Ecophysiological Response of Vitis vinifera L. in an Urban Agrosystem: Preliminary Assessment of Genetic Variability
Abstract
1. Introduction
2. Results and Discussion
2.1. The Urban Environment
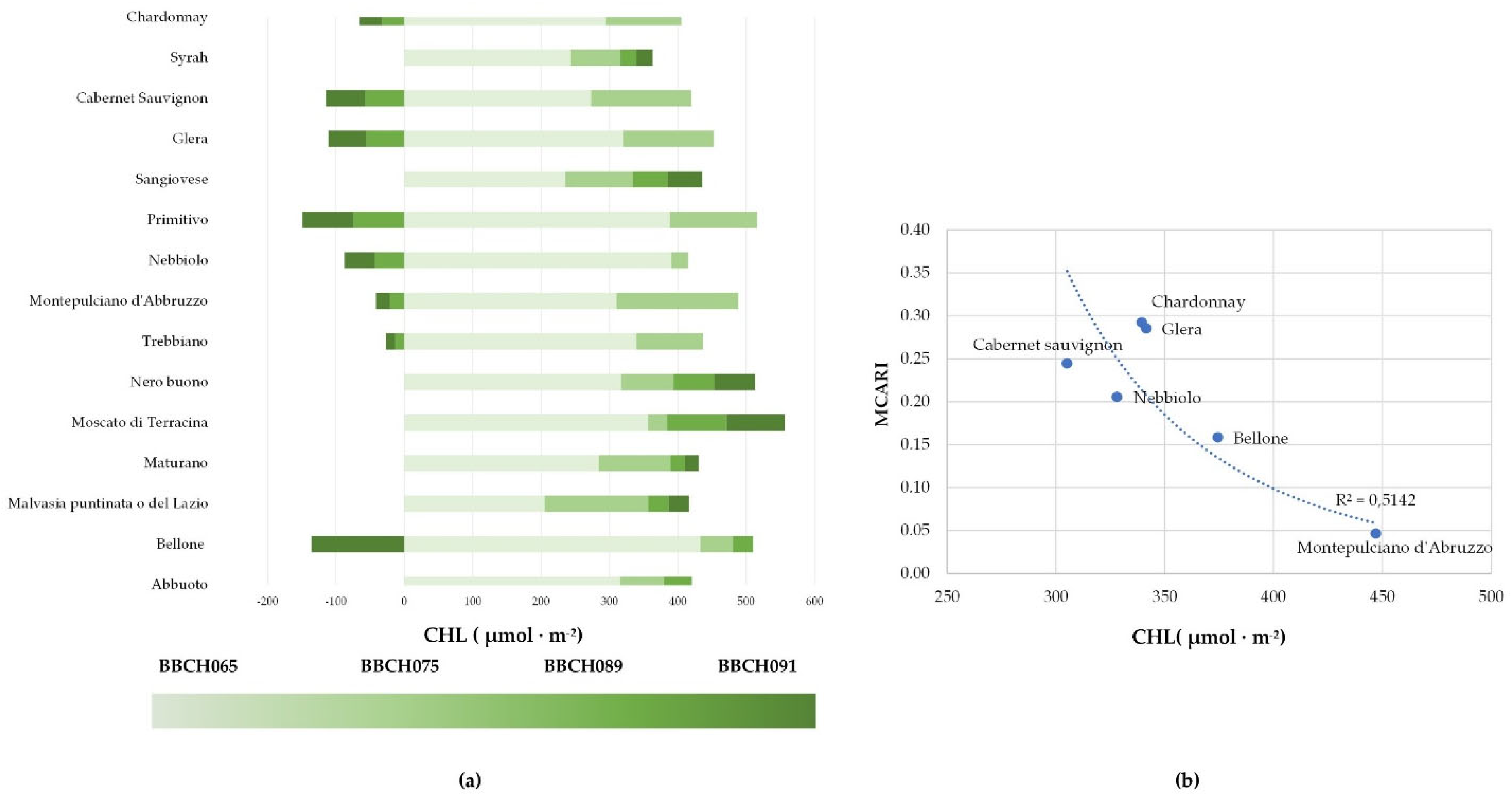
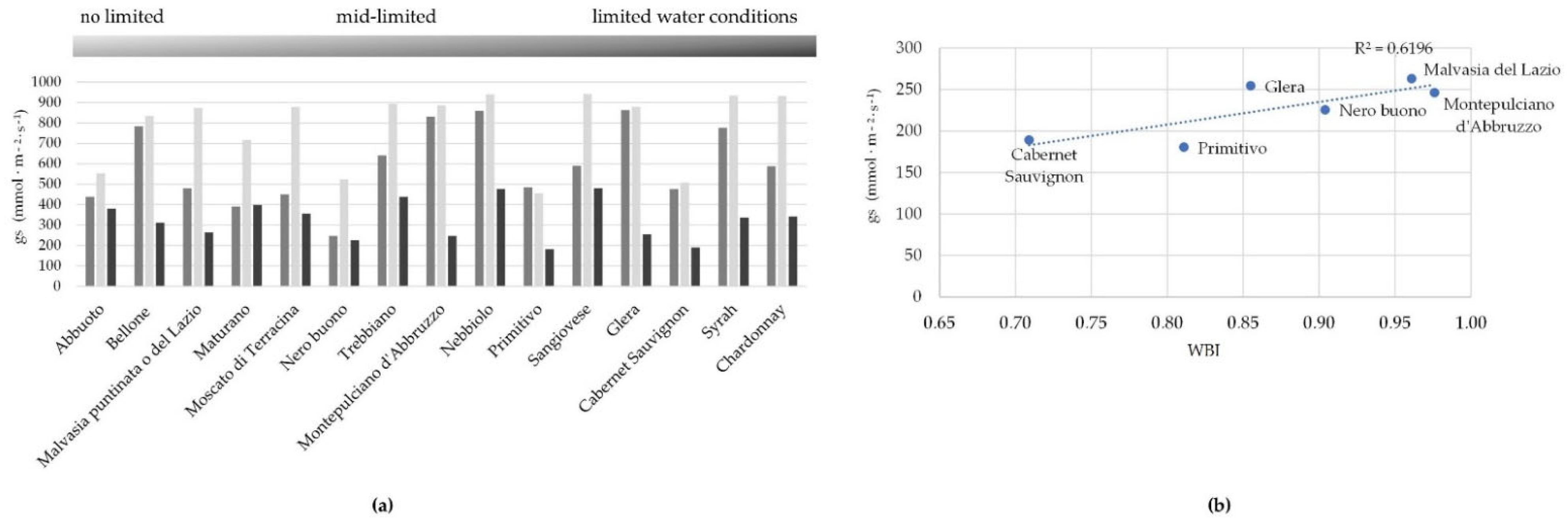
2.2. Physiological Responses of Grapevine Cultivars to Stressors
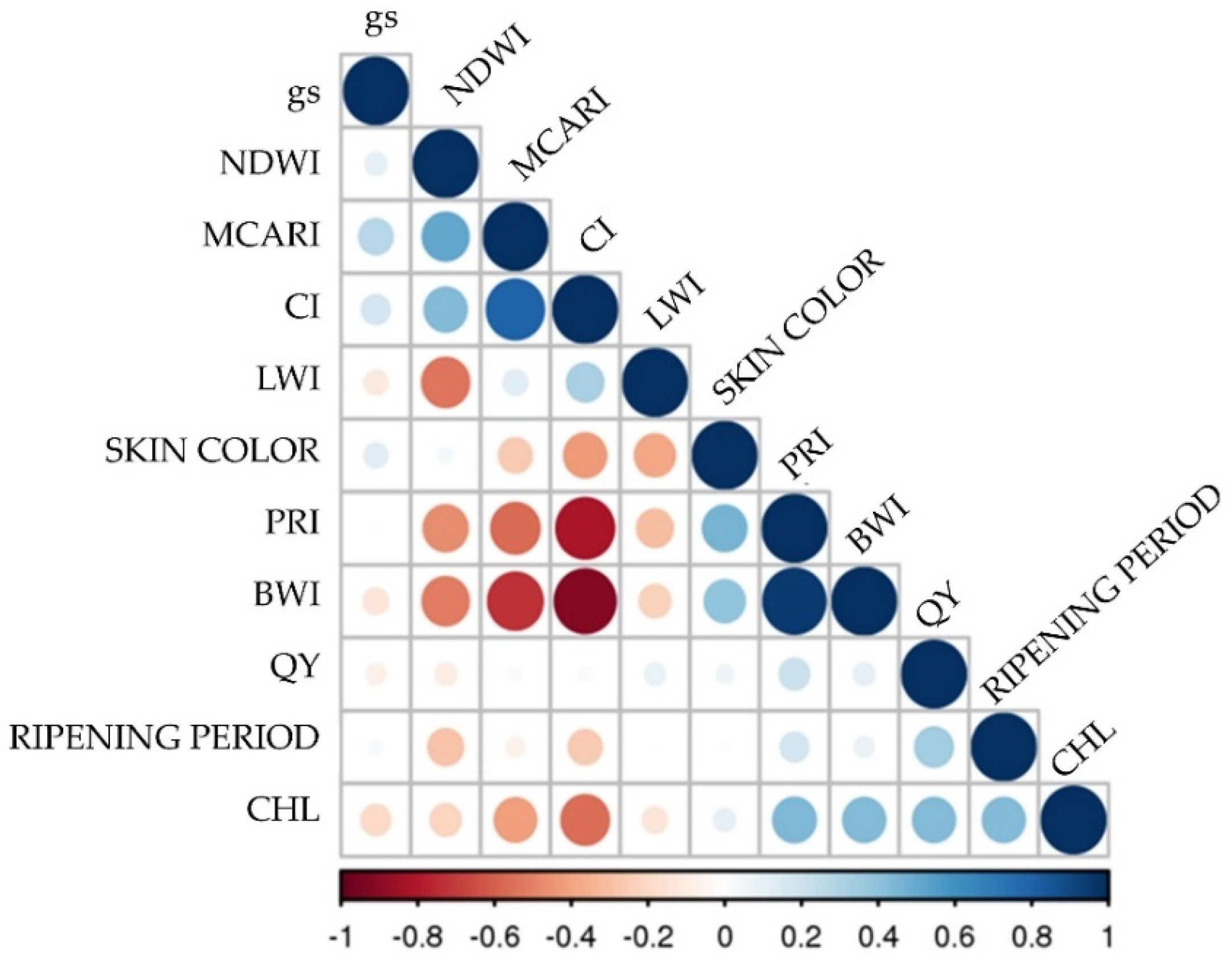
