Abstract
The aim of this study is to compare the functions of the physiologically active compounds of three types of mulberry leaf by cultivar, and to confirm the changes using hot-melt extrusion (HME−ML). The active components of mulberry leaf were analyzed using the HPLC system, and total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity were measured. Among the three varieties, the highest contents of rutin and isoquercetin were detected in Cheongil, of TPC in Cheongol, and of TFC in Cheongil. It was confirmed that this bio-accessibility was increased in HME−ML compared with the control. The DPPH radical scavenging activity of Cheongol showed greater antioxidant properties, and HME showed improvement in the antioxidant properties of all mulberry leaves. These results suggest that the application of HME technology can improve the biological activities of mulberry leaf.
1. Introduction
Mulberry leaf (Morus alba L.) has been used in traditional medicine and is an edible plant used to grow silkworms [1]. It is currently used to make diet foods or to develop products, such as fortified beverages, yogurts, and teas [2]. Mulberry leaf is used in traditional Chinese medicine to treat diabetes and hyperlipidemia and contains secondary metabolites, such as flavonoids, organic acids, alkaloids (1-deoxynojirimycin, fagomine), and polyphenols [3,4,5]. Mulberry leaf includes the effects of lowering blood sugar, antioxidant, anti-inflammatory, high-density lipoprotein–cholesterol increase, and low-density lipoprotein–cholesterol reduction [6,7,8]. A previous study found that the functional components of mulberry leaves differ between varieties [9]. Therefore, it is important to select suitable mulberry leaves through activity studies according to variety.
The active components of the mulberry leaf include rutin, isoquercetin, and quercetin [4,10]. Isoquercetin and rutin are flavonoids, which are a family of polyphenolic compounds. The content difference between total phenol content (TPC) and total flavonoid content (TFC) may be caused by growth environment, contamination, and pathogens [11,12]. The water solubility of rutin and isoquercetin was 0.125 g/L and 0.095 g/L. Although many biological activities have been revealed due to poor water solubility, they have the disadvantage of showing low bioavailability and absorption when applied to food development [13,14].
Hot-melt extrusion (HME) has proven to be a successful technique in drug delivery systems (DDS) and several applications through properties such as the improved solubility of poorly soluble compounds, targeting, drug delivery, and the preparation of nanoparticles [15]. HME is widely used in the food industry and pharmaceuticals. HME converts a mixture into having certain properties, such as uniform shape and density, by forcing the mixture through a die [16]. The principle of HME is to induce melting and further solubilization and fusion by applying heat and friction (interparticle friction, sample and wall friction, sample, and screw friction) to the mixture [17]. The advantage of HME is that it is an eco-friendly technology that does not use organic solvents, improves the dispersal of the drug and the solubility of the poorly soluble drugs, and increases bioavailability. However, the disadvantage of HME is that it requires high temperature and must be stable under thermal decomposition [18]. Charunuch et al. compared antioxidant activity and TPC according to the various conditions of HME by mixing mulberry leaf and instant cereal beverage powder. As the content of mulberry leaf in the mixture increased, antioxidant activity and TPC increased [19].
The HME increases solubility by producing a stable amorphous solid dispersion and increased energy form through a combination of processing and excipients [20]. Whey protein isolate (WPI) is receiving attention as a food biopolymer in the food industry, and heat treatment above 60 degrees causes the denaturation of WPI, exposing many hydrophobic functional groups on the surface of protein particles [21]. Among natural emulsifiers, lecithin is an amphiphilic surfactant that can bind proteins through hydrophobic interactions [22]. Lecithin, used as a surfactant, improves the solubility of active ingredients by improving solubility, and is also used as a plasticizer for the matrix [23]. During the HME process, the addition of plasticizer could decrease processing temperature and achieve higher dispersion [24,25]. Citric acid was used as a pH adjuster to control drug release in hydroxypropyl methylcellulose (HPMC) matrix tablets [26] and was used as a solid-state plasticizer when preparing a solid dispersion through HME [27,28,29]. Ascorbyl palmitate, an amphiphilic synthetic derivative of ascorbic acid, is used as a natural antioxidant in the food industry [30,31] and can be used as an emulsifier [32]. In HME studies with Moringa oleifera Lam, ascorbyl palmitate was used as a plasticizer [33]. Vitamin C is widely used in food as a naturally occurring antioxidant and has been used as a plasticizer for biodegradable polymers [34]. Ascorbyl palmitate, Vitamin E, and WPI were mixed and coated to protect peanuts from lipid oxidation [35].
