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Guan, Z.; Wang, S.; Zhang, Y.; Wang, W.; Zhang, X.; Fan, X.; Li, F.; Ye, N. Elevated CO2 Concentrations Promote Growth and Photosynthesis of the Brown Alga Saccharina japonica. J. Appl. Phycol. 2020, 32, 1949–1959. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, E.J.; Edwards, M.S.; Lee, K.; Jeong, H.J.; Kim, K.Y. Species-Specific Responses of Temperate Macroalgae with Different Photosynthetic Strategies to Ocean Acidification: A Mesocosm Study. Algal Res. 2016, 31, 243–256. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, J.; Chu, Y.; Liu, Y.; Wang, Q.; Gong, Q.; Li, J. The Brown Algae Saccharina Japonica and Sargassum Horneri Exhibit Species-Specific Responses to Synergistic Stress of Ocean Acidification and Eutrophication. J. Ocean Univ. China 2021, 20, 1253–1262. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, G.; Xu, J.; Wu, H. Physiological Response of a Golden Tide Alga (Sargassum Muticum) to the Interaction of Ocean Acidification and Phosphorus Enrichment. Biogeochemistry 2016, 14, 671–681. [Google Scholar]
- Olischläger, M.; Bartsch, I.; Gutow, L.; Wiencke, C. Effects of Ocean Acidification on Different Life-Cycle Stages of the Kelp Laminaria Hyperborea (Phaeophyceae). Bot. Mar. 2012, 55, 511–525. [Google Scholar] [CrossRef]
- Barakat, K.M.; El-Sayed, H.S.; Khairy, H.M.; El-Sheikh, M.A.; Al-Rashed, S.A.; Arif, I.A.; Elshobary, M.E. Effects of Ocean Acidification on the Growth and Biochemical Composition of a Green Alga (Ulva Fasciata) and Its Associated Microbiota. Saudi J. Biol. Sci. 2021, 28, 5106–5114. [Google Scholar] [CrossRef]
- Gao, G.; Gao, Q.; Bao, M.; Xu, J.; Li, X. Nitrogen Availability Modulates the Effects of Ocean Acidification on Biomass Yield and Food Quality of a Marine Crop Pyropia Yezoensis. Food Chem. 2019, 271, 623–629. [Google Scholar] [CrossRef]
- Zou, D. Effects of Elevated Atmospheric CO2 on Growth, Photosynthesis and Nitrogen Metabolism in the Economic Brown Seaweed, Hizikia Fusiforme (Sargassaceae, Phaeophyta). Aquaculture 2005, 250, 726–735. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and Plant Responses to Salinity Stress: A Review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Xie, X.J.; Wang, X.L.; Lin, L.D.; He, L.W.; Gu, W.H.; Gao, S.; Yan, X.F.; Pan, G.H.; Wu, M.J.; Wang, G.C. Effects of Hypo- and Hypersalinity on Photosynthetic Performance of Sargassum Fusiforme (Fucales, Heterokontophyta). Photosynthetica 2016, 54, 210–218. [Google Scholar] [CrossRef]
- Takolander, A.; Leskinen, E.; Cabeza, M. Synergistic Effects of Extreme Temperature and Low Salinity on Foundational Macroalga Fucus Vesiculosus in the Northern Baltic Sea. J. Exp. Mar. Biol. Ecol. 2017, 495, 110–118. [Google Scholar] [CrossRef]
- Gao, G.; Qu, L.; Xu, T.; Burgess, J.G.; Li, X.; Xu, J. Future CO2-Induced Ocean Acidification Enhances Resilience of a Green Tide Alga to Low-Salinity Stress. ICES J. Mar. Sci. 2019, 76, 2437–2445. [Google Scholar] [CrossRef]
- Harris, C.M.; McClelland, J.W.; Connelly, T.L.; Crump, B.C.; Dunton, K.H. Salinity and Temperature Regimes in Eastern Alaskan Beaufort Sea Lagoons in Relation to Source Water Contributions. Estuaries Coasts 2017, 40, 50–62. [Google Scholar] [CrossRef]
- Shindo, A.; Nishihara, G.N.; Borlongan, I.A.; Terada, R. The Effects of Desiccation and Salinity Gradients in the PSII Photochemical Efficiency of a Subtidal Brown Alga, Saccharina Japonica (Laminariales) from Hokkaido, Japan. Phycol. Res. 2022, 70, 89–96. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, D.; Xu, X.T.; Gao, X.X.; Sun, X.; Xu, N.J. Physiological Responses of a Green Algae (Ulva Prolifera) Exposed to Simulated Acid Rain and Decreased Salinity. Photosynthetica 2017, 55, 623–629. [Google Scholar] [CrossRef]
- Van Ginneken, V. Some Mechanism Seaweeds Employ to Cope with Salinity Stress in the Harsh Euhaline Oceanic Environment. Am. J. Plant Sci. 2018, 9, 1191–1211. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Beardall, J.; Gao, K. Lower Salinity Leads to Improved Physiological Performance in the Coccolithophorid Emiliana Huxleyi, Which Partly Ameliorates the Effects of Ocean Acidification. Front. Mar. Sci. 2020, 7, 704. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Wang, Y.; Xu, D.; Zhang, J.; Ye, N. Exploring Core Response Mechanisms to Multiple Environmental Stressors Via A Genome-Wide Study in the Brown Alga Saccharina Japonica (Laminariales, Phaeophyceae). J. Phycol. 2021, 57, 345–354. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate Production from Marine Macroalgae, with Emphasis on Kelp Farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2018; Volume 11, pp. 27–66. ISBN 978-981-10-6909-3. [Google Scholar]