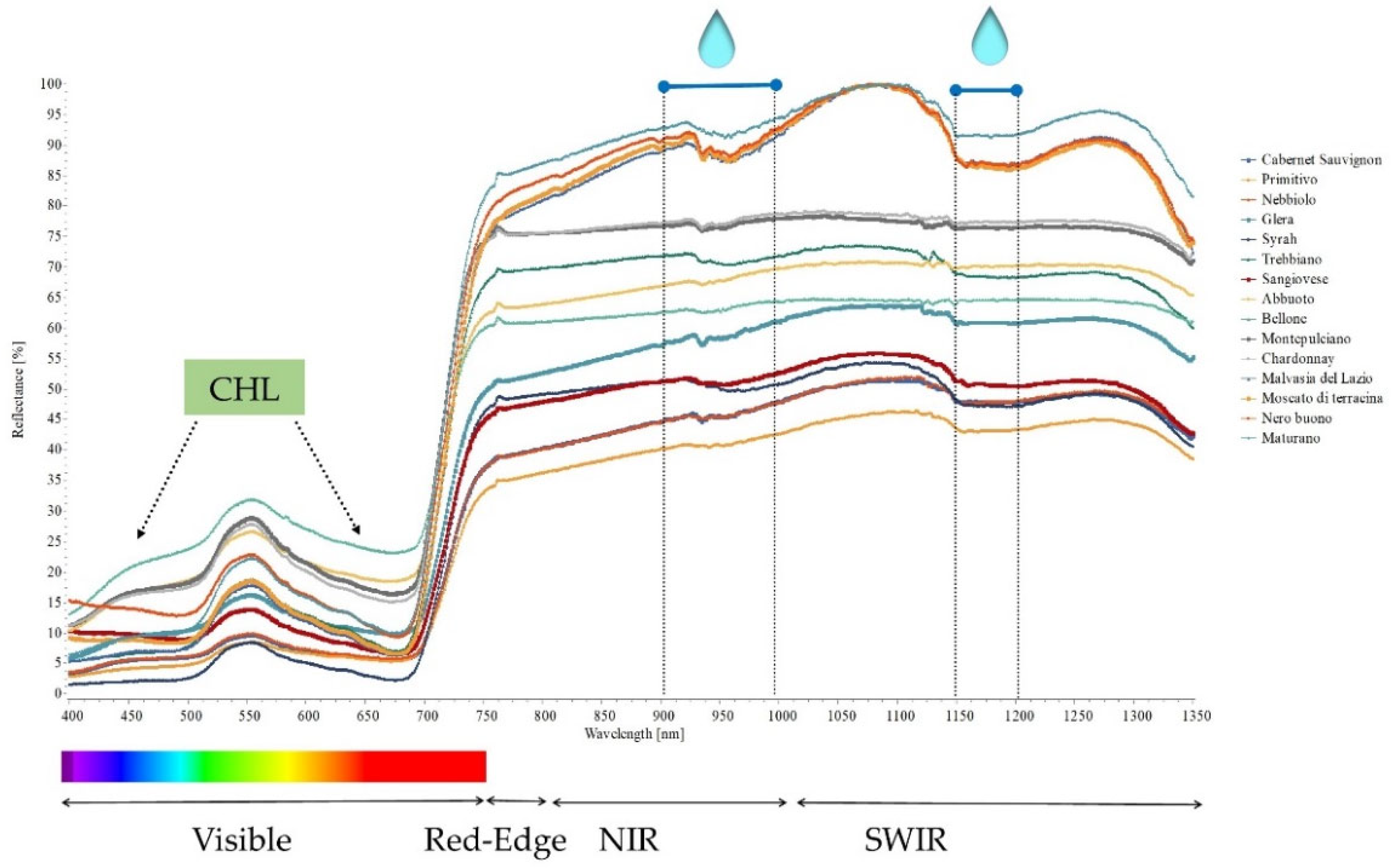
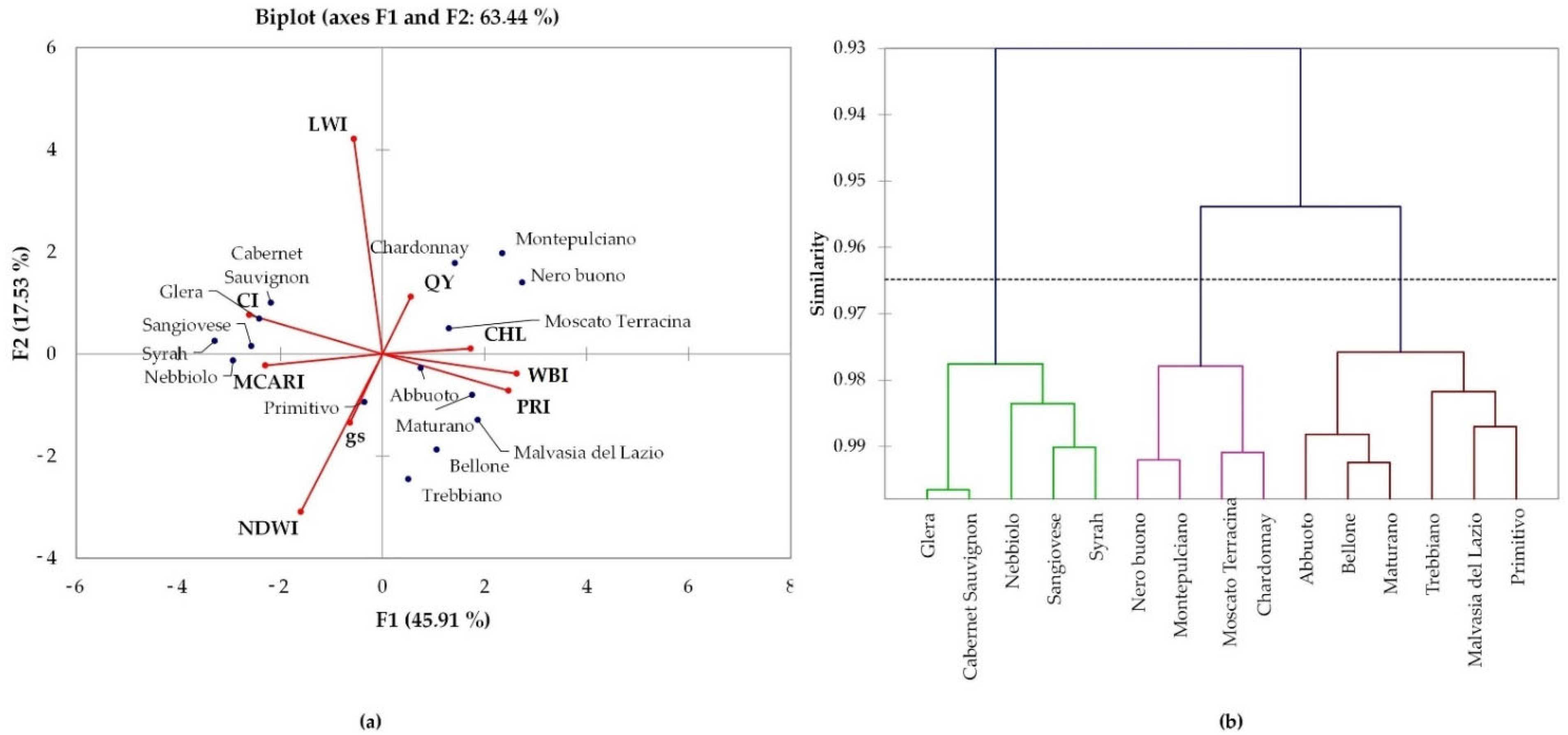
2.3. Hyperspectral Genotype Signature
3. Materials and Methods
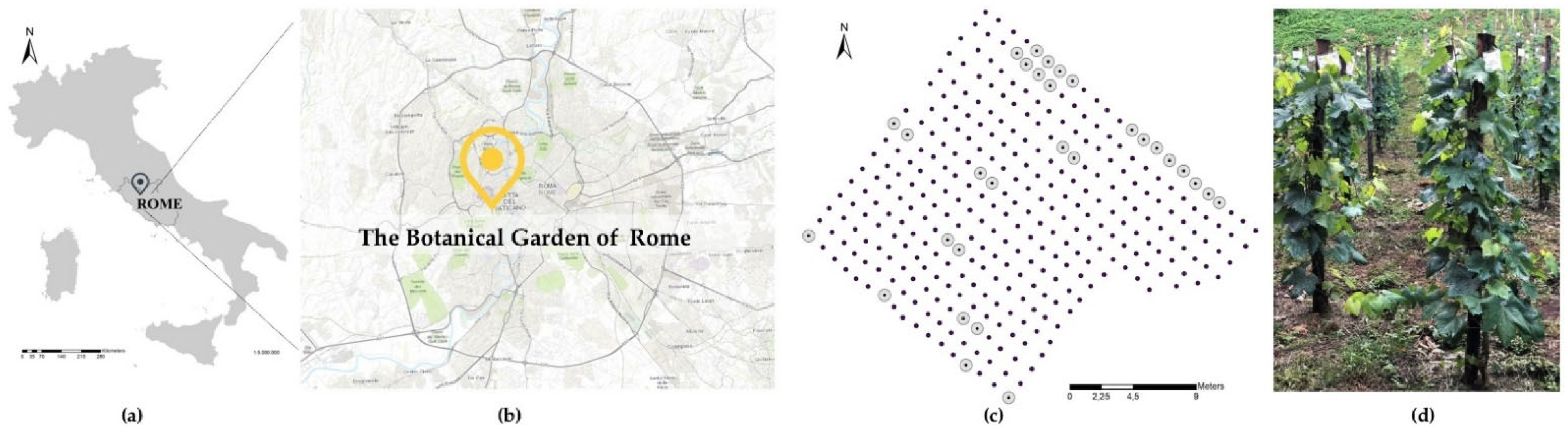
3.1. Study Site and Plant Material
3.2. Ecophysiological Determinations
3.3. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Department of Economic and Social Affairs, United Nation. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; Department of Economic and Social Affairs, United Nation: New York, NY, USA, 2016. [Google Scholar]
- European Commission. A European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 1 September 2022).
- Clinton, N.; Stuhlmacher, M.; Miles, A.; Uludere, N.; Wagner, M.; Georgescu, M.; Herwig, C.; Gong, P. A global geospatial ecosystem services estimate of urban agriculture. Earth’s Future 2018, 6, 40–60. [Google Scholar] [CrossRef]
- FAO; Rikolto; RUAF. Urban and PERI-Urban Agriculture Sourcebook–From Production to Food Systems; FAO and Rikolto: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Singh, R.P. Integration and commercialization of local varieties under sub-optimal environments for food security, promoting sustainable agriculture and agrobiodiversity conservation. MOJ Ecol. Environ. Sci. 2018, 3, 65–67. [Google Scholar] [CrossRef]
- OIV Statistical Report on World Vitiviniculture. Available online: http://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 23 April 2019).
