Anther Culture-Derived Haploids of Citrus aurantium L. (Sour Orange) and Genetic Verification of Haploid-Derived Regenerated Plants
Abstract
:1. Introduction
2. Results
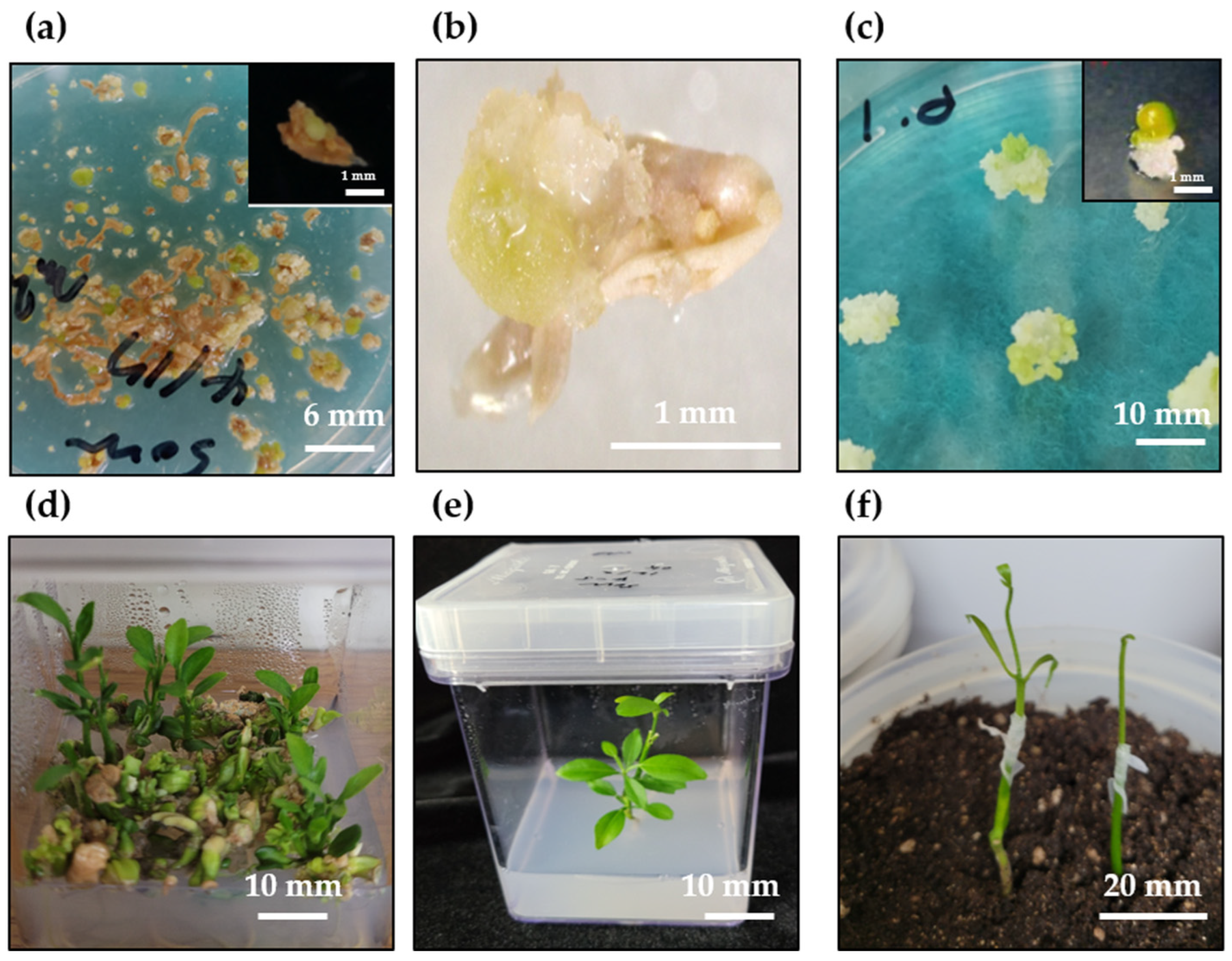
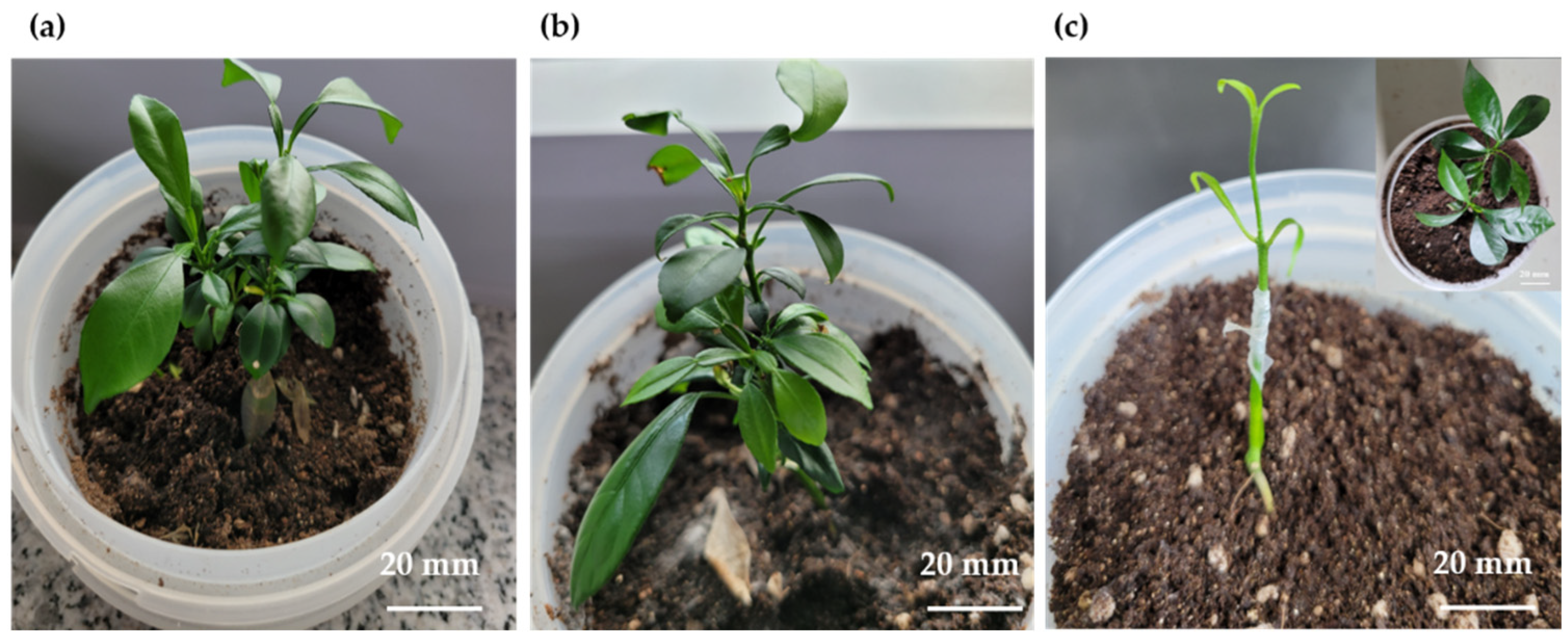
2.1. Plant Regeneration System
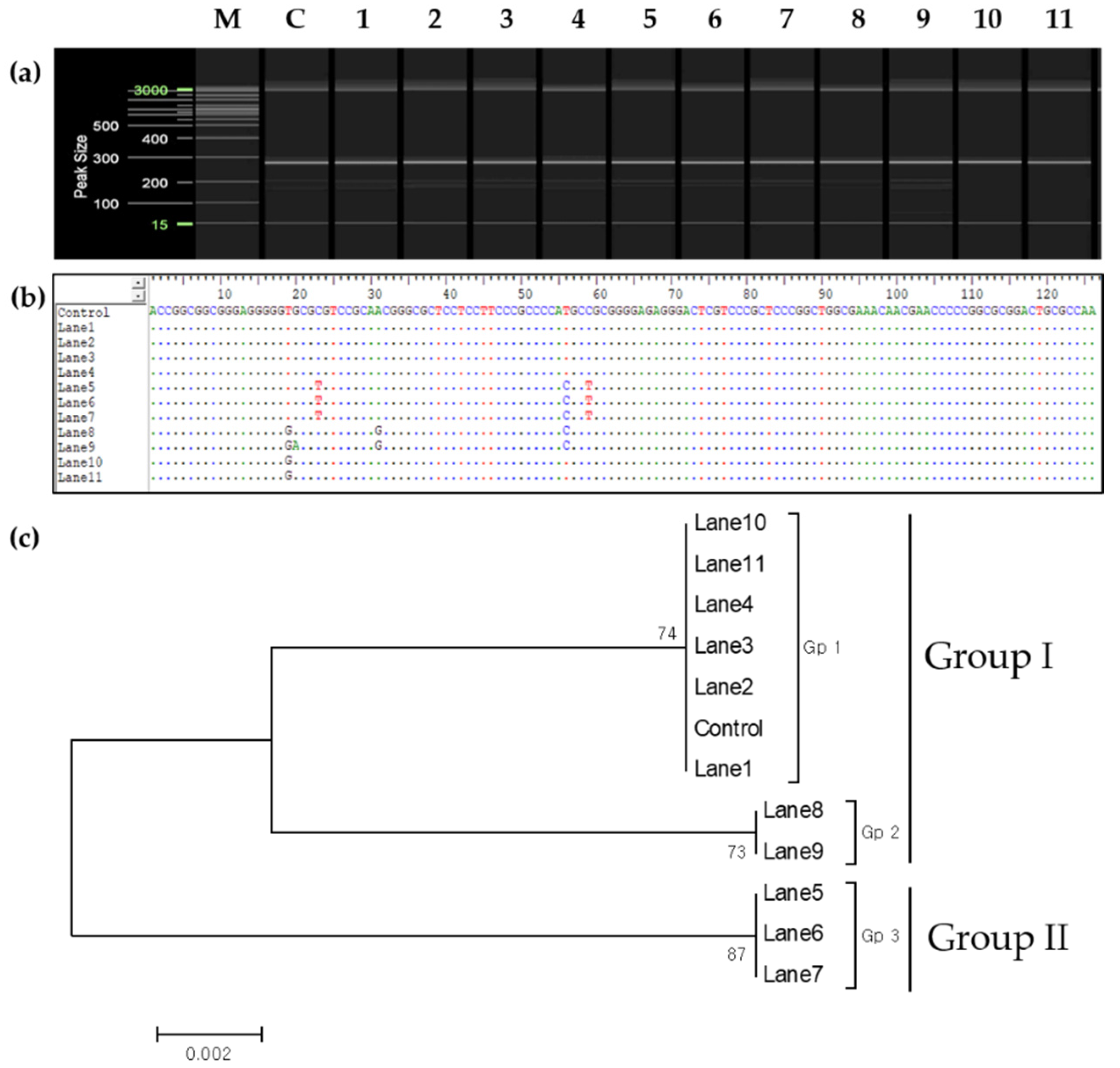
2.2. Genetic Verification
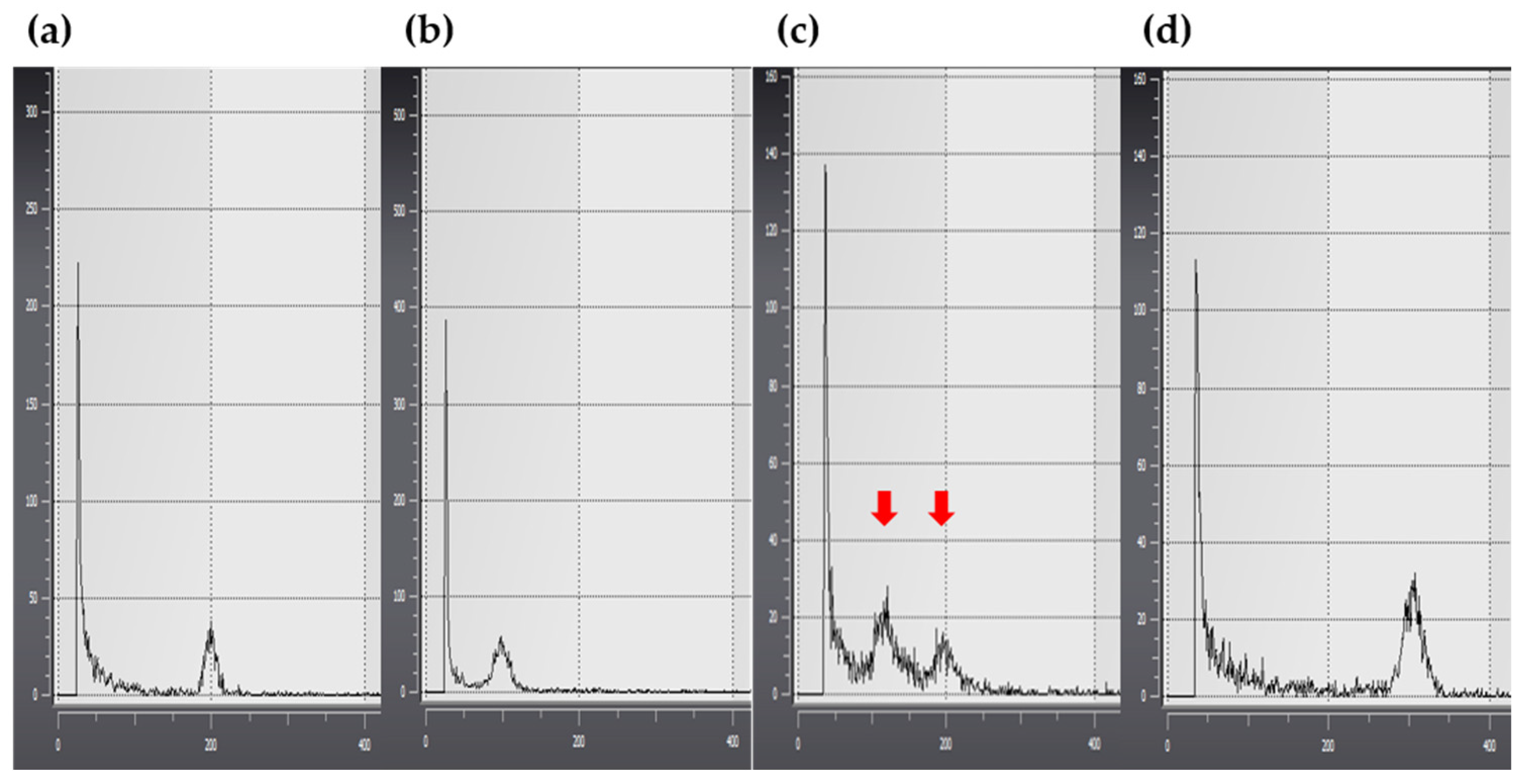
2.3. Polyploidy Verification and Morphological Selection
2.4. Chromosomal Analysis
2.5. Phylogenetic Analysis
3. Discussion
4. Materials and Methods
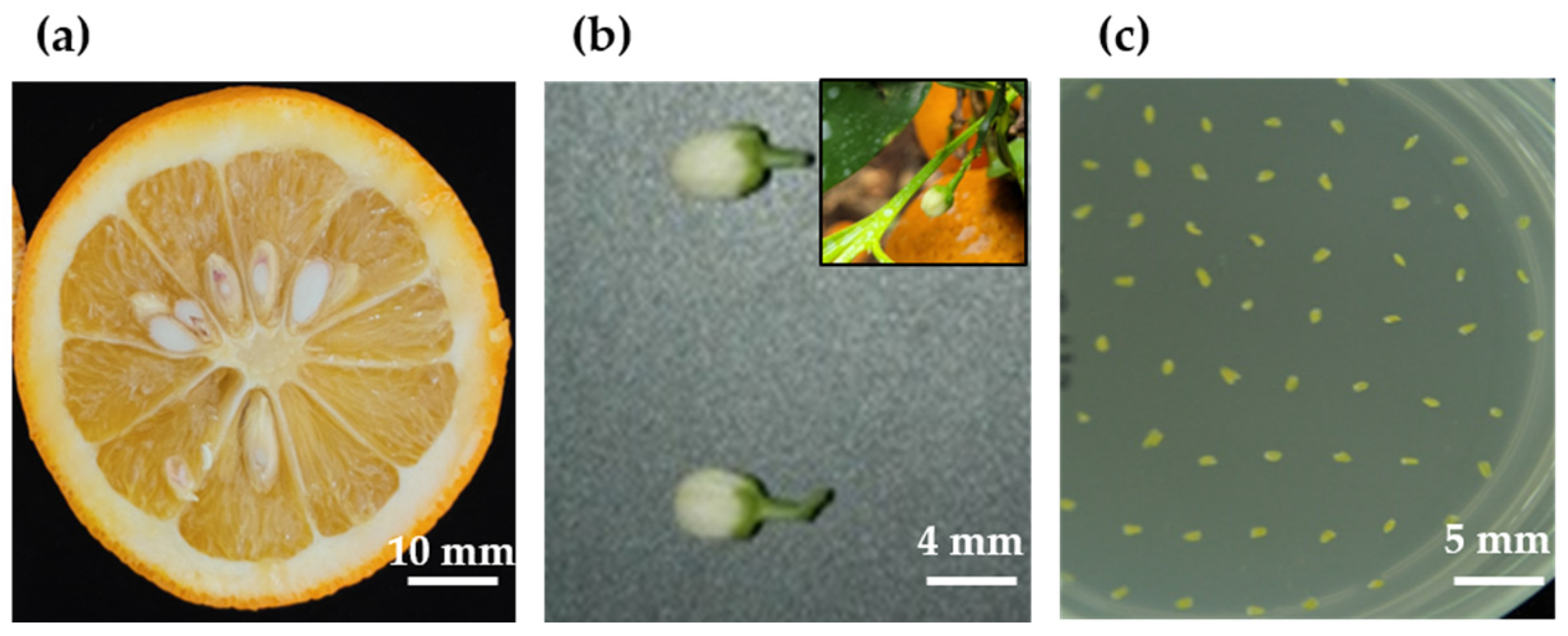
4.1. Plant Materials and Pretreatment
