Impact of Water Supply Reduction and Cold Storage on Phenolic Compounds from Mango (Mangifera indica L. cv. Cogshall) Pulp and Peel
Abstract
1. Introduction
2. Results
2.1. Identification of the Phenolic Compounds in Mango Peel
2.1.1. Gallic Acid Derivatives
2.1.2. Xanthone Glycoside
2.1.3. Flavonoids
2.1.4. Gallotannins
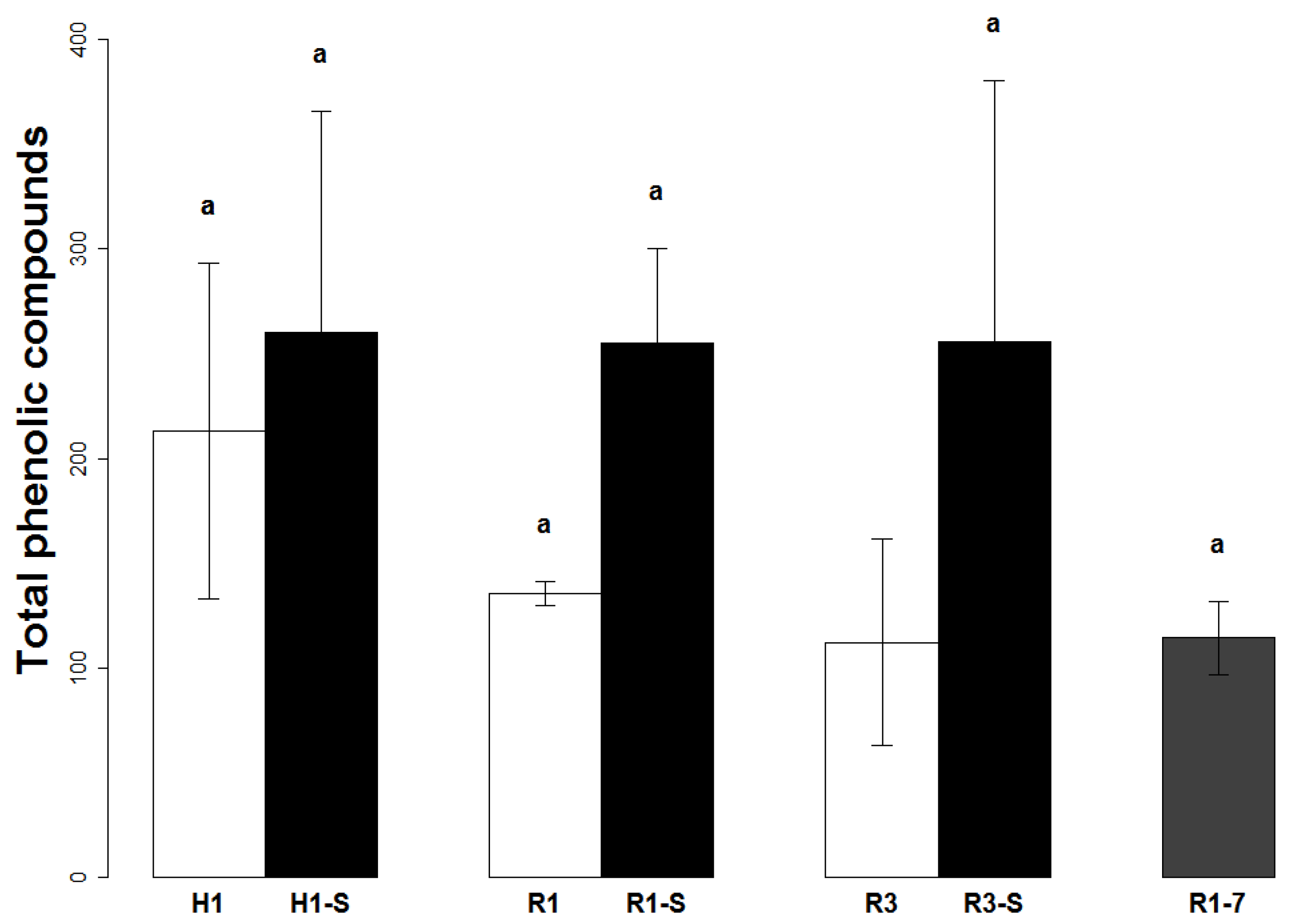
2.2. Quantification of the Phenolic Compounds in Mango Peel
2.3. Quantification of the Phenolic Compounds in Mango Pulp
3. Discussion
4. Material and Methods
4.1. Crops and Fruits
4.1.1. Contrasting Irrigation
4.1.2. Harvests
4.2. Cold Storage
4.3. Post-Harvest Measurements and Quality Descriptors
4.4. Samples
4.5. Extraction of Phenolic Compounds
4.6. Identification of Phenolic Compounds
4.7. Quantification of Phenolic Compounds
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, C.; Chachin, K.; Ueda, Y. Metabolism of phenolic compounds during loquat fruit development. J. Agric. Food Chem. 2001, 49, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoïds and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Silva, F.A.M.; Borges, F.; Guimara, C.; Matos, C.; Reis, S.; Williams, B. Phenolic Acids and Derivatives: Studies on the Relationship among Structure, Radical Scavenging Activity, and Physicochemical Parameters. J. Agric. food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef]
- Zheng, M.S.; Lu, Z.Y. Antiviral effect of mangiferin and isomangiferin on herpes simplex virus. Chin. Med. J. 1990, 103, 160–165. [Google Scholar]
- Masibo, M.; Qian, H. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Ovadia, R.; Galsarker, O.; Maoz, I.; Sela, N.; Maurer, D.; Oren Shamir, M.; Alkan, N. Glycosylated flavonoids: Fruit’s concealed antifungal arsenal. New Phytol. 2020, 225, 1788–1798. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Jabri-karoui, I.; Hamrouni-sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crop. Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Navarro, J.M.; Pérez-Pérez, J.G.; Romero, P.; Botía, P. Analysis of the changes in quality in mandarin fruit, produced by deficit irrigation treatments. Food Chem. 2010, 119, 1591–1596. [Google Scholar] [CrossRef]
- Buendia, B.; Allende, A.; Nicolas, E.; Alarcon, J.J.; Gil, M.I. Effect of Regulated Deficit Irrigation and Crop Load on the Antioxidant Compounds of Peaches. J. Agric. Food Chem. 2008, 56, 3601–3608. [Google Scholar] [CrossRef] [PubMed]
- Sanches, A.G.; da Silva, M.B.; Fernandes TF, S.; Pedrosa VM, D.; Wong MC, C.; Gratão, P.L.; Teixeira, G.H.D.A. Reducing chilling injury in ’Palmer’ mangoes submitted to quarantine cold treatment. J. Sci. Food Agric. 2022, 102, 6112–6122. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, S.; Navarro, P.; Monterde, A.; Benabda, J.; Salvador, A. Effect of postharvest degreening followed by a cold-quarantine treatment on vitamin C, phenolic compounds and antioxidant activity of early-season citrus fruit. Postharvest Biol. Technol. 2012, 65, 13–21. [Google Scholar] [CrossRef]