- Siahaan, E.A.; Pendleton, P.; Woo, H.-C.; Chun, B.-S. Brown Seaweed (Saccharina Japonica) as an Edible Natural Delivery Matrix for Allyl Isothiocyanate Inhibiting Food-Borne Bacteria. Food Chem. 2014, 152, 11–17. [Google Scholar] [CrossRef]
- Borlongan, I.A.; Arita, R.; Nishihara, G.N.; Terada, R. The Effects of Temperature and Irradiance on the Photosynthesis of Two Heteromorphic Life History Stages of Saccharina Japonica (Laminariales) from Japan. J. Appl. Phycol. 2020, 32, 4175–4187. [Google Scholar] [CrossRef]
- Shindo, A.; Borlongan, I.A.; Nishihara, G.N.; Terada, R. Interactive Effects of Temperature and Irradiance Including Spectral Light Quality on the Photosynthesis of a Brown Alga Saccharina Japonica (Laminariales) from Hokkaido, Japan. Algal Res. 2022, 66, 102777. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Han, W.; Wang, Y.; Fan, X.; Loladze, I.; Gao, G.; Zhang, Y.; Tong, S.; Ye, N. Elevated CO2 Affects Kelp Nutrient Quality: A Case Study of Saccharina Japonica from CO2-enriched Coastal Mesocosm Systems. J. Phycol. 2021, 57, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.F.; Bonsell, C.; Dunton, K.H. Inherent Tolerance of Extreme Seasonal Variability in Light and Salinity in an Arctic Endemic Kelp (Laminaria Solidungula). J. Phycol. 2021, 57, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.S. Ocean Acidification Induced Changes in Ulva Fasciata Biochemistry May Improve Dicentrarchus Labrax Aquaculture via Enhanced Antimicrobial Activity. Aquaculture 2022, 560, 738474. [Google Scholar] [CrossRef]
- Shi, X.; Zou, D.; Hu, S.; Mai, G.; Ma, Z.; Li, G. Photosynthetic Characteristics of Three Cohabitated Macroalgae in the Daya Bay, and Their Responses to Temperature Rises. Plants 2021, 10, 2441. [Google Scholar] [CrossRef]
- Kirst, G.O. Salinity Tolerance of Eukaryotic Marine Algae. Annu. Rev. Plant Biol. 1990, 41, 21–53. [Google Scholar] [CrossRef]
- Benjamina, K.J.; Walker, D.I.; McComb, A.J.; Kuo, J. Structural Response of Marine and Estuarine Plants of Halophila Ovalis (R. Br.) Hook. f. to Long-Term Hyposalinity. Aquat. Bot. 1999, 64, 1–17. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, Y.; Xu, K.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Investigating the Mechanisms Underlying the Hyposaline Tolerance of Intertidal Seaweed, Pyropia Haitanensis. Algal Res. 2020, 47, 101886. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Luo, M.B.; Liu, F. Salinity-Induced Oxidative Stress and Regulation of Antioxidant Defense System in the Marine Macroalga Ulva Prolifera. J. Exp. Mar. Biol. Ecol. 2011, 409, 223–228. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae Cultivation and Metabolites Production: A Comprehensive Review. Biofuels Bioprod. Bioref. 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Y.; Li, J.; Wang, Q.; Gong, Q. Nutrient Enrichment Regulates the Growth and Physiological Responses of Saccharina Japonica to Ocean Acidification. J. Ocean Univ. China 2020, 19, 895–901. [Google Scholar] [CrossRef]
- Kang, J.W.; Chung, I.K. The Interactive Effects of Elevated CO2 and Ammonium Enrichment on the Physiological Performances of Saccharina Japonica (Laminariales, Phaeophyta). Ocean Sci. J. 2018, 53, 487–497. [Google Scholar] [CrossRef]
- Ordoñez, A.; Wangpraseurt, D.; Lyndby, N.H.; Kühl, M.; Diaz-Pulido, G. Elevated CO2 Leads to Enhanced Photosynthesis but Decreased Growth in Early Life Stages of Reef Building Coralline Algae. Front. Mar. Sci. 2019, 5, 495. [Google Scholar] [CrossRef]
- Liu, T.; Chen, J.A.; Wang, W.; Simon, M.; Wu, F.; Hu, W.; Chen, J.B.; Zheng, H. A Combined Proteomic and Transcriptomic Analysis on Sulfur Metabolism Pathways of Arabidopsis Thaliana under Simulated Acid Rain. PLoS ONE 2014, 9, e90120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zou, D.; Lou, W.; Deng, Y.; Zeng, X. Effects of Seawater Acidification and Alkalization on the Farmed Seaweed, Pyropia Haitanensis (Bangiales, Rhodophyta), Grown under Different Irradiance Conditions. Algal Res. 2018, 31, 413–420. [Google Scholar] [CrossRef]