4.2. Composition of Anther Culture Medium and Culture Conditions
4.3. Plant Regeneration
4.4. Genetic Analysis of Regenerants
4.5. Analysis of Polyploidy
4.6. Chromosomal Analysis
4.7. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, J.C.; Martinelli, A.P.; Germanà, M.A.; Latado, R.R. In vitro anther culture of sweet orange (Citrus sinensis L. Osbeck) genotypes and of a C. clementina × C. sinensis ‘Hamlin’ hybrid. Plant Cell Tissue Organ Cult. 2014, 117, 455–464. [Google Scholar] [CrossRef]
- Ezrari, S.; Radouane, N.; Tahiri, A.; El Housni, Z.; Mokrini, F.; Özer, G.; Lazraq, A.; Belabess, Z.; Amiri, S.; Lahlali, R. Dry root rot disease, an emerging threat to citrus industry worldwide under climate change: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101753. [Google Scholar] [CrossRef]
- Huh, M.K.; Yoon, H.J.; Choi, J.S. Phylogenic study of genus citrus and two relative genera in Korea by trnL-trnF sequence. J. Life Sci. 2011, 21, 1452–1459. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.H.; Kwon, H.M.; Kang, S.H.; Kang, J.H.; Kim, S.C. Investigation of the phylogenetic relationships within the genus Citrus (Rutaceae) and related species in Korea using plastid trnL-trnF sequences. Sci. Hortic. 2005, 104, 179–188. [Google Scholar] [CrossRef]
- Chung, W.-H. An analysis of policy effect of the orchard scale improvement project. J. Agric. Life Sci. 2022, 56, 127–135. [Google Scholar] [CrossRef]
- Abbate, L.; Panno, S.; Mercati, F.; Davino, S.; Del Bosco, S.F. Citrus rootstock breeding: Response of four allotetraploid somatic hybrids to Citrus tristeza virus induced infections. Eur. J. Plant Pathol. 2019, 153, 837–847. [Google Scholar] [CrossRef]
- Mekki, M.H.; Elamin, O.M.; Elkashif, M.E. Vegetative growth of some grapefruit cultivars, grafted on sour orange and grown in the heavy clay soils of Gezira State, Sudan. SUJ 2017, 5. Available online: http://repo.uofg.edu.sd/handle/123456789/2066 (accessed on 3 October 2022).
