Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation
Abstract
:1. Introduction
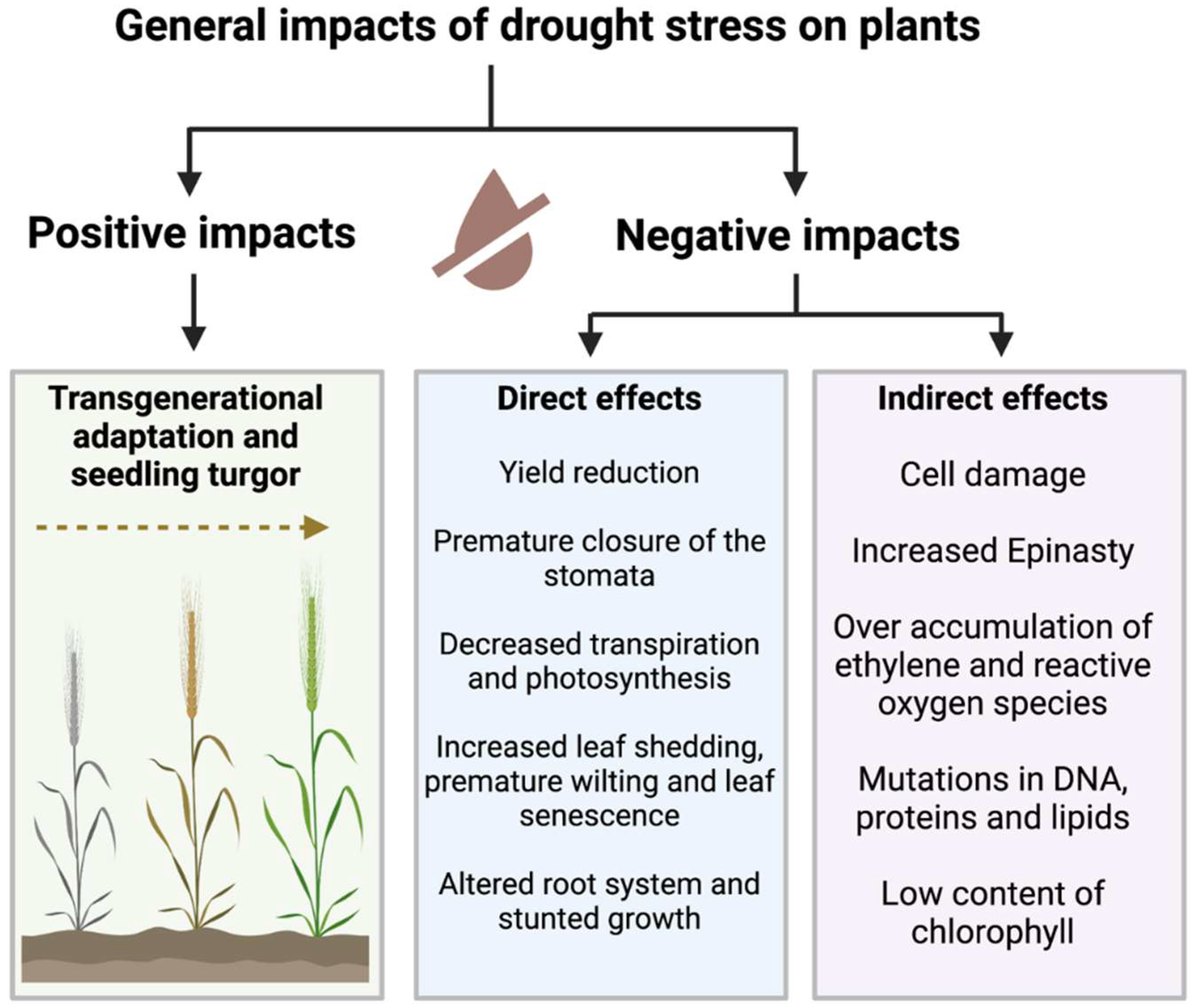
2. Drought’s Detrimental Effects on Crop Plants
3. Mechanistic Outlook of PGPR in Drought-Stressed Plants
3.1. Physiological Components
3.1.1. Osmotic Tuning
3.1.2. VOCs Production
3.1.3. Enhanced Nutrient Uptake
3.1.4. Modulation of the Phytohormonal Level
3.1.5. Fabrication of Exopolysaccharides (EPS)
3.2. Biochemical and Morphological Strategy
3.2.1. Protection by Improved Antioxidants Stature
3.2.2. The Re-Establishment of Turgor Pressure
3.2.3. Attuned Stomatal Conductivity
3.3. Molecular Strategy
3.4. Aspect of System Biology
4. Advancement in the Molecular Study of Drought-Responsive Genes
| PGPR | Host Plant | Role | Notable Genes Identified | Reference |
|---|---|---|---|---|
| Pseudomonas strains | A. thaliana | Improved drought resistance | ACS, ACO, (biosynthesis of ethylene), CPA, ADC, SAMDC, SPMS, SPDS, and AIH (biosynthesis of polyamine), Pdf1.2 (JA marker gene), VSP1 (ethylene-responsive gene), and PR1 (a gene involved in the regulation of SA) | Wang, et al. [145] |
| Bacillus spp. | P. nigrum | Improved drought resistance | VA, Cadhn, and Shsp | Guterman [146] |
| P. florescens | O. sativa | Improved drought resistance | COC1, COX1 ERD15, Hsp20, bZIP1, and PKDB | Wang, et al. [147] |
| Pseudomonas strains | L. barium | Improved drought resistance | RAB18, LbSKOR, and LbKT1 | Kaushal [26] |
| B. amyloliquefaciens 5113, and A. brasilense N040 | T. aestivum | Increment in the redox cycle of ascorbate-glutathione | SAMS1, HSP 17.8, and APX1 | Wu, et al. [148] |
5. Seed Priming with PGPR Bioinoculants in the Mitigation of Drought Stress
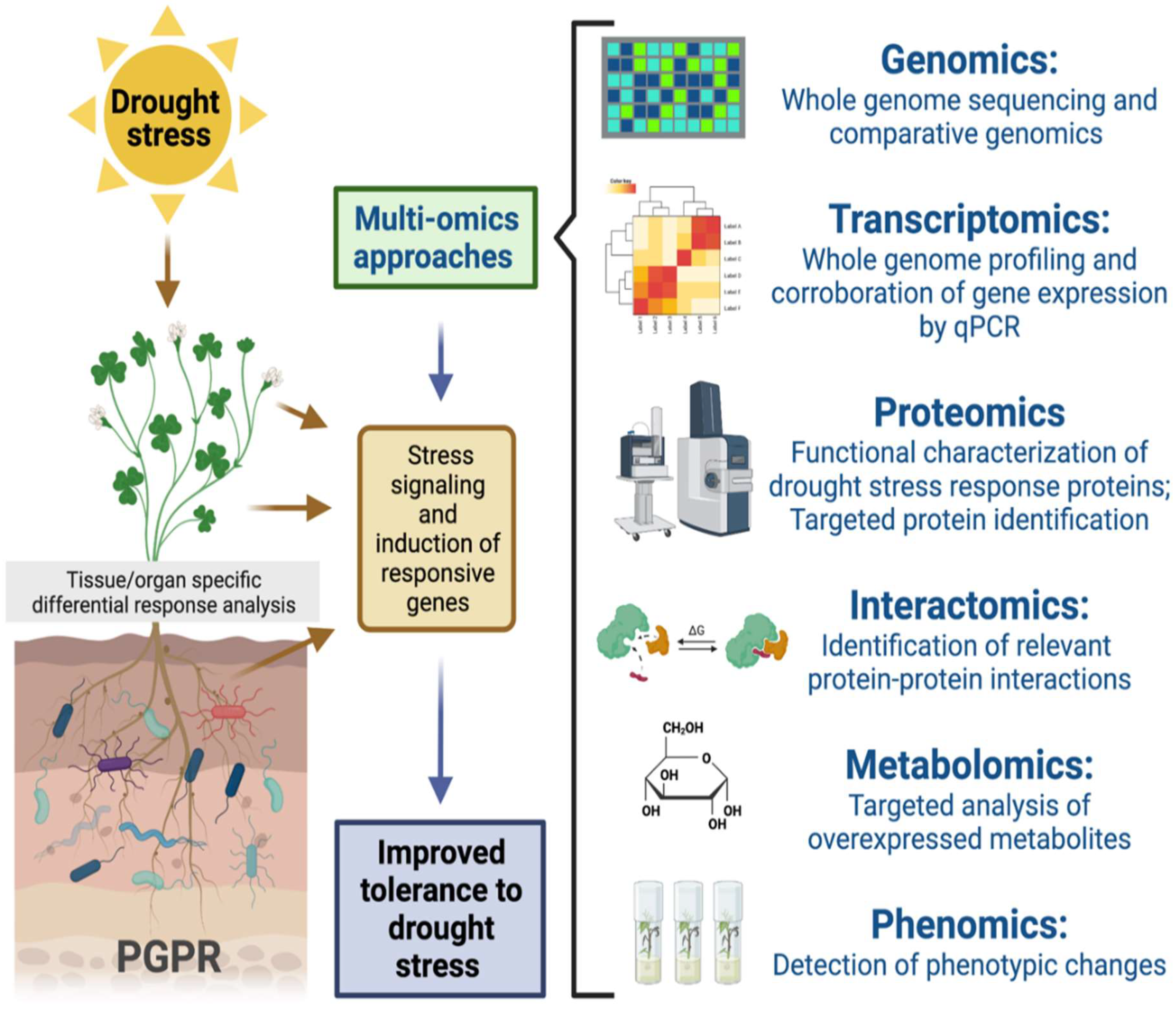
6. Omics Approaches Employed in the Microbe-Mediated Mitigation of Drought Stress
Major Constraints and Future Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The potential role of microbial biostimulants in the amelioration of climate change-associated abiotic stresses on crops. Front. Microbiol. 2022, 12, 829099. [Google Scholar] [CrossRef] [PubMed]