- Xu, K.; Chen, H.; Wang, W.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Responses of Photosynthesis and CO2 Concentrating Mechanisms of Marine Crop Pyropia Haitanensis Thalli to Large PH Variations at Different Time Scales. Algal Res. 2017, 28, 200–210. [Google Scholar] [CrossRef]
- Iñiguez, C.; Carmona, R.; Lorenzo, M.R.; Niell, F.X.; Wiencke, C.; Gordillo, F.J.L. Increased CO2 Modifies the Carbon Balance and the Photosynthetic Yield of Two Common Arctic Brown Seaweeds: Desmarestia Aculeata and Alaria Esculenta. Polar Biol. 2016, 39, 1979–1991. [Google Scholar] [CrossRef]
- Iñiguez, C.; Heinrich, S.; Harms, L.; Gordillo, F.J.L. Increased Temperature and CO2 Alleviate Photoinhibition in Desmarestia Anceps: From Transcriptomics to Carbon Utilization. J. Exp. Bot. 2017, 68, 3971–3984. [Google Scholar] [CrossRef]
- Caron, L.; Douady, D.; Martino, A.D.; Quinet, M. Light Harvesting in Brown Algae. Cah. De Biol. Mar. 2001, 42, 109–124. [Google Scholar]
- Halsey, K.H.; Jones, B.M. Phytoplankton Strategies for Photosynthetic Energy Allocation. Annu. Rev. Mar. Sci. 2015, 7, 265–297. [Google Scholar] [CrossRef] [PubMed]
- Takolander, A.; Cabeza, M.; Leskinen, E. Seasonal Interactive Effects of PCO2 and Irradiance on the Ecophysiology of Brown Macroalga Fucus Vesiculosus L. Eur. J. Phycol. 2019, 54, 380–392. [Google Scholar] [CrossRef]
- Li, S.F.; Fanesi, A.; Martin, T.; Lopes, F. Biomass Production and Physiology of Chlorella Vulgaris during the Early Stages of Immobilized State Are Affected by Light Intensity and Inoculum Cell Density. Algal Res. 2021, 59, 102453. [Google Scholar] [CrossRef]
- Xu, J.; Fan, X.; Li, X.; Liu, G.; Zhang, Z.; Zhu, Y.; Fu, Z.; Qian, H. Effect of Salicylic Acid on Fatty Acid Accumulation in Phaeodactylum Tricornutum during Stationary Growth Phase. J. Appl. Phycol. 2017, 29, 2801–2810. [Google Scholar] [CrossRef]
- Garcia-Mendoza, E.; Ocampo-Alvarez, H. Govindjee Photoprotection in the Brown Alga Macrocystis Pyrifera: Evolutionary Implications. J. Photochem. Photobiol. B Biol. 2011, 104, 377–385. [Google Scholar] [CrossRef]
- Zhang, D.; Beer, S.; Li, H.; Gao, K. Photosystems I and II in Ulva Lactuca Are Well Protected from High Incident Sunlight. Algal Res. 2020, 52, 102094. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate Metabolism and Respiration in Algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2003; Volume 14, pp. 205–224. ISBN 978-94-010-3772-3. [Google Scholar]
- Graiff, A.; Karsten, U. Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus Vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment. Biology 2021, 10, 1330. [Google Scholar] [CrossRef]
- Evans, L.V.; Simpson, M.; Callow, M.E. Sulphated Polysaccharide Synthesis in Brown Algae. Planta 1973, 110, 237–252. [Google Scholar] [CrossRef]
- Cesário, M.T.; da Fonseca, M.M.R.; Marques, M.M.; de Almeida, M.C.M.D. Marine Algal Carbohydrates as Carbon Sources for the Production of Biochemicals and Biomaterials. Biotechnol. Adv. 2018, 36, 798–817. [Google Scholar] [CrossRef]
- Borburema, H.D.S.; Graiff, A.; Karsten, U.; Marinho-Soriano, E. Photobiological and Biochemical Responses of Mangrove-Associated Red Macroalgae Bostrychia Calliptera and Bostrychia Montagnei to Short-Term Salinity Stress Related to Climate Change. Hydrobiologia 2022, 9, 989454. [Google Scholar] [CrossRef]
- Floreto, E.A.T.; Teshima, S. The Fatty Acid Composition of Seaweeds Exposed to Different Levels of Light Intensity and Salinity. Bot. Mar. 1998, 41, 467–482. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L. Microalgae for Use in Tropical Aquaculture II: Effect of Salinity on Growth, Gross Chemical Composition and Fatty Acid Composition of Three Species of Marine Microalgae. J. Appl. Phycol. 1994, 6, 347–356. [Google Scholar] [CrossRef]
- Tatewaki, M. Formation of a Crustose Sporophyte with Unilocular Sporangia in Scitosiphon Lomentaria. Phycologia 1966, 6, 62–66. [Google Scholar] [CrossRef]
- Lewis, E.R.; Wallace, D.W.R. Program Developed for CO2 System Calculations; United States Department of Energy: Washington, DC, USA, 1998.