The purpose of the study was to improve the active compound water solubility of three mulberry leaf varieties through the HME. The mulberry leaf and HME-mulberry leaf (HME−ML) were compared through high-performance liquid chromatography (HPLC), antioxidant, and TPC and TFC analysis.
2. Materials and Methods
2.1. Materials
The materials used in this study are three types of mulberry leaf grown in Korea, namely the Cheongol, Iksu, and Cheongil varieties. To avoid any effect from pedoclimatic factors, the three varieties were grown and collected at the same place, the National Institute of Agricultural Sciences, (Wanju, Korea) in early May. Acetic acid was purchased from Duchefa (Haarlem, The Netherlands). Acetonitrile (ACN) was purchased from Fisher (A9984, Waltham, MA, USA). Folin–Ciocalteu’s phenol reagent (F9252), gallic acid (G7384), and quercetin (Q4951) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Sodium carbonate was purchased from Daejung (7541-3300, Siheung, Korea). Potassium acetate was purchased from TCI (P2786, Tokyo, Japan). Aluminum chloride hexahydrate, 2,2-diphenyl-1-picrylhydrazyl (DPPH) (044150), and L-ascorbic acid (011188) were purchased from Alfa Aesar (Ward Hill, MA, USA).
2.2. Extraction Method
The weights of the mulberry leaf and HME−ML were all calibrated to 1 g (100%) and weighed. Deionized water (D.W.; 50 mL) was used as an extraction solvent. An ultrasonic cleaner (UCP-20, JeioTech Co., Ltd., Daejeon, Korea) was used for ultrasonic extraction (40 °C, 30 min). The extract was centrifuged at 3000 rpm, and the supernatant was filtered by Whatman filter paper No. 6 (Cytiva, Marlborough, MA, USA) and recovered by rotary pressure concentration (Eyela Co., Ltd., Tokyo, Japan).
2.3. Preparation of HME-ML
After mixing the mulberry leaf and each additive, the HME process (STS-25HS tween screw extruder, Hankook E.M. Ltd., Pyoung-Taek, Korea) was performed. As additives, whey protein isolate, soy lecithin, vitamin C, vitamin E 50%, citric acid, and ascorbyl palmitate were used, after mixing in predetermined ratios (Table 1). The ratio of additives in HME processing was carried out under the same conditions to compare the activity according to the varieties of mulberry leaves. The processing temperature was fixed at 100 °C. The conditions of HME process were 40~50 bar of pressure and 50 rpm of screw speed. The HME-ML was used for subsequent experiments after drying.

Table 1.
The composition of HME−ML.
2.4. HPLC Analysis
The mulberry leaf was extracted with distilled water to prepare 1 mg/mL and analyzed. The calibration curve was prepared with rutin and isoquercetin (Sigma–Aldrich Co., St. Louis, MO, USA). The instrument used for HPLC analysis was a Simadzu LC-20AT HPLC system (Tokyo, Japan). Samples were filtered with a syringe filter (0.45 µm) before analysis. Water containing 0.5% acetic acid and acetonitrile (ACN) was used as a solvent. It proceeded according to the analysis conditions (Table 2).

Table 2.
The HPLC analysis conditions.
2.5. Total Phenolic Content (TPC)
TPC was tested using the Folin–Denis method [36]. The concentration of the sample was 1 mg/mL, and in 20 µL of the sample, 100 µL of Folin–Ciocalteu’s phenol reagent and 80 µL of 7.5% sodium carbonate were added. Absorbance was measured at 760 nm using a microplate reader (Epoch, Agilent Technologies Inc., Santa Clara, CA, USA) at room temperature (RT) for 45 min in the dark. The standard curve was prepared at 20, 40, 60, 80, and 100 µg/mL using gallic acid, respectively, and a calibration curve was then prepared.