- Biasi, R.; Barbera, G.; Marino, E.; Brunori, E.; Nieddu, G. Viticulture as crucial cropping system for counteracting the desertification of coastal land. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People: International Symposium on the Effect of Climate Change on Production and Quality of Grapevines and their Products, Lisbon Portugal, 22–27 August 2012; Volume 931, pp. 71–77. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Moresi, F.V.; Maesano, M.; Cipriani, F.; Carpentieri, S.; Rossetti, L.; Attorre, F. Resilience and resistance of viticultural biodiversity in the urban ecosystem: The case of the grapevine collection of the Botanical Garden of Rome. Acta Hortic. 2022, 1345, 75–82. [Google Scholar] [CrossRef]
- Brunori, E.; Farina, R.; Biasi, R. Sustainable viticulture: The carbon-sink function of the vineyard agro-ecosystem. Agric. Ecosyst. Environ. 2016, 223, 10–21. [Google Scholar] [CrossRef]
- Candiago, S.; Winkler, K.J.; Giombini, V.; Giupponi, C.; Vigl, L.E. An ecosystem service approach to the study of vineyard landscapes in the context of climate change: A review. Sustain. Sci. 2022. [Google Scholar] [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripochen, A.; Valdés-Gómez, H.; Metay, A. Management of service crops for the provision of ecosystem services in vineyards: A review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Brunori, E.; Salvati, L.; Mancinelli, R.; Smiraglia, D.; Biasi, R. Multi-temporal land use and cover changing analysis: The environmental impact in Mediterranean area. Int. J. Sustain. Dev. World Ecol. 2017, 24, 276–288. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Biol. Sci. 2013, 110, 6907–6912. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Assessing impacts of climate change on phenology and quality traits of Vitis vinifera L.: The contribution of local knowledge. Plants 2019, 8, 121. [Google Scholar] [CrossRef]
- Santos, J.A.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Bindi, M.; Leolini, L.; et al. Long-Term Adaptation of European Viticulture to Climate Change: An Overview from the H2020 Clim4Vitis Action; IVES Technical Reviews: Villenave d’Ornon, France, 2021. [Google Scholar] [CrossRef]
- Hunter, J.J.K.; Tarricone, L.; Volschenk, C.; Giacalone, C.; Susete Melo, M.; Zorer, R. Grapevine physiological response to row orientation-induced spatial radiation and microclimate changes. OENO One 2020, 54, 411–433. [Google Scholar] [CrossRef]
- De la Salle, J.; Holland, M. Agricultural Urbanism: Handbook for Building Sustainable Food & Agriculture Systems in 21st Century Cities; Green Frigate Books; Libri Publishing: Faringdon, UK, 2010; ISBN 978-0-9812434-2-9. [Google Scholar]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.A.; Justes, E.; Sarthou, J.P. How to implement biodiversity-based agriculture to enhance ecosystem services: A review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Ibañez, D.; Guallart, V.; Salka, M. On pedagogical prototyping of advanced ecological buildings and biocities at Valldaura Labs. AGATHÓN Int. J. Archit. Art Des. 2022, 11, 136–149. [Google Scholar] [CrossRef]
- Viljoen, A.; Bohn, K.; Howe, J. Continuous Productive Urban Landscapes: Designing Urban Agriculture for Sustainable Cities; Elsevier, Architectural Press: Oxford, UK, 2005. [Google Scholar]
- Tabari, H.; Mendoza Paz, S.; Buekenhout, D.; Willems, P. Comparison of statistical downscaling methods for climate change impact analysis on precipitation-driven drought. Hydrol. Earth Syst. Sci. 2021, 25, 3493–3517. [Google Scholar] [CrossRef]
- Pogačar, T.; Zupanc, V.; Kajfež Bogataj, L.; Črepinšek, Z. Soil temperature analysis for various locations in Slovenia. Ital. J. Agrometeorol. 2018, 1, 25–34. [Google Scholar] [CrossRef]
- Wei, J.; Li, X.; Liu, L.; Røjle Christensen, T.; Jiang, Z.; Ma, Y.; Wu, X.; Yao, H.; López-Blanco, E. Radiation, soil water content, and temperature effects on carbon cycling in an alpine swamp meadow of the northeastern Qinghai–Tibetan Plateau. Biogeosciences 2022, 19, 861–875. [Google Scholar] [CrossRef]