- Nagappan, A.; Park, H.-S.; Hong, G.-E.; Yumnam, S.; Lee, H.-J.; Kim, D.-H.; Kim, E.-H.; Kim, G.-S. Anti-cancer and anti-inflammatory properties of Korean citrus fruits (Citrus aurantium L.). J. Korean Clin. Health Sci. 2014, 2, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An overview on Citrus aurantium L.: Its functions as food ingredient and therapeutic agent. Oxid. Med. Cell Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef] [Green Version]
- Davies, F.S.; Albrigo, L.G. Citrus; CAB International: Wallingford, UK, 1998; pp. 154–196. [Google Scholar]
- Bowman, K.D.; Faulkner, L.; Kesinger, M. New citrus rootstocks released by USDA 2001–2010: Field performance and nursery characteristics. HortScience 2016, 51, 1208–1214. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, T.; Yamada, Y.; Shichijo, T. Plantlet formation by anther culture of Citrus aurantium L. Jpn. J. Breed. 1982, 32, 247–252. [Google Scholar] [CrossRef]
- Benelli, C.; Germanà, M.; Ganino, T.; Beghè, D.; Fabbri, A. Morphological and anatomical observations of abnormal somatic embryos from anther cultures of Citrus reticulata. Biol. Plant. 2010, 54, 224–230. [Google Scholar] [CrossRef]
- Chiancone, B.; Karasawa, M.M.G.; Gianguzzi, V.; Abdelgalel, A.M.; Bárány, I.; Testillano, P.S.; Marinoni, D.T.; Botta, R.; Germanà, M.A. Early embryo achievement through isolated microspore culture in Citrus clementina Hort. ex Tan., cvs. ‘Monreal Rosso’ and ‘Nules’. Front. Plant Sci. 2015, 6, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiancone, B.; Germanà, M.A. Microspore embryogenesis through anther culture in Citrus clementina Hort. ex Tan. In In Vitro Embryogenesis in Higher Plants; Germana, M.A., Lambardi, M., Eds.; Humana Press: New York, NY, USA, 2016; pp. 475–487. [Google Scholar]
- Germanà, M.A. Haploidy. In Citrus Genetics, Breeding and Biotechnology; Khan, I., Ed.; CABI Publishing: Wallingford, UK, 2007; pp. 167–196. [Google Scholar]
- Germanà, M.A. Haploids and doubled haploids in fruit trees. In Advances in Haploid Production in Higher Plants; Touraev, A., Forster, B.P., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 241–263. [Google Scholar]
- Lee, S.; Kim, J.S.; Kang, S.-H.; Sohn, S.-H.; Won, S.Y. Rediscovery of haploid breeding in the genomics era. J. Plant Biotechnol. 2016, 43, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Karjee, S.; Mahapatra, S.; Singh, D.; Saha, K.; Viswakarma, P.K. Production of double haploids in ornamental crops. J. Pharmacogn. Phytochem. 2020, 9, 555–565. [Google Scholar]
- Burbulis, N.; Blinstrubienė, A.; Sliesaravičius, A.; Venskutonienė, E. Influence of genotype, growth regulators, sucrose level and preconditioning of donor plants on flax (Linum usitatissimum L.) anther culture. Acta Biol. Hung. 2005, 56, 323–331. [Google Scholar] [CrossRef]
- Niroula, R.K.; Bimb, H.P. Overview of wheat X maize system of crosses for dihaploid induction in wheat. World Appl. Sci. J. 2009, 7, 1037–1045. [Google Scholar]
- Cimò, G.; Casamento, D.; Marinoni, D.T.; Botta, R.; Germanà, M.A. Gametic embryogenesis through isolated microspore culture in mandarin (Citrus reticulata Blanco), Mandarino Tardivo Di Ciaculli: Effect of meta-Topolin and temperature treatments. Citrus Res. Technol. 2016, 37, 113–122. [Google Scholar] [CrossRef]
- Germanà, M.A.; Aleza, P.; Carrera, E.; Chen, C.; Chiancone, B.; Costantino, G.; Dambier, D.; Deng, X.; Federici, C.T.; Froelicher, Y.; et al. Cytological and molecular characterization of three gametoclones of Citrus clementina. BMC Plant Biol. 2013, 13, 129. [Google Scholar] [CrossRef]
- Das, A.; Kumar, K.; Tribhuvan, K.U.; Das, S.S.; Mishra, M. Development of haploid and double haploid in fruit crops—A review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2119–2132. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. Haploid production in higher plant. Int. J. Chem. Biol. Sci. 2014, 1, 26–45. [Google Scholar]
- Aboshama, H.M. Anther culture and plant regeneration in Citrus (Citrus volkameriana). J. Plant Prod. 2011, 2, 1717–1729. [Google Scholar] [CrossRef]