2.6. Total Flavonoid Content (TFC)
The TFC was tested using the Dowd method [37]. The concentration of the sample was 1 mg/mL, and after adding 60 µL of 95% ethanol, 4 µL of 10% aluminum chloride hexahydrate, 4 µL of 1 M potassium acetate, and 112 µL of D.W. to 20 µL of the sample, it was reacted at RT for 40 min. Absorbance was measured at 415 nm using a microplate reader. The standard curve was prepared at 20, 40, 60, 80, and 100 µg/mL using quercetin, respectively, and a calibration curve was then prepared.
2.7. Antioxidant Activity
Antioxidant activity was tested using 2,2-diphenyl-1-picrylhydrazyl (DPPH) [38]. Samples were prepared at 0, 1000, 2000, 3000, 4000, and 5000 µg/mL, and then 150 µL of 0.4 mM DPPH solution that was prepared in methanol was added to 5 µL of each sample, followed by reaction in the dark at RT for 30 min, and the absorbance was measured at 517 nm. L-ascorbic acid was used as a control, and a comparative experiment was performed after preparation at 0, 20, 40, 60, 80, and 100 µg/mL. DPPH radical scavenging activity was measured by IC50 (µg/mL), which is a concentration that reduces DPPH radical by 50%. The inhibition rate of the mulberry leaf was calculated using the following equation:
2.8. Statistical Processing
The results of repeated HPLC, TPC, TFC, and DPPH radical scavenging activity experiments using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) were expressed as the mean ± standard deviation. Significant differences between samples were tested for significance at the Duncan’s Multiple Range Test (DMRT) 5% level (p < 0.05).
3. Results and Discussion
3.1. Analysis of Isoquercetin and Rutin Contents of Mulberry Leaf and HME-ML
The isoquercetin and rutin content of mulberry leaf and HME−ML before and after HME showed different results in Figure 1. Cheongil had the highest isoquercetin content, and it was confirmed that there was a difference of about two times compared to other varieties. After HME processing, isoquercetin content was increased in all varieties, and HME−Cheongil showed the highest content in the HME formulation. The rutin content was the highest in Cheongil, and after HME processing, the rutin content was increased in all varieties, and HME−Cheongil showed the highest content in the HME formulation.
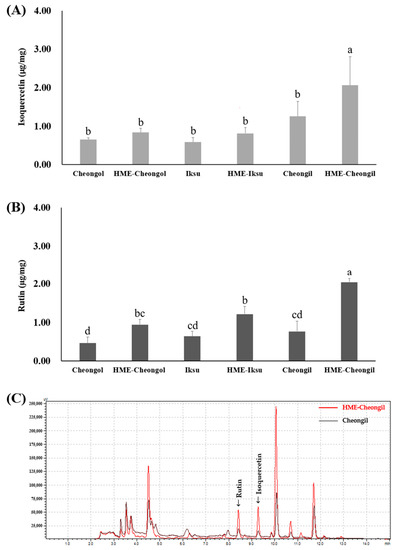
Figure 1.
(A) Isoquercetin and (B) rutin contents of mulberry leaf and HME-ML extract; and (C) HPLC chromatogram of rutin and isoquercetin in Cheongil and HME-Cheongil extract. Data are expressed as mean ± standard deviation (n = 3). Means with different letters vary significantly with Duncan’s Multiple Range Test (DMRT) at 5% level (p < 0.05) within samples.
The rutin content of mulberry leaf extracted using methanol was reported to be the highest in Cheongol and the lowest in Choengil [39]. Rutin and isoquercetin are generally poorly soluble in water [14,40], and HME increases the solubility of liposoluble components [41,42,43]. Park et al. confirmed the increased rutin content through HME processing of buckwheat flour, and the rutin content increased as the mixed yeast content increased [44]. When lecithin and vitamin E were added in the HME processing of buckwheat and potato, the content of rutin was increased. In addition to rutin, the increase in phenol and flavonoid contents may be due to the encapsulation of active components through lecithin liposome formation [45]. The results show that through the HME process, the solubility of liposoluble components, rutin, and isoquercetin increased in water, resulting in an enhanced amount of hydrophobic active compounds.