- Vaio, C.; Di Graziani, G.; Marra, L. Antioxidant capacities, carotenoids and polyphenols evaluation of fresh and refrigerated peach and nectarine cultivars from Italy. Eur. Food Res. Technol. 2008, 227, 1225–1231. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, W.; Cao, J. Effect of cold-shock treatment on chilling injury in mango (Mangifera indica L. cv. ‘Wacheng’) fruit. J. Sci. Food Agric. 2006, 86, 2458–2462. [Google Scholar] [CrossRef]
- Berardini, N.; Fezer, R.; Conrad, J.; Beifuss, U.; Carle, R.; Scheiber, A. Screening of mango (Mangifera indica L.) cultivars for their contents of flavonol O-and xanthone C-glycosides, anthocyanins, and pectin. J. Agric. Food Chem. 2005, 53, 1563–1570. [Google Scholar] [CrossRef]
- Rocha Ribeiro, S.M.; Queiroz, J.H.; Lopes Ribeiro de Queiroz, M.E.; Campos, F.M.; Pinheiro Sant’ana, H.M. Antioxidant in mango (Mangifera indica L.) pulp. Plant Foods Hum. Nutr. 2007, 62, 13–17. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Sgroppo, S.C.; Sánchez Moreno, C.; de Ancos Siguero, B. Mango ‘criollo’ by-products as a source of polyphenols with antioxidant capacity. Ultrasound assisted extraction evaluated by response surface methodology and HPLC-ESI-QTOF-MS/MS characterization. Food Chem. 2022, 396, 133738. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. In vitro bioaccessibility of phenolic compounds and alpha-glucosidase inhibition activity in yoghurts enriched with mango peel powder. Food Biosci. 2022, 50, 102011. [Google Scholar] [CrossRef]
- Luximon-ramma, A.; Bahorun, T.; Crozier, A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. J. Sci. Food Agric. 2003, 502, 496–502. [Google Scholar] [CrossRef]
- Schieber, A.; Berardini, N.; Carle, R. Identification of Flavonol and Xanthone Glycosides from Mango (Mangifera indica L. Cv. “Tommy Atkins ”) Peels by High-Performance Liquid Chromatography-Electrospray. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Berardini, N.; Carle, R.; Schieber, A. Characterization of gallotanins and benzophenone derivatives from mango (Mangifera indica L. cv. ‘Tommy Atkins’) peels, pulp and kernels by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Brecht, J.K.; Talcott, S.T. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007, 105, 1327–1334. [Google Scholar] [CrossRef]
- Palafox-carlos, H.; Yahia, E.; Islas-osuna, M.A.; Gutierrez-martinez, P.; Robles-sánchez, M. Effect of ripeness stage of mango fruit (Mangifera indica L., cv. Ataulfo) on physiological parameters and antioxidant activity. Sci. Hortic. 2012, 135, 7–13. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Stander M a Fawole, O.; Opara, U.L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolic acids, vitamin C and antioxidant activity of pomegranate juice (cv. Wonderful). Sci. Hortic. 2014, 179, 36–45. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Khuong, T.; Lovatt, C.J. Effect of harvest date on the nutritional quality and antioxidant capacity in “Hass” avocado during storage. Food Chem. 2012, 135, 694–698. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gurjar, P.S.; Beer, K.; Pongener, A.; Ravi, S.C.; Singh, S.; Verma, A.; Singh, A.; Tripathy, S.; Verma, D.K.; et al. A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food Biosci. 2022, 48, 101783. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Anal, A.K. Standardization of ultrasound assisted extraction for the recovery of phenolic compounds from mango peels. J. Food Sci. Technol. 2022, 59, 2813–2820. [Google Scholar] [CrossRef]
- Macedo, A.; Gomes, T.; Ribeiro, C.; Moldão-Martins, M.; Duarte, E.; Alves, V.D. Membrane Technology for Valorization of Mango Peel Extracts. Foods 2022, 11, 2581. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC-DAD-ESI-MS n: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Hollecker, L.; Pinna, M.; Filippino, G.; Scrugli, S.; Pinna, B.; Argiolas, F.; Murru, M. Simultaneous determination of polyphenolic compounds in red and white grapes grown in Sardinia by high performance liquid chromatography-electron spray ionisation-mass spectrometry. J. Chromatogr. A 2009, 1216, 3402–3408. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, J.; Wang, Q.; Jiang, W. Postharvest infiltration of BTH reduces infection of mango fruits (Mangifera indica L. cv. Tainong) by Colletotrichum gloeosporioides and enhances resistance inducing compounds. J. Phytopathol. 2008, 156, 68–74. [Google Scholar] [CrossRef]
- Rosalie, R.; Joas, J.; Deytieux-Belleau, C.; Vulcain, E.; Payet, B.; Dufossé, L.; Léchaudel, M. Antioxidant and enzymatic responses to oxidative stress induced by pre-harvest water supply reduction and ripening on mango (Mangifera indica L. cv. “Cogshall”) in relation to carotenoid content. J. Plant Physiol. 2015, 184, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Takahama, U.; Oniki, T. A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol. Plant. 1997, 101, 845–852. [Google Scholar] [CrossRef]
- Manthey, J.; Perkins-Veazie, P. Influences of harvest date and location on the levels of beta-carotene, ascorbic acid, total phenols, the in vitro antioxidant capacity, and phenolic profiles of five commercial varieties of mango (Mangifera indica L.). J. Agric. Food Chem. 2009, 57, 10825–10830. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Giordani, E.; Pippucci, L.; Checchini, L.; Galvan, P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J. Food Compos. Anal. 2009, 22, 668–677. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Dew, T.P.; Orfila, C.; Urwin, P.E. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2011, 59, 9673–9682. [Google Scholar] [CrossRef]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Tovar, M.J.; Romero, M.P.; Girona, J.; Motilva, M.J. L-phenylalanine ammonia-lyase activity and concentration of phenolics in developing olive (Olea europaea L. cv Arbequina) fruit grown under different irrigation regimes. J. Sci. Food Agric. 2002, 82, 892–898. [Google Scholar] [CrossRef]