- Webb, W.L.; Newton, M.; Starr, D. Carbon Dioxide Exchange of Alnus Rubra. Oecologia 1974, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]



| Treatment | pCO2 (μatm) | pH | HCO3− (μmol kg−1) | CO32− (μmol kg−1) | CO2 (μmol kg−1) | DIC (μmol kg−1) | TA (μmol kg−1) |
|---|---|---|---|---|---|---|---|
| LC-30 | 400 | 7.84 ± 0.01 f | 2060 ± 13 d | 76.5 ± 1.1 e | 30.6 ± 0.4 a | 2167 ± 14 e | 2249 ± 14 c |
| LC-25 | 7.80 ± 0.00 e | 1798 ± 15 b | 52.6 ± 0.4 d | 31.5 ± 0.3 ab | 1882 ± 15 c | 1928 ± 15 b | |
| LC-20 | 7.72 ± 0.03 d | 1568 ± 23 a | 33.1 ± 1.6 b | 35.4 ± 2.4 b | 1636 ± 23 a | 1650 ± 20 a | |
| HC-30 | 1000 | 7.62 ± 0.01 c | 2123 ± 10 e | 46.7 ± 0.4 c | 53.2 ± 0.9 c | 2223 ± 10 f | 2238 ± 9 c |
| HC-25 | 7.55 ± 0.01 b | 1857 ± 22 c | 30.8 ± 0.5 b | 57.4 ± 2.2 d | 1945 ± 23 d | 1934 ± 20 b | |
| HC-20 | 7.48 ± 0.01 a | 1609 ± 18 a | 19.5 ± 0.1 a | 63.0 ± 1.4 e | 1691 ± 19 b | 1657 ± 18 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Shi, Y.; He, L.; Chen, X.; Hu, F.; Chen, Y.; Pang, Y.; Li, S.; Chu, Y. Decreased Salinity Offsets the Stimulation of Elevated pCO2 on Photosynthesis and Synergistically Inhibits the Growth of Juvenile Sporophyte of Saccharina japonica (Laminariaceae, Phaeophyta). Plants 2022, 11, 2978. https://doi.org/10.3390/plants11212978
Zhang W, Shi Y, He L, Chen X, Hu F, Chen Y, Pang Y, Li S, Chu Y. Decreased Salinity Offsets the Stimulation of Elevated pCO2 on Photosynthesis and Synergistically Inhibits the Growth of Juvenile Sporophyte of Saccharina japonica (Laminariaceae, Phaeophyta). Plants. 2022; 11(21):2978. https://doi.org/10.3390/plants11212978
Chicago/Turabian StyleZhang, Wenze, Yunyun Shi, Lianghua He, Xinhua Chen, Fengxiao Hu, Yinrong Chen, Yun Pang, Sufang Li, and Yaoyao Chu. 2022. "Decreased Salinity Offsets the Stimulation of Elevated pCO2 on Photosynthesis and Synergistically Inhibits the Growth of Juvenile Sporophyte of Saccharina japonica (Laminariaceae, Phaeophyta)" Plants 11, no. 21: 2978. https://doi.org/10.3390/plants11212978
APA StyleZhang, W., Shi, Y., He, L., Chen, X., Hu, F., Chen, Y., Pang, Y., Li, S., & Chu, Y. (2022). Decreased Salinity Offsets the Stimulation of Elevated pCO2 on Photosynthesis and Synergistically Inhibits the Growth of Juvenile Sporophyte of Saccharina japonica (Laminariaceae, Phaeophyta). Plants, 11(21), 2978. https://doi.org/10.3390/plants11212978