- Aryal, J.P.; Sapkota, T.B.; Khurana, R.; Khatri-Chhetri, A.; Rahut, D.B.; Jat, M.L. Climate change and agriculture in South Asia: Adaptation options in smallholder production systems. Environ. Dev. Sustain. 2020, 22, 5045–5075. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security; Food and Agricultural Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Rivera-Ferre, M.G. From agriculture to food systems in the IPCC. Glob. Change Biol. 2020, 26, 2731–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- IPCC. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Samant, M.; Pandey, S.C.; Pandey, A. Impact of hazardous waste material on environment and their management strategies. In Microbial Biotechnology in Environmental Monitoring and Cleanup; IGI Global: Hershey, PA, USA, 2018; pp. 175–192. [Google Scholar] [CrossRef] [Green Version]
- Pande, V.; Pandey, S.C.; Sati, D.; Pande, V.; Samant, M. Bioremediation: An emerging effective approach towards environment restoration. Environ. Sustain. 2020, 3, 91–103. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Tariq, H.; Latif, S.; Yasmin, H.; Mehmood, A.; Shahid, M.A. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy 2020, 10, 1683. [Google Scholar] [CrossRef]
- Khan, A.; Pan, X.; Najeeb, U.; Tan, D.K.Y.; Fahad, S.; Zahoor, R.; Luo, H. Coping with drought: Stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 2018, 51, 47. [Google Scholar] [CrossRef]
- Omomowo, I.O.; Fadiji, A.E.; Omomowo, O.I. Assessment of bio-efficacy of Glomus versiforme and Trichoderma harzianum in inhibiting powdery mildew disease and enhancing the growth of cowpea. Ann. Agric. Sci. 2018, 63, 9–17. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Vigani, G.; Borin, S.; Sorlini, C.; Ouzari, H.; Zocchi, G.; Daffonchio, D. Are drought-resistance promoting bacteria cross-compatible with different plant models? Plant Signal. Behav. 