- Steele, M.; Gitelson, A.A.; Rundquist, D. Nondestructive estimation of leaf chlorophyll content in grapes. Am. J. Enol. Vitic. 2008, 59, 299–305. [Google Scholar]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.) An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Sellami, M.H.; Albrizio, R.; Colovíc, M.; Hamze, M.; Cantore, V.; Ťodorovic, M.; Piscitelli, L.; Stellacci, A.M. Selection of hyperspectral vegetation indices for monitoring yield and physiological response in sweet maize under different water and nitrogen availability. Agronomy 2022, 12, 489. [Google Scholar] [CrossRef]
- Riffle, V.; Palmer, N.; Casassa, L.F.; Dodson Peterson, J.C. The effect of grapevine age (Vitis vinifera L. cv. Zinfandel) on phenology and gas exchange parameters over consecutive growing seasons. Plants 2021, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable Viticulture: Effects of Soil Management in Vitis vinifera. Agronomy 2020, 10, 1949. [Google Scholar] [CrossRef]
- Mattila, H.; Valev, D.; Havurinne, V.; Khorobrykh, S.; Virtanen, O.; Antinluoma, M.; Mishra, K.B.; Tyystjärvi, E. Degradation of chlorophyll and synthesis of flavonols during autumn senescence—The story told by individual leaves. AoB Plants 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 2011, 1807, 977–988. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Silvestroni, O.; Lanari, V.; D’Onofrio, C.; Matarese, F.; Bellincontro, A.; Poni, S. Physiological parameters and protective energy dissipation mechanisms expressed in the leaves of two Vitis vinifera L. genotypes under multiple summer stresses. J. Plant Physiol. 2015, 185, 84–92. [Google Scholar] [CrossRef]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Collins, M.J.; Fuentes, S.; Barlow, E.W.R. Partial rootzone drying and deficit irrigation increase stomatal sensitivity to vapour pressure deficit in anisohydric grapevines. Funct. Plant Biol. 2010, 37, 128–138. [Google Scholar] [CrossRef]
- Bianchi, D.; Grossi, D.; Tincani, D.T.G.; Di Lorenzo, G.S.; Brancadoro, L.; Rustioni, L. Multi-parameter characterization of water stress tolerance in Vitis hybrids for new rootstock selection. Plant Physiol. Biochem. 2018, 132, 333–340. [Google Scholar] [CrossRef]
- Bota, J.M.; Tomás, J.; Flexas, J.; Medrano, H.; Escalona, J.M. Differences among grapevine cultivars in their stomatal behavior and water use efficiency under progressive water stress. Agric. Water Manag. 2016, 164, 91–99. [Google Scholar] [CrossRef]
- Cogato, A.; Wu, L.; Jewan, S.Y.Y.; Meggio, F.; Marinello, F.; Sozzi, M.; Pagay, V. Evaluating the spectral and physiological responses of grapevines (Vitis vinifera L.) to heat and water stresses under different vineyard cooling and irrigation strategies. Agronomy 2021, 11, 1940. [Google Scholar] [CrossRef]
- Laroche-Pinel, E.; Albughdadi, M.; Duthoit, S.; Chéret, V.; Rousseau, J.; Clenet, H. Understanding vine hyperspectral signature through different irrigation plans: A first step to monitor vineyard water status. Remote Sens. 2021, 13, 536. [Google Scholar] [CrossRef]
- Imanishi, J.; Sugimoto, K.; Morimoto, Y. Detecting drought status and LAI of two Quercus species canopies using derivative spectra. Comput. Electron. Agric. 2004, 43, 109–129. [Google Scholar] [CrossRef]
- Ronay, I.; Ephrath, J.E.; Eizenberg, H.; Blumberg, D.G.; Maman, S. Hyperspectral reflectance and indices for characterizing the dynamics of crop–weed competition for water. Remote Sens. 2021, 13, 513. [Google Scholar] [CrossRef]
- Florez-Sarasa, I.; Clemente-Moreno, M.J.; Cifre, J.; Capó, M.; Llompart, M.; Fernie, A.R.; Bota, J. Differences in metabolic and physiological responses between local and widespread grapevine cultivars under water deficit stress. Agronomy 2020, 10, 1052. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate change impacts and adaptation strategies of agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Jones, G.V. Climate and terroir: Impacts of climate variability and change on wine. In Fine Wine and Terroir—The Geoscience Perspective; Macqueen, R.W., Meinert, L.D., Eds.; Series No. 9; Geoscience Canada Reprint; Geological Association of Canada: St. John’s, NL, Canada, 2007; pp. 1–19. [Google Scholar]
- Johnson, D.M.; Woodruff, D.R.; McCulloh, K.A.; Meinzer, F.C. Leaf hydraulic conductance, measured in situ, declines and recovers daily: Leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiol. 2009, 29, 879–887. [Google Scholar] [CrossRef]