- Hidaka, T. Effects of sucrose concentration, pH of media, and culture temperature on anther culture of Citrus. Jpn. J. Breed. 1984, 34, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Biswas, M.K.; Lü, Y.; Amar, M.H.; Tong, Z.; Xu, Q.; Xu, J.; Guo, W.; Deng, X. Doubled haploid callus lines of Valencia sweet orange recovered from anther culture. Plant Cell Tissue Organ Cult. 2011, 104, 415–423. [Google Scholar] [CrossRef]
- Froelicher, Y.; Ollitrault, P. Effects of the hormonal balance on Clausena excavata androgenesis. Acta Hortic. 2000, 535, 139–146. [Google Scholar] [CrossRef]
- Germanà, M.A.; Chiancone, B.; Lain, O.; Testolin, R. Anther culture in Citrus clementina: A way to regenerate tri-haploids. Aust. J. Agric. Res. 2005, 56, 839–845. [Google Scholar] [CrossRef]
- Germanà, M.A.; Chiancone, B. Improvement of Citrus clementina Hort. ex Tan. microspore-derived embryoid induction and regeneration. Plant Cell Rep. 2003, 22, 181–187. [Google Scholar] [CrossRef]
- Wang, S.-M.; Lan, H.; Cao, H.-B.; Xu, Q.; Chen, C.-L.; Deng, X.-X.; Guo, W.-W. Recovery and characterization of homozygous lines from two sweet orange cultivars via anther culture. Plant Cell Tissue Organ Cult. 2015, 123, 633–644. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Park, S.-M. Growth and fruit characteristics of aneuploid apple obtained from crosses between diploid and triploid. J. Bio-Environ. Control 2009, 18, 481–491. [Google Scholar]
- Germanà, M.A. Protocol of somatic embryogenesis from Citrus spp. anther culture. In Protocol for Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 191–207. [Google Scholar]
- Makarevitch, I.; Harris, C. Aneuploidy causes tissue-specific qualitative changes in global gene expression patterns in maize. Plant Physiol. 2010, 152, 927–938. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Tsai, H.J.; Gordon, M.R.; Li, R. Cellular stress associated with aneuploidy. Dev. Cell 2018, 44, 420–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, C.J. Down syndrome (trisomy 21). In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 1123–1256. [Google Scholar]
- Birchler, J.A.; Bhadra, U.; Bhadra, M.P.; Auger, D.L. Dosage-dependent gene regulation in multicellular eukaryotes: Implications for dosage compensation, aneuploid syndromes, and quantitative traits. Dev. Biol. 2001, 234, 275–288. [Google Scholar] [CrossRef] [Green Version]
- Birchler, J.A.; Veitia, R.A. The gene balance hypothesis: From classical genetics to modern genomics. Plant Cell 2007, 19, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.; Wu, T.; Liang, G.; Wu, D.; He, Q.; Guo, Q. Identification and characterization of a loquat aneuploid with novel leaf phenotypes. HortScience 2019, 54, 804–808. [Google Scholar] [CrossRef] [Green Version]
- Makarevitch, I.; Phillips, R.L.; Springer, N.M. Profiling expression changes caused by a segmental aneuploid in maize. BMC Genom. 2008, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, D.-H.; Qu, Y.; Xu, D.-X.; Li, J.; Chen, Z.-L. Analysis on chromosome number and morphology of varieties in Phalaenopsis. Acta Hortic. Sin. 2007, 34, 1257–1262. [Google Scholar]
- Chagné, D.; Kirk, C.; Whitworth, C.; Erasmuson, S.; Bicknell, R.; Sargent, D.J.; Kumar, S.; Troggio, M. Polyploid and aneuploid detection in apple using a single nucleotide polymorphism array. Tree Genet. Genomes 2015, 11, 94. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, J.H. Development of endosperm and embryo of aneuploid seeds obtained from 3x × 2x and 3x × 4x crosses and its rescue in grapes. J. Korean Soc. Hortic. Sci. 2001, 42, 315. [Google Scholar]
- Simard, M.H.; Guisnel, R.; Daguin, F.; Demilly, D.; Billy, B.; Honoré, D. Is dwarfing in pear rootstocks due to aneuploid genetic structures? Acta Hortic. 2011, 909, 59–66. [Google Scholar] [CrossRef]