- Rinaldo, D.; Mbéguié-A-Mbéguié, D.; Fils-Lycaon, B. Advances on polyphenols and their metabolism in sub-tropical and tropical fruits. Trends Food Sci. Technol. 2010, 21, 599–606. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, W.; Wang, B.; Liu, S.; Gong, Z.; Luo, Y. Partial properties of polyphenol oxidase in mango (Mangifera Indica L. cv. “Tainong”) pulp. J. Food Biochem. 2007, 31, 45–55. [Google Scholar] [CrossRef]
- Zhu, J.J.; Li, Y.R.; Liao, J.X. Involvement of anthocyanins in the resistance to chilling-induced oxidative stress in Saccharum officinarum L. leaves. Plant Physiol. Biochem. 2013, 73, 427–433. [Google Scholar] [CrossRef]
- Franz, G.; Grün, M. Chemistry, occurrence and biosynthesis of C-glycosyl compounds in plants. Planta Med. 1983, 47, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V. Bioactive polyphenols: Their role in quality and storability of fruit and vegetables. J. Appl. Bot. 2003, 77, 128–146. [Google Scholar]
- Nadeem, A.; Ahmed, Z.F.R.; Hussain, S.B.; Amin, M.; Javed, S.; Alam-Eldein, S.M.; Mira, A.M. On-Tree Fruit Bagging and Cold Storage Maintain the Postharvest Quality of Mango Fruit. Horticulturae 2022, 8, 814. [Google Scholar] [CrossRef]
- Monteith, J.L. Evaporation and environment. Symp. Soc. Exp. Biol. 1965, 19, 205–234. [Google Scholar] [PubMed]
- Chopart, J.L.; Vauclin, M. Water balance estimation model: Field test and sensitivity analysis. Soil Sci. Soc. Am. J. 1990, 54, 1377–1384. [Google Scholar] [CrossRef]
- Chopart, J.L.; Le Mézo, L.; Mézino, M. PROBE-w (PROgramme de Bilan de l’Eau): Logiciel de modélisation du bilan hydrique dans un sol cultivé. In Presentation and User Guide; CIRAD-PERSYST: Montpellier, France, 2009; 18p. [Google Scholar]
- Léchaudel, M.; Urban, L.; Joas, J. Chlorophyll fluorescence, a nondestructive method to assess maturity of mango fruits (Cv. “Cogshall”) without growth conditions bias. J. Agric. Food Chem. 2010, 58, 7532–7538. [Google Scholar] [CrossRef] [PubMed]
- Joas, J.; Caro, Y.; Léchaudel, M. Ripening behaviour of mango (Cv Lirfa) in relation to carbon nutrition stress and harvest period. In Proceedings of the International Conference Postharvest Unlimited Downunder, Sydney, Australia, 9–12 November 2004; Volume 687, pp. 401–404. [Google Scholar]
- Rosalie, R.; Léchaudel, M.; Dhuique-Mayer, C.; Dufossé, L.; Joas, J. Antioxidant and enzymatic responses to oxidative stress induced by cold temperature storage and ripening in mango (Mangifera indica L. cv. ‘Cogshall’) in relation to carotenoid content. J. Plant Physiol. 2018, 224–225, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Joas, J.; Caro, Y.; Lechaudel, M. Comparison of postharvest changes in mango (cv Cogshall ) using a Ripening class index (Rci) for different carbon supplies and harvest dates. Postharvest Biol. Technol. 2009, 54, 25–31. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 10 October 2022).