3.2. Total Phenolic Content
The TPC results are presented using gallic acid as a standard. Among the three mulberry leaf varieties, Cheongol had the highest TPC content. In all mulberry leaf varieties, TPC was higher after HME, and HME−Cheongol showed the highest TPC content of 31.14 ± 4.63 mg/g (Table 3). High temperature and shear forces applied to phenolic compounds can form amorphous structures and lead to increased solubility through the destruction of cellular components [46]. An increase in TPC by HME was also reported in another study [45]. It was confirmed that for all mulberry leaf varieties, TPC was higher after extrusion than before extrusion. This suggests that when HME is applied to mulberry leaf, TPC is increased. This result could be explained as the increased water solubility of rutin and isoquercetin correlating with the total amount of phenolic content.

Table 3.
Total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity by DPPH of mulberry leaf extract.
3.3. Total Flavonoid Content
The TFC results are presented using quercetin as a standard. Among the three mulberry leaf varieties, Cheongil had the highest TFC content, and in HME−ML, HME−Iksu showed the highest TFC content of 22.12 ± 3.9 mg/g (Table 3). It was confirmed that for all mulberry leaf varieties, TFC was higher after extrusion than before extrusion. This suggests that when HME is applied to mulberry leaf, TFC is increased. Wang et al. suggested that the total flavonoid solubility of Ginkgo biloba extract was increased through HME and could increase oral bioavailability [47].
3.4. Antioxidant Activity
The antioxidant activity of mulberry leaf extracts was compared by DPPH radical scavenging activity (Table 3). Among the three mulberry leaf varieties, Cheongol had the lowest IC50, and in HME−ML, HME−Cheongol showed the lowest IC50 of 4480.83 ± 35.63 µg/mL. HME−ML had a higher IC50 value than ascorbic acid, but all mulberry leaf varieties showed a lower IC50 value than non-HME−ML. The DPPH radical scavenging activity was increased after HME processing in all mulberry leaf varieties, and it was confirmed that the inhibition rate was increased in a concentration-dependent manner. This suggests that the application of HME to mulberry leaf increases antioxidant activity. Among the natural antioxidants, phenolic compounds, which are secondary metabolites produced by plants, are contained the most. The TFC and TPC in mulberry leaf have been found to have a significant effect on antioxidant activity [12]. Phaseolus bulgaris L. shows a difference in antioxidant activity depending on the cultivar, and the free radical scavenging activity was faster in the extrudate extract [48].
4. Conclusions
In this study, HME with proper additives enabled improved water solubility of rutin and isoquercetin. The isoquercetin content of the three kinds of the mulberry leaf was high in the order Cheongil > Cheongol > Iksu, and it was confirmed that the rutin content was high in the order Cheongil > Iksu > Cheongol. Through HME, it was confirmed that the content of isoquercetin and rutin in all mulberry leaves was increased, and through this, it was confirmed that the solubility of the active ingredient, which showed low water solubility, was improved. TPC was the highest in the order Cheongol > Iksu > Cheongil, and TFC was the highest in the order Cheongil > Cheongol > Iksu. In antioxidant activity using DPPH assay, Cheongol showed the lowest IC50 value in the order Cheongol < Iksu < Cheongil, and after HME processing, all mulberry leaves showed a decrease in IC50 value. HME-treated Cheongil was found to have the highest contents of rutin and isoquercetin. Moreover, it showed the highest antioxidant activity in the DPPH assay, suggesting that the correlation between the active compound and antioxidant activity contributed to the improvement of antioxidant capacity. The results provide information that different mulberry leaf varieties may be used for different purposes in the future. Furthermore, this suggests that HME can be used as a candidate to enhance the biological activities of the mulberry leaf.