- Yasuda, K.; Yahata, M.; Komatsu, H.; Kurogi, Y.; Kunitake, H. Triploid and aneuploid hybrids from diploid-diploid intergeneric crosses between citrus cultivar ‘Kiyomi’ tangor and Meiwa kumquat (Fortunella crassifolia Swingle) for seedless breeding of kumquats. J. Jpn. Soc. Hortic. Sci. 2010, 79, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Niizeki, M.; Kita, F. Production of aneuploid plants by anther culture in Nicotiana tabacum L. Jpn. J. Breed. 1975, 25, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xu, X.; Jin, W.; Chen, S. Morphological and molecular evidences for DNA introgression in haploid induction via a high oil inducer CAUHOI in maize. Planta 2009, 230, 367–376. [Google Scholar] [CrossRef]
- Winarto, B.; Rachmawati, F.; Pramanik, D.; Teixeira da Silva, J.A. Morphological and cytological diversity of regenerants derived from half-anther cultures of anthurium. Plant Cell Tissue Organ Cult. 2011, 105, 363–374. [Google Scholar] [CrossRef]
- Chiancone, B.; Germanà, M.A. Micropropagation of Citrus spp. by organogenesis and somatic embryogenesis. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 99–118. [Google Scholar]
- Antonietta, G.M.; Ahmad, H.I.; Maurizio, M.; Alvaro, S. Preliminary research on conversion of encapsulated somatic embryos of Citrus reticulata Blanco, cv. Mandarino Tardivo di Ciaculli. Plant Cell Tissue Organ Cult. 2007, 88, 117–120. [Google Scholar] [CrossRef]
- Parra-Vega, V.; Renau-Morata, B.; Sifres, A.; Seguí-Simarro, J.M. Stress treatments and in vitro culture conditions influence microspore embryogenesis and growth of callus from anther walls of sweet pepper (Capsicum annuum L.). Plant Cell Tissue Organ Cult. 2013, 112, 353–360. [Google Scholar] [CrossRef]
- Prem, D.; Solís, M.T.; Bárány, I.; Rodríguez-Sanz, H.; Risueño, M.C.; Testillano, P.S. A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Biol. 2012, 12, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguí-Simarro, J.M.; Corral-Martínez, P.; Parra-Vega, V.; González-García, B. Androgenesis in recalcitrant solanaceous crops. Plant Cell Rep. 2011, 30, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Wedzony, M.; Forster, B.P.; Żur, I.; Golemiec, E.; Szechyńska-Hebda, M.; Dubas, E.; Gotębiowska, G.; Wędzony, M. Progress in doubled haploid technology in higher plants. In Advances in Haploid Production in Higher Plants; Touraev, A., Forster, B.P., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–33. [Google Scholar]
- Germanà, M.A. Doubled haploid production in fruit crops. Plant Cell Tissue Organ Cult. 2006, 86, 131–146. [Google Scholar] [CrossRef] [Green Version]
- Chiancone, B.; Tassoni, A.; Bagni, N.; Germanà, M.A. Effect of polyamines on in vitro anther culture of Citrus clementina Hort. ex Tan. Plant Cell Tissue Organ Cult. 2006, 87, 145–153. [Google Scholar] [CrossRef]
- Jin, S.B.; Park, J.H.; Park, S.M.; Lee, D.H.; Yun, S.H. Production of citrus plants from ovule cell culture and verification of CTV-free plants. Korean J. Hortic. Sci. Technol. 2017, 35, 121–130. [Google Scholar] [CrossRef]
- Dunwell, J.M. Haploids in flowering plants: Origins and exploitation. Plant Biotechnol. J. 2010, 8, 377–424. [Google Scholar] [CrossRef] [PubMed]
- Ziauddin, A.; Simion, E.; Kasha, K.J. Improved plant regeneration from shed microspore culture in barley (Hordeum vulgare L.) cv. igri. Plant Cell Rep. 1990, 9, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Touraev, A.; Indrianto, A.; Wratschko, I.; Vicente, O.; Heberle-Bors, E. Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex. Plant Reprod. 1996, 9, 209–215. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Thurling, N. Role of sucrose in microspore embryo production in Brassica napus ssp. oleifera. J. Exp. Bot. 1985, 36, 1478–1491. [Google Scholar] [CrossRef]
- Supena, E.D.J.; Suharsono, S.; Jacobsen, E.; Custers, J.B.M. Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep. 2006, 25, 1–10. [Google Scholar] [CrossRef]
- Germanà, M.A. Anther culture for haploid and doubled haploid production. Plant Cell Tissue Organ Cult. 2011, 104, 283–300. [Google Scholar] [CrossRef]
- Jin, S.B.; Park, J.H.; Park, S.M.; Lee, D.H.; Yun, S.H. Genetic phylogenetic relationship of the Jeju native citrus ‘Byungkyool’ (Citrus platymamma Hort. ex Tanaka) using ITS (internal transcribed spacer) region of nuclear ribosomal DNA. Korean J. Breed. Sci. 2016, 48, 241–253. [Google Scholar] [CrossRef]
