Preliminary Evaluation of the Application of Algae-Based Biostimulants on Almond
Abstract
:1. Introduction
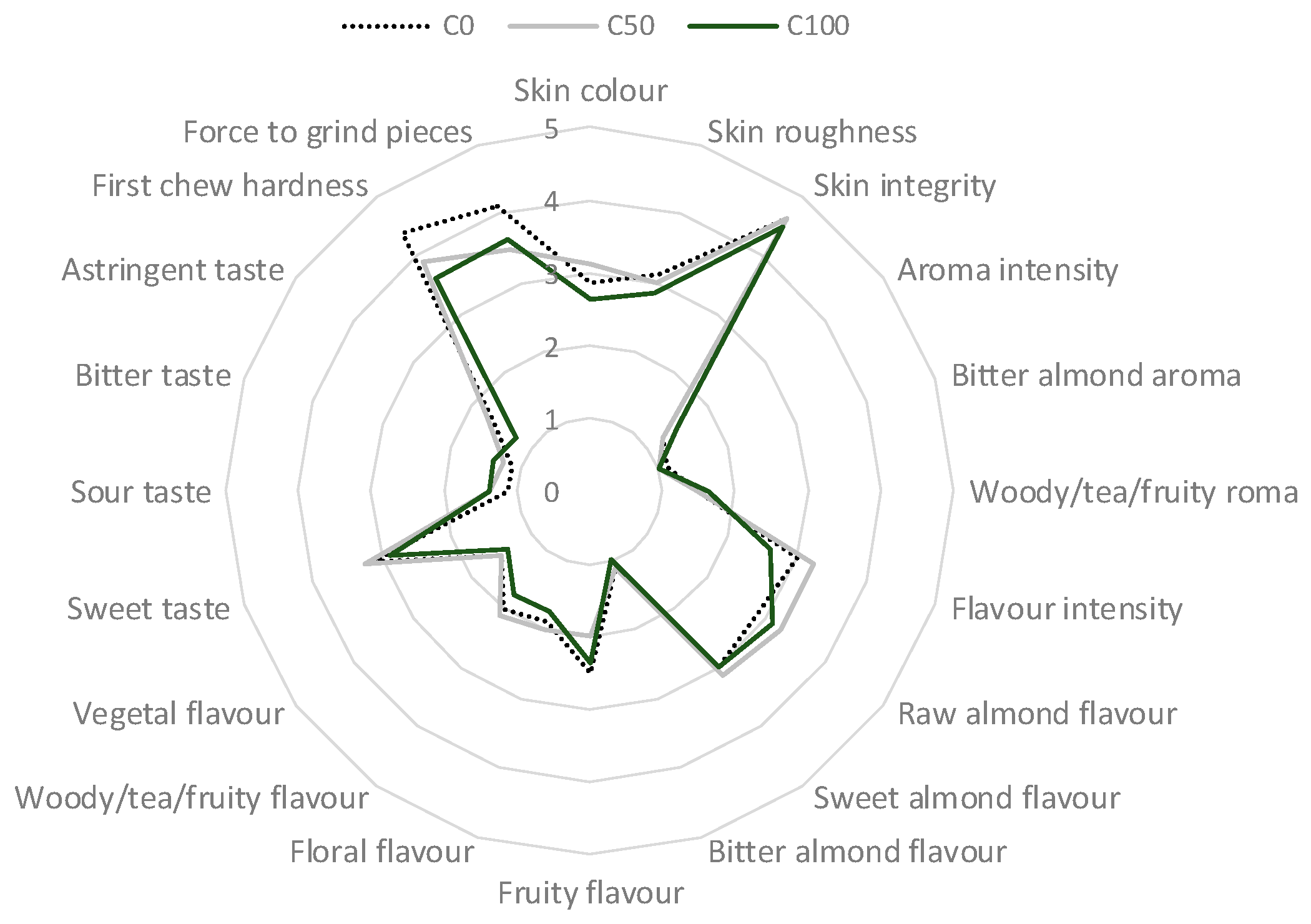
2. Results and Discussion
2.1. Bioactive Composition
2.2. Antioxidant Activity
2.3. Chemical Composition
3. Materials and Methods
3.1. Samples
3.2. Preparation of Extracts for Bioactive Compounds and Antioxidant Assays
3.3. Bioactive Compounds: Ortho-Diphenols, Total Phenolic and Total Flavonoid Content
3.4. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabadán, A.; Álvarez-Ortí, M.; Pardo, J. A comparison of the effect of genotype and weather conditions on the nutritional composition of most important commercial nuts. Sci. Hortic. 2019, 244, 218–224. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Meyer, A.S.; Gonçalves, B. Biostimulants to Improved Tree Physiology and Fruit Quality: A Review with Special Focus on Sweet Cherry. Agronomy 2022, 12, 659. [Google Scholar] [CrossRef]
- Basile, B.; Rouphael, Y.; Colla, G.; Soppelsa, S.; Andreotti, C. Appraisal of emerging crop management opportunities in fruit trees, grapevines and berry crops facilitated by the application of biostimulants. Sci. Hortic. 2020, 267, 109330. [Google Scholar] [CrossRef]
- Souri, M.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Venkatachalan, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond polyphenols: Methods of analysis, contribution to food quality, and health promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef] [Green Version]
- Maatallah, S.; Guizani, M.; Elloumi, O.; Ghrab, M. Phenological and Biochemical Characteristics of Almond Cultivars in Arid Climate of Central Tunisia. Environ. Sci. Proc. 2022, 16, 7. [Google Scholar]
- Lipan, L.; Cano-Lamadrid, M.; Vazquez-Araújo, L.; Sendra, E.; Hernandez, F.; Corell, M.; Moriana, A.; Carbonell-Barrachina, A.A. How does water stress and roasting temperature affect the physicochemical parameters of almonds? Food Sci. Technol. 2021, 150, 112073. [Google Scholar] [CrossRef]
- Čolić, S.D.; Bakić, I.V.; Zagorac, D.Č.D.; Natić, M.M.; Smailagić, A.T.; Pergal, M.V.; Pešić, M.B.; Milinčić, D.D.; Rabrenović, B.B.; Akšić, M.M.F. Chemical fingerprint and kernel quality assessment in different grafting combinations of almond under stress condition. Sci. Hortic. 2021, 275, 109705. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of different processing treatments on almond (Prunus dulcis) bioactive compounds, antioxidant activities, fatty acids, and sensorial characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Bolling, B.; Chen, C.; McKay, D.; Blumberg, J. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.C.; Pinto, D.C.; Figueiredo, C.; Santos, C.; Silva, A.M. Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signal. Mol. 2019, 157–168. [Google Scholar]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Aires, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Phenolic and fatty acid profiles, α-tocopherol and sucrose contents, and antioxidant capacities of understudied Portuguese almond cultivars. J. Food Biochem. 2019, 43, e12887. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Ribeiro, C.; Gonçalves, B. Morphological, mechanical and antioxidant properties of Portuguese almond cultivars. J. Food Sci. Technol. 2018, 55, 467–478. [Google Scholar] [CrossRef] [Green Version]
- Barreira, J.; Ferreira, I.; Oliveira, M.; Pereira, J. Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem. Toxicol. 2008, 46, 2230–2235. [Google Scholar] [CrossRef]