Author Contributions
Conceptualization, H.-B.K. and S.R.; methodology, H.-B.K. and S.R.; formal analysis, H.-B.K. and S.R.; writing—original draft preparation, H.-B.K. and S.R.; writing—review and editing, J.-S.B.; project administration, H.-B.K. and J.-S.B.; funding acquisition, H.-B.K. and J.-S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant (PJ015570012022) from the National Institute of Agricultural Sciences, Rural Development Administration, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Liu, J.; Liao, S.; Zou, Y. Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients 2019, 11, 1017. [Google Scholar] [CrossRef]
- Wen, L.; Shi, D.; Zhou, T.; Tu, J.; He, M.; Jiang, Y.; Yang, B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020, 315, 126236. [Google Scholar] [CrossRef]
- Hao, J.Y.; Wan, Y.; Yao, X.H.; Zhao, W.G.; Hu, R.Z.; Chen, C.; Li, L.; Zhang, D.-Y.; Wu, G.-H. Effect of different planting areas on the chemical compositions and hypoglycemic and antioxidant activities of mulberry leaf extracts in Southern China. PLoS ONE 2018, 13, e0198072. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Wan, Y.; Hao, J.-Y.; Hu, R.-Z.; Chen, C.; Yao, X.-H.; Zhao, W.-G.; Liu, Z.-Y.; Li, L. Evaluation of the alkaloid, polyphenols, and antioxidant contents of various mulberry cultivars from different planting areas in eastern China. Ind. Crops Prod. 2018, 122, 298–307. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Tassotti, M.; del Rio, D.; Hernández, F.; Martínez, J.J.; Mena, P. (Poly)phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC-MS approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Ying, C.; Liu, L.; Lou, C. Effects of mulberry leaf on experimental hyperlipidemia rats induced by high-fat diet. Exp. Ther. Med. 2018, 16, 547–556. [Google Scholar] [CrossRef]
- Tu, J.; Shi, D.; Wen, L.; Jiang, Y.; Zhao, Y.; Yang, J.; Liu, H.; Liu, G.; Yang, B. Identification of moracin N in mulberry leaf and evaluation of antioxidant activity. Food Chem. Toxicol. 2019, 13, 110730. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, W.; Gu, Y.; Yu, S. 1-Deoxynojirimycin in Mulberry (Morus indica L.) Leaves Ameliorates Stable Angina Pectoris in Patients with Coronary Heart Disease by Improving Antioxidant and Anti-inflammatory Capacities. Front. Pharmacol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Chae, J.Y.; Lee, J.Y.; Hoang, I.S.; Whangbo, D.; Choi, P.W.; Lee, W.C.; Kim, J.-W.; Kim, S.-Y.; Choi, S.-W.; Rhee, S.-J. Analysis of Functional Components of Leaves of Different Mulberry Cultivars. J. Korean Soc. Food Sci. Nutr. 2003, 32, 15–21. [Google Scholar] [CrossRef][Green Version]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Zhu, M.; Zhao, W.; Li, F.; Zou, Y.; Yang, L. Chemical composition, nutritional value, and antioxidant activities of eight mulberry cultivars from China. Pharmacogn. Mag. 2012, 8, 215–224. [Google Scholar] [CrossRef]
- He, X.; Chen, X.; Ou, X.; Ma, L.; Xu, W.; Huang, K. Evaluation of flavonoid and polyphenol constituents in mulberry leaves using HPLC fingerprint analysis. Int. J. Food Sci. Technol. 2019, 55, 526–533. [Google Scholar] [CrossRef]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Chapter 2.14—Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 111–117. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Simões, M.F.; Pinto, R.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug Discov. 2019, 24, 1749–1768. [Google Scholar] [CrossRef]