- Jin, S.B.; Park, J.H.; Park, S.M.; Lee, D.H.; Lee, W.J.; Yun, S.H. A phylogenic analysis of citrus cultivars native to Jeju using chloroplast DNA trnL-trnF and internal transcribed spacer region sequences. Korean J. Hortic. Sci. Technol. 2018, 36, 585–597. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Kim, M.S.; Shin, K.; Park, S.; Choi, C.; Yun, S.H.; Jin, S.B. Comparative genetic analysis between the Jeju ‘Inchangkyool’ and Chinese ‘Ichangensis’ (Citrus ichangensis) using internal chloroplast trnL-trnF intergenic spacers and transcribed spacer sequence regions. Korean J. Breed. Sci. 2021, 53, 16–31. [Google Scholar] [CrossRef]
- Li, J.R.; Zhuang, F.Y.; Ou, C.G.; Hu, H.; Zhao, Z.W.; Mao, J.H. Microspore embryogenesis and production of haploid and doubled haploid plants in carrot (Daucus carota L.). Plant Cell Tissue Organ Cult. 2013, 112, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.C. The N6 medium and its applications to anther culture of cereal crops. In Proceedings of the Symposium on Plant Tissue Culture, Beijing, China, 25–30 May 1978; Peking Science Press: Beijing, China, 1978; pp. 43–50. [Google Scholar]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jin, S.B.; Song, K.J.; Riu, K.Z. Several factors affecting embryogenic culture maintenance and shoot regeneration in ‘Miyagawa Wase’ Satsuma Mandarin (Citrus unshiu). Hortic. Environ. Biotechnol. 2007, 48, 165–170. [Google Scholar]
- Woo, J.K.; Yun, S.H.; Yi, K.U.; Park, Y.C.; Lee, H.Y.; Kim, M.; Lee, Y.; Song, K.J.; Kim, H.B. Identification of Citrus varieties bred in Korea using microsatellite markers. Korean J. Hortic. Sci. Technol. 2020, 38, 374–384. [Google Scholar] [CrossRef]
- Ha, M.K.T.T.; Sevilleno, S.S.; Cabahug, R.A.M.; Yi, J.H.; Oh, H.J.; Kwon, H.H.; Kim, S.Y.; Hwang, Y.J. Cytogenetic analysis and genome size estimation of Korean native Veronica taxa. Flower Res. J. 2022, 30, 41–52. [Google Scholar] [CrossRef]
- Kirov, I.; Divashuk, M.; Laere, K.V.; Soloviev, A.; Khrustaleva, L. An easy “SteamDrop” method for high quality plant chromosome preparation. Mol. Cytogenet. 2014, 7, 21. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]




| No. | Product Name | Primer Sequence (5′-3′) | Repeat Motif | Homozygous Cultivars [References] | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| 1 | BM-CiSSR-P1 | CCCCCTCTTCTTTCACACAA | GGTGAGCAGCCATCTTCTTC | (TA)6 | C. clementina ‘Fina Sodea’, C. erythrosa ‘Dingjeongkyul’ |
| 2 | BM-CiSSR-P2 | GAATTGGGAGGACGAACTGA | CGAGCCCTAGACAGAGATGG | (AGA)7 | C. pseudogulgul, Citrus hybrid ‘Haruka’ |
| 3 | BM-CiSSR-043 | ATTAGTGCGGGTAAGATGAA | AAGGATTTGGTGTAGGAAGTAA | (AAAAT)3 | Woo et al. [73] |
| 4 | BM-CiSSR-226 | ATTAAGGCTGGAAATGCCAC | ATTCTGCTGACGCTTCAATG | (ATT)9 | Woo et al. [73] |
| 5 | BM-CiSSR-246 | CCCTAGGGAAATTTGGGAAT | GCACTCGAGAGTTCTCGTTAAG | (CAT)11 | Woo et al. [73] |
| 6 | BM-CiSSR-253 | AATTTCCTGCTCCAAACCAG | TCCAACAACTTGAACACGGT | (TAA)14 | Woo et al. [73] |
| 7 | BM-CiSSR-254 | TAAAATCCCTCGGAAACAGG | CTTTGCATGTTCAACGTTCC | (ATC)6 | Woo et al. [73] |
| 8 | BM-CiSSR-260 | TCATCTGAACGGACCACAAT | TAACTGCACTTGCTTCCCTG | (TTC)6 | Woo et al. [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.B.; Kim, M.J.; Choi, C.W.; Park, S.M.; Yun, S.H. Anther Culture-Derived Haploids of Citrus aurantium L. (Sour Orange) and Genetic Verification of Haploid-Derived Regenerated Plants. Plants 2022, 11, 3022. https://doi.org/10.3390/plants11223022
Jin SB, Kim MJ, Choi CW, Park SM, Yun SH. Anther Culture-Derived Haploids of Citrus aurantium L. (Sour Orange) and Genetic Verification of Haploid-Derived Regenerated Plants. Plants. 2022; 11(22):3022. https://doi.org/10.3390/plants11223022
Chicago/Turabian StyleJin, Seong Beom, Min Ju Kim, Cheol Woo Choi, Suk Man Park, and Su Hyun Yun. 2022. "Anther Culture-Derived Haploids of Citrus aurantium L. (Sour Orange) and Genetic Verification of Haploid-Derived Regenerated Plants" Plants 11, no. 22: 3022. https://doi.org/10.3390/plants11223022