- Vinson, J.A.; Hao, Y.; Su, X.; Zubik, L. Phenol antioxidant quantity and quality in foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Messina, G.A.; Bertolino, F.A.; Pereira, S.V.; Raba, J.; Nazareno, M.A. Comparative study of different methodologies for the determination the antioxidant activity of Venezuelan propolis. Microchem. J. 2020, 158, 105244. [Google Scholar] [CrossRef]
- Sakar, E.; El Yamani, M.; Boussakouran, A.; Ainane, A.; Ainane, T.; Gharby, S.; Rharrabti, Y. Variability of oil content and its physicochemical traits from the main almond [Prunus dulcis Mill. DA Webb] cultivars grown under contrasting environments in north-eastern Morocco. Biocatal. Agric. Biotechnol. 2021, 32, 101952. [Google Scholar] [CrossRef]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Review about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Vargas, F.J.; Tous, J.; Ninot, A.; Miarnau, X. New almond varieties from IRTA’s breeding programme:(1) chemical composition. Acta Hortic 2009, 2009. 912, 477–484. [Google Scholar] [CrossRef]
- Pascoalino, L.A.; Reis, F.S.; Barros, L.; Rodrigues, M.Â.; Correia, C.M.; Vieira, A.L.; Ferreira, I.C.F.R.; Barreira, J.C.M. Effect of Plant Biostimulants on Nutritional and Chemical Profiles of Almond and Hazelnut. Appl. Sci. 2021, 11, 7778. [Google Scholar] [CrossRef]
- Lipan, L.; Cano-Lamadrid, M.; Hernández, F.; Sendra, E.; Corell, M.; Vázquez-Araújo, L.; Moriana, A.; Carbonell-Barrachina, A.A. Long-Term Correlation between Water Deficit and Quality Markers in HydroSOStainable Almonds. Agronomy 2020, 10, 1470. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Rook, F.; Hadingham, S.A.; Li, Y.; Bevan, M.W. Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ. 2006, 29, 426–434. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Garcia, B.; Coelho, J.; Costa, M.; Pinto, J.; Paiva-Martins, F. A simple method for the determination of bioactive antioxidants in virgin olive oils. J. Sci. Food Agric. 2013, 93, 1727–1732. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Siddhraju, P.; Becker, K. Antioxidant properties of various solvents extracts of total phenolic constituents from three differe agroclimatic origins of drumstick tree (Moringa oleifera Lam) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Salleh, W.; Ahmad, F.; Yen, K.; Sirat, H. Chemical compositions, antioxidant and antimicrobial activity of the essential oils of Piper officinarum (Piperaceae). Nat. Prod. Commun. 2012, 7, 1659–1662. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.; Afonso, S.; Pinto, L.; Vieira, S.; Vilela, A.; Silva, A.P. Preliminary Evaluation of the Application of Algae-Based Biostimulants on Almond. Plants 2022, 11, 3083. https://doi.org/10.3390/plants11223083
Oliveira I, Afonso S, Pinto L, Vieira S, Vilela A, Silva AP. Preliminary Evaluation of the Application of Algae-Based Biostimulants on Almond. Plants. 2022; 11(22):3083. https://doi.org/10.3390/plants11223083
Chicago/Turabian StyleOliveira, Ivo, Sílvia Afonso, Luís Pinto, Sofia Vieira, Alice Vilela, and Ana Paula Silva. 2022. "Preliminary Evaluation of the Application of Algae-Based Biostimulants on Almond" Plants 11, no. 22: 3083. https://doi.org/10.3390/plants11223083
APA StyleOliveira, I., Afonso, S., Pinto, L., Vieira, S., Vilela, A., & Silva, A. P. (2022). Preliminary Evaluation of the Application of Algae-Based Biostimulants on Almond. Plants, 11(22), 3083. https://doi.org/10.3390/plants11223083