| Peak No. | Tr (min) | λ max | MS (-) | MS2 | MS3 | Identification |
|---|---|---|---|---|---|---|
| 1 | 6.7 | 281 | 331 | 169 | galloyl hexose | |
| 2 | 8.6 | 274 | 169 | 125 | gallic acid | |
| 3 | 11.9 | 258, 300 | 331 | 169 | not identified | |
| 4 | 13.1 | 278 | 325 | 169, 281, 125 | galloyl shikimic acid a | |
| 5 | 13.9 | 278 | 325 | 169, 281, 125 | galloyl shikimic acid a | |
| 6 | 15.6 | 290sh, 321 | 423 | 333, 303, 193 | maclurin 3-C-β-D glycosyl b | |
| 7 | 17.5 | 298 | 325 | 163 | p-coumarate glycosyl | |
| 8 | 19.5 | 270 | 479 | 443, 281, 237 | not identified | |
| 9 | 21.4 | 276, 325sh | 575 | 423, 303 | maclurin mono O galloyl glycosyl b | |
| 10 | 22.4 | 282 | 289 | 245, 205, 179 | standard catechin | |
| 11 | 25.7 | 278 | 477 | 325, 169 | di-galloyl shikimic acid a | |
| 12 | 27.8 | 283, 320sh | 727 | 575 | 485, 405, 313 | maclurin di-O-galloyl glycosyl b |
| 13 | 28.2 | 260, 321, 369 | 421 | 331, 301 | Mangiferin c | |
| 14 | 32.6 | 282 | 441 | 289 | 245, 205, 137 | catechin-gallate d |
| 15 | 33.7 | 277 | nd | nd | ||
| 16 | 35.1 | 280 | 787 | 617, 635 | tetra O galloyl glycosyl b | |
| 17 | 35.9 | 280 | 787 | 617, 635 | tetra O galloyl glycosyl b | |
| 18 | 37.3 | 256, 357 | 463 | 301 | 179, 151 | quercetin O glycosyl c |
| 19 | 37.9 | 257, 356 | 463 | 301 | 179, 151 | quercetin O glycosyl c |
| 20 | 38.4 | 282 | 939 | 769, 617 | penta O galloyl glycosyl b | |
| 21 | 39.1 | 280 | 939 | 769, 617 | penta O galloyl glycosyl b | |
| 22 | 39.2 | 258, 358 | 433 | 301 | quercetin pentose e | |
| 23 | 39.8 | 280 | 939 | 769, 617 | penta O galloyl glycosyl b | |
| 24 | 40 | 257, 356 | 433 | 301 | quercetin pentose e | |
| 25 | 40.5 | 281 | 1091 | hexa O galloyl glycosyl b | ||
| 26 | 40.7 | 256, 355 | 433 | 301 | quercetin pentose e | |
| 27 | 40.8 | 281 | 1091 | hexa O galloyl glycosyl b | ||
| 28 | 41.2 | 268, 346 | 447 | 285 | kaempferol hexose f | |
| 29 | 41.4 | 256, 357 | 447 | 301 | quercetin rhamnoside f | |
| 30 | 41.5 | 280 | 1091 | hexa O galloyl glycosyl b | ||
| 31 | 41.8 | 280 | 1091 | hexa O galloyl glycosyl b |
| Tr (min) | λ max | MS/2 (-) | MS (-) | Identification |
|---|---|---|---|---|
| 23.6 | 280 | 545 | 1091 | hexa O galloyl glycosyl |
| 24.7 | 280 | 545 | 1091 | hexa O galloyl glycosyl |
| 26.8 | 280 | 545 | 1091 | hexa O galloyl glycosyl |
| 31.6 | 280 | 621 | 1243 | hepta O galloyl glycosyl |
| 33.6 | 280 | 621 | 1243 | hepta O galloyl glycosyl |