- Stavrinides, M.C.; Daane, K.M.; Lampinen, B.D.; Mills, N.J. Plant water stress, leaf temperature, and spider mite (Acari: Tetranychidae) outbreaks in California vineyards. Environ. Entomol. 2010, 39, 1232–1241. [Google Scholar] [CrossRef]
- Cavallo, P.; Poni, S.; Rotundo, A. Ecophysiology and vine performance of cv. “Aglianico” under various training systems. Sci. Hortic. 2001, 87, 21–32. [Google Scholar] [CrossRef]
- Darra, N.; Psomiadis, E.; Kasimati, A.; Anastasiou, A.; Anastasiou, E.; Fountas, S. Remote and proximal sensing-derived spectral indices and biophysical variables for spatial variation determination in vineyards. Agronomy 2021, 11, 741. [Google Scholar] [CrossRef]

| Water Condition | ||||
|---|---|---|---|---|
| Unlimited | Mid-Limited | Limited | Reference | |
| Dry Spell Duration | Very short to short | Medium | Long to very long | [22] |
| dry days (n) | 7 ÷ 13 | 14 ÷ 19 | >20 | |
| Soil temperature | [23] | |||
| (°C) | <22 | 22 ÷ 30 | >30 | |
| Soil water content | Saturated | Medium | Low | [24] |
| (%) | >70 | 20 ÷ 70 | <20 | |
| Growing Area | Cultivar | Berry Color/Ripening Period 1 |
|---|---|---|
| Northern Italy | ||
| Piemonte | Nebbiolo | B/E |
| Veneto | Glera | W/L |
| Central Italy | ||
| Lazio | Abbuoto | B/L |
| Malvasia del Lazio | W/L | |
| Moscato Terracina | W/M | |
| Bellone | W/M | |
| Maturano | W/M | |
| Nero buono | B/M | |
| Abbruzzo | Trebbiano | W/M |
| Montepulciano | B/M | |
| Toscana | Sangiovese | B/L |
| Southern Italy | ||
| Puglia | Primitivo | B/E |
| International varieties | Chardonnay | W/E |
| Syrah | B/M | |
| Cabernet Sauvignon | B/L |
| Category | ||||
|---|---|---|---|---|
| Physiological Criterion | High | Medium | Low | Reference |
| Leaf pigments | [CHL] > 350 mol m−2 | 350 μmol · m−2 ≤ [CHL] > 250 μmol m−2 | [CHL] ≤ 250 μmol m−2 | [25] |
| Leaf chlorophyll content (CHL) | Malvasia del Lazio, Maturano, Moscato di Terracina, Nero buono, Trebbiano, Sangiovese | Syrah | Chardonnay, Cabernet Sauvignon, Glera, Nebbiolo, Montepulciano, Bellone, Primitivo, Abbuoto | |
| Stomatal behavior under drought stress | 700 > gs > 200 mmol H2O m−2 s−1 | 200 > gs > 50 mmol H2O m−2 s−1 | gs ≤ 50 mmol H2O m−2 s−1 | [26] |
| Stomatal conductance (gs) | Trebbiano, Nebbiolo, Sangiovese | Syrah, Chardonnay, Moscato di Terracina, Abbuoto, Maturano | Primitivo, Cabernet Sauvignon, Nero buono, Montepulciano, Glera, Malvasia del Lazio | |
| Functionality of photosynthetic apparatus | QY > 0.75 | 0.75 ≤ QY > 0.50 | QY ≤ 0.50 | [27] |
| Photosynthetic activity (QY) | Glera, Chardonnay, Abbuoto, Cabernet Sauvignon, Nero buono, Sangiovese, Maturano, Malvasia del Lazio | Nebbiolo, Primitivo, Syrah, Moscato di Terracina, Trebbiano, Montepulciano | Bellone | |
| Photochemical reflectance index (PRI) | Montepulciano, Maturano, Malvasia del Lazio, Glera, Syrah | Abbuoto, Primitivo, Sangiovese, Nebbiolo, Moscato di Terracina, Nero buono, Trebbiano, Cabernet Sauvignon | Bellone, Cabernet Sauvignon | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunori, E.; Bernardini, A.; Moresi, F.V.; Attorre, F.; Biasi, R. Ecophysiological Response of Vitis vinifera L. in an Urban Agrosystem: Preliminary Assessment of Genetic Variability. Plants 2022, 11, 3026. https://doi.org/10.3390/plants11223026
Brunori E, Bernardini A, Moresi FV, Attorre F, Biasi R. Ecophysiological Response of Vitis vinifera L. in an Urban Agrosystem: Preliminary Assessment of Genetic Variability. Plants. 2022; 11(22):3026. https://doi.org/10.3390/plants11223026
Chicago/Turabian StyleBrunori, Elena, Alessandra Bernardini, Federico Valerio Moresi, Fabio Attorre, and Rita Biasi. 2022. "Ecophysiological Response of Vitis vinifera L. in an Urban Agrosystem: Preliminary Assessment of Genetic Variability" Plants 11, no. 22: 3026. https://doi.org/10.3390/plants11223026
APA StyleBrunori, E., Bernardini, A., Moresi, F. V., Attorre, F., & Biasi, R. (2022). Ecophysiological Response of Vitis vinifera L. in an Urban Agrosystem: Preliminary Assessment of Genetic Variability. Plants, 11(22), 3026. https://doi.org/10.3390/plants11223026