- Censi, R.; Gigliobianco, M.R.; Casadidio, C.; di Martino, P. Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process. Pharmaceutics 2018, 10, 89. [Google Scholar] [CrossRef]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: A comprehensive review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef]
- Go, E.-J.; Ryu, B.-R.; Ryu, S.-J.; Kim, H.-B.; Lee, H.-T.; Kwon, J.-W.; Baek, J.-S.; Lim, J.-D. An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. Int. J. Mol. Sci. 2021, 22, 12377. [Google Scholar] [CrossRef]
- Kanikkannan, N. Technologies to Improve the Solubility, Dissolution and Bioavailability of Poorly Soluble Drugs. J. Anal. Pharm. Res. 2018, 7, 00198. [Google Scholar] [CrossRef]
- Charunuch, C.; Tangkanakul, P.; Limsangouan, N.; Sonted, V. Effects of Extrusion Condition on the Physical and Functional Properties of Instant Cereal Beverage Powders Admixed with Mulberry (Morus alba L.) Leaves. Food Sci. Technol. Res. 2008, 14, 421–430. [Google Scholar] [CrossRef][Green Version]
- Zelikina, D.V.; Gureeva, M.D.; Chebotarev, S.A.; Samuseva, Y.V.; Antipova, A.S.; Martirosova, E.I.; Semenova, M.G. Functional food compositions based on whey protein isolate, fish oil and soy phospholipids. Food Syst. 2020, 3, 16–20. [Google Scholar] [CrossRef]
- Wu, F.; Chen, F.; Pu, Y.; Qian, F.; Leng, Y.; Mu, G.; Zhu, X. Effects of soy lecithin concentration on the physicochemical properties of whey protein isolate, casein-stabilised simulated infant formula emulsion and their corresponding microcapsules. Int. J. Dairy Technol. 2022, 75, 513–526. [Google Scholar] [CrossRef]
- Chuah, A.M.; Jacob, B.; Jie, Z.; Ramesh, S.; Mandal, S.; Puthan, J.K.; Deshpande, P.; Vaidyanathan, V.V.; Gelling, R.W.; Patel, G.; et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014, 1, 227–233. [Google Scholar] [CrossRef]
- Yang, F.; Su, Y.; Zhang, J.; Dinunzio, J.; Leone, A.; Huang, C.; Brown, C.D. Rheology Guided Rational Selection of Processing Temperature to Prepare Copovidone–Nifedipine Amorphous Solid Dispersions via Hot Melt Extrusion (HME). Mol. Pharm. 2016, 13, 3494–3505. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, R.; Chen, Y.; Ke, X.; Hu, D.; Han, M. Application of Carrier and Plasticizer to Improve the Dissolution and Bioavailability of Poorly Water-Soluble Baicalein by Hot Melt Extrusion. AAPS PharmSciTech 2014, 15, 560–568. [Google Scholar] [CrossRef]
- Nie, S.; Pan, W.; Li, X.; Wu, X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev. Ind. Pharm. 2004, 30, 627–635. [Google Scholar] [CrossRef]
- Schilling, S.U.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Citric acid as a solid-state plasticizer for Eudragit RS PO. J. Pharm. Pharmacol. 2007, 59, 1493–1500. [Google Scholar] [CrossRef]
- Rajput, A.S.; Jha, D.K.; Gurram, S.; Shah, D.S.; Amin, P.D. RP-HPLC method development and validation for the quantification of Efonidipine hydrochloride in HME processed solid dispersions. Futur. J. Pharm. Sci. 2020, 6, 70. [Google Scholar] [CrossRef]
- Avgerinos, T.; Kantiranis, N.; Panagopoulou, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Mechanical properties and drug release of venlafaxine HCl solid mini matrices prepared by hot-melt extrusion and hot or ambient compression. Drug Dev. Ind. Pharm. 2018, 44, 338–348. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Panahi-Azar, V.; Barzegar, A.; Ezzati Nazhad Dolatabadi, J.; Dehghan, P. Spectroscopic, thermodynamic and molecular docking studies of bovine serum albumin interaction with ascorbyl palmitate food additive. Bioimpacts 2017, 7, 241–246. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Mohammadzadeh-Aghdash, H.; Baghbani, E.; Dehghan, P.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and Genotoxicity Assessment of Ascorbyl Palmitate (AP) Food Additive. Adv. Pharm. Bull. 2018, 8, 341–346. [Google Scholar] [CrossRef]