| 34.9 | 280 | 621 | 1243 | hepta O galloyl glycosyl |
| 40 | 280 | 697 | 1395 | octo O galloyl glycosyl |
| 43.4 | 280 | 697 | 1395 | octo O galloyl glycosyl |
| 47–49.1 | 280 | 697, 773 | 1395, 1547 | Octo/nona O galloyl glycosyl |
| 53–57 | 280 | 773, 849 | 1547, 1699 | Nona/deca O galloyl glycosyl |
| 61–64 | 280 | 849, 925 | 1699, 1851 | Deca/undeca O galloyl glycosyl |
| 72 | 280 | 1001 | Dodeca O galloyl glycosyl |
| Peels | n | n-galloyl-O-glycoside | Derivatives of Maclurin | Galloyl-Shikimic Acid | Galloyl-Catechin | Gallotannins | Mangiferin | Derivatives of Quercetin | Derivatives of Kaempferol |
|---|---|---|---|---|---|---|---|---|---|
| Harvest | |||||||||
| H1 | 3 | 2105.17 ± 240.24 a | 113.87 ± 16.27 b | 120.14 ± 0.5 a | 204.43 ± 49.26 a | 40027.7 ± 103.27 a | 327.65 ± 144.26 a | 365.78 ± 96.53 a | 16.36 ± 9.17 a |
| H1-S | 3 | 4428.74 ± 244.97 A | 192.94 ± 56.65 A | 122.2 ± 2.79 A | 165.3 ± 13.47 A | 39365.89 ± 7046.38 B | 32.36 ± 7.39 B | 294.73 ± 61.66 A | n.d |
| Effect | * | n.s | n.s. | n.s. | n.s. | * | n.s | - | |
| Ripe | |||||||||
| R1 | 3 | 1782.19 ± 57.08 a a | 302.44 ± 26.08 a a | 87.35 ± 30.22 a a | 139.51 ± 90.1 a a | 41682.98 ± 4646.9 a a | 157.65 ± 48 a a | 368.09 ± 27.53 a a | 18.21 ± 5.03 a a |
| R1-S | 3 | 3116.5 ± 1586.09 A A | 381.34 ± 132.72 A A | 130.3 ± 25.73 A A | 606.04 ± 302.91 A A | 56810.97 ± 3597.52 A A | 546.71 ± 327.74 A A | 434.25 ± 100.4 A A | 25.87 ± 14.13 A A |
| Effect | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. | |
| R3 | 4 | 1412.13 ± 1080.23 a | 138.13 ± 33.98 a | 59.62 ± 12.44 a | 66.34 ± 29.25 a | 23362.8 ± 8763.63 b | 213.05 ± 14.96 a | 230.82 ± 108.91 a | 7.33 ± 2.44 b |
| R3-S | 3 | 1765.1 ± 416.73 A | 163.39 ± 16.59 A | 78.56 ± 22.13 A | 154.54 ± 35.31 A | 37016.5 ± 6985.17 B | 198.32 ± 25.98 A | 412.37 ± 149.42 A | 27.98 ± 9.52 A |
| Effect | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. | - |
| Peels | n | n-galloyl-O-glycoside | Derivatives of Maclurin | Galloyl-Shikimic Acid | Galloyl-Catechin | Gallotannins | Mangiferin | Derivatives of Quercetin | Derivatives of Kaempferol |
|---|---|---|---|---|---|---|---|---|---|
| R1 | 3 | 1782.19 ± 57.08 | 302.44 ± 26.08 | 87.35 ± 30.22 | 139.51 ± 90.1 | 41682.98 ± 4646.9 | 157.65 ± 48 | 368.09 ± 27.53 | 18.21 ± 5.03 |
| R1-12 | 4 | 1717.17 ± 534.22 | 152.02 ± 52.98 | 133.23 ± 16.82 | 186.31 ± 41.51 | 40903.15 ± 4372.17 | 191.13 ± 43.53 | 278.83 ± 35.83 | 7.25 ± 5.64 |