- Fratter, A.; Mason, V.; Pellizzato, M.; Valier, S.; Cicero, A.F.G.; Tedesco, E.; Meneghetti, E.; Benetti, F. Lipomatrix: A Novel Ascorbyl Palmitate-Based Lipid Matrix to Enhancing Enteric Absorption of Serenoa Repens Oil. Int. J. Mol. Sci. 2019, 20, 669. [Google Scholar] [CrossRef]
- Park, M.-O.; Park, C.-I.; Jin, S.-J.; Park, M.-R.; Choi, I.-Y.; Park, C.-H.; Adnan, M. Comparison in Content of Total Polyphenol, Flavonoid, and Antioxidant Capacity from Different Organs and Extruded Condition of Moringa oleifera Lam. Processes 2022, 10, 819. [Google Scholar] [CrossRef]
- Yoon, S.-D. Cross-Linked Potato Starch-Based Blend Films Using Ascorbic Acid as a Plasticizer. J. Agric. Food Chem. 2014, 62, 1755–1764. [Google Scholar] [CrossRef]
- Han, J.H.; Hwang, H.M.; Min, S.; Krochta, J.M. Coating of peanuts with edible whey protein film containing alpha-tocopherol and ascorbyl palmitate. J. Food Sci. 2008, 73, E349–E355. [Google Scholar] [CrossRef]
- Lim, J.D.; Yu, C.Y.; Kim, M.J.; Yun, S.J.; Lee, S.J.; Kim, N.Y.; Chung, I.-M. Comparison of SOD Activity and Phenolic Compound Contents in Various Korean Medicinal Plants. Korean J. Med. Crop Sci. 2004, 12, 191–202. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem. Cent. J. 2012, 6, 12. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, S.L.; Seok, Y.S.; Lee, S.H.; Jo, Y.Y.; Kweon, H.Y.; Lee, K.-G. Quantitative analysis of rutin with mulberry leaves (I). J. Sericult. Entomol. Sci. 2014, 52, 52–58. [Google Scholar] [CrossRef][Green Version]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Techonol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McCinity, J.W.; Martin, C. Pharmaceutical applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2008, 33, 909–926. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rana, M.M.; Boateng, J.S.; Mitchell, J.C.; Douroumis, D. Dissolution enhancement of poorly water-soluble APIs processed by hot-melt extrusion using hydrophilic polymers. Drug Dev. Ind. Pharm. 2013, 39, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.O.K.; Kang, W.S.; Lim, J.D.; Park, C.H. Bio-Fortification of Angelica gigas Nakai Nano-Powder Using Bio-Polymer by Hot Melt Extrusion to Enhance the Bioaccessibility and Functionality of Nutraceutical Compounds. Pharmaceuticals 2019, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Park, M.O.; Park, C.I.; Jin, S.J.; Park, M.R.; Choi, I.Y.; Park, C.H. Yeast and hot melt extrusion enhance polyphenol and flavonoids. Fagopyrum 2022, 39, 13–18. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Adnan, M.; Sung, I.J.; Lim, J.D.; Baek, J.S.; Lim, Y.S.; Park, C.H. Development of value-added functional food by fusion of colored potato and buckwheat flour through hot-melt extrusion. J. Food Processs. Preserv. 2022, 46, e15312. [Google Scholar] [CrossRef]
- Jurisic, V.; Julson, J.L.; Kricka, T.; Curic, D.; Voca, N.; Karunanithy, C. Effect of Extrusion Pretreatment on Enzymatic Hydrolysis of Miscanthus for the Purpose of Ethanol Production. J. Agric. Sci. 2015, 7, 132–142. [Google Scholar] [CrossRef]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef]
- Korus, J.; Gumul, D.; Czechowsak, K. Effect of Extrusion on the Phenolic Composition and Antioxidant Activity of Dry Beans of Phaseolus vulgaris L. Food Technol. Biotechnol. 2007, 45, 139–146. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).