| R1-7 | 3 | 2252.85 ± 723.35 | 188.63 ± 108.86 | 137.36 ± 8.31 | 238.35 ± 38.65 | 44014.11 ± 7276.26 | 297.57 ± 43.67 | 447.09 ± 49.71 | 26.36 ± 5.63 |
| Effect | n.s. | ** | n.s. | n.s. | n.s. | * | ** | * |
| Pulps | n-galloyl-O-glycoside | Derivatives of Maclurin | p-Coumaric Acid | Gallotannins |
|---|---|---|---|---|
| Harvest | ||||
| H1 | 90.29 ± 6.73 a | 15.39 ± 0.55 a | 2.07 ± 0.53 a | 105.7 ± 72.96 a |
| H1-S | 103.64 ± 18.67 A | 16.27 ± 6.77 A | 3.91 ± 0.58 A | 136.59 ± 82.76 A |
| Effect | n.s | n.s | * | n.s |
| Ripe | ||||
| R1 | 73.98 ± 3.16 b a | 8.9 ± 0.38 b a | 2.22 ± 0.4 a a | 50.46 ± 2.7 a a |
| R1-S | 94.67 ± 2.22 A A | 15.75 ± 3.34 A A | 4.73 ± 1.07 A A | 140.1 ± 41.34 A A |
| Effect | ** | n.s. | * | * |
| R3 | 42.58 ± 33.45 a | 9.17 ± 1.84 a | n.d | 60.54 ± 13.88 a |
| R3-S | 103.43 ± 20.71 A | 14.75 ± 2.08 A | 8.22 ± 2.57 A | 105.7 ± 72.96 A |
| Effect | n.s | n.s. | - | n.s |
| Pulps | n-galloyl-O-glycoside | Derivatives of Maclurin | p-Coumaric Acid | Gallotannins |
|---|---|---|---|---|
| R1 | 73.98 ± 3.16 | 8.9 ± 0.38 b | 2.22 ± 0.4 | 50.46 ± 2.7 a |
| R1-7 | 61.63 ± 7.29 | 9.62 ± 1.47 | 1.09 ± 0.33 | 42.21 ± 10.67 |
| Effect | * | n.s | * | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosalie, R.; Joas, J.; Mertz, C.; Dufossé, L.; Léchaudel, M. Impact of Water Supply Reduction and Cold Storage on Phenolic Compounds from Mango (Mangifera indica L. cv. Cogshall) Pulp and Peel. Plants 2022, 11, 3038. https://doi.org/10.3390/plants11223038
Rosalie R, Joas J, Mertz C, Dufossé L, Léchaudel M. Impact of Water Supply Reduction and Cold Storage on Phenolic Compounds from Mango (Mangifera indica L. cv. Cogshall) Pulp and Peel. Plants. 2022; 11(22):3038. https://doi.org/10.3390/plants11223038
Chicago/Turabian StyleRosalie, Rémy, Jacques Joas, Christian Mertz, Laurent Dufossé, and Mathieu Léchaudel. 2022. "Impact of Water Supply Reduction and Cold Storage on Phenolic Compounds from Mango (Mangifera indica L. cv. Cogshall) Pulp and Peel" Plants 11, no. 22: 3038. https://doi.org/10.3390/plants11223038
APA StyleRosalie, R., Joas, J., Mertz, C., Dufossé, L., & Léchaudel, M. (2022). Impact of Water Supply Reduction and Cold Storage on Phenolic Compounds from Mango (Mangifera indica L. cv. Cogshall) Pulp and Peel. Plants, 11(22), 3038. https://doi.org/10.3390/plants11223038