2013, 8, e26741. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Chiappero, J.; del Rosario Cappellari, L.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts toward overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.M.; Trexler, R.V.; Malik, R.J.; Hockett, K.L.; Bell, T.H. The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol. 2019, 37, 140–151. [Google Scholar] [CrossRef]
- Naylor, D.; DeGraaf, S.; Purdom, E.; Coleman-Derr, D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017, 11, 2691–2704. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.; Ali, B.; Sajid, I. Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front. Microbiol. 2016, 7, 1334. [Google Scholar] [CrossRef] [Green Version]
- WWAP. United Nations World Water Assessment Programme, 2018. The United Nations World Water Development Report 2018: Nature-Based Solutions; UNESCO: Paris, France, 2018. [Google Scholar]
- Howitt, R.; Medellín-Azuara, J.; MacEwan, D.; Lund, J.R.; Sumner, D. Economic Analysis of the 2015 Drought for California Agriculture; Center for Watershed Sciences: Davis, CA, USA, 2015. [Google Scholar]
- Juana, J.S.; Makepe, M.; Mangadi, K.; Narayana, N. The socio-economic impact of drought in Botswana. Int. J. Environ. Dev. 2014, 11, 43–60. [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Kaushal, M. Microbes in cahoots with plants: MIST to hit the jackpot of agricultural productivity during drought. Int. J. Mol. Sci. 2019, 20, 1769. [Google Scholar] [CrossRef]
- Lovelace, A.H.; Ma, W. How do bacteria transform plants into their oasis? Cell Host Microbe 2022, 30, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Gall, H.L.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 1–16. [Google Scholar]
- Steiner, F.; Zuffo, A.M.; Zoz, T.; Zoz, A.; Zoz, J. Drought tolerance of wheat and black oat crops at early stages of seedling growth. Rev. Ciênc. Agrár. 2017, 40, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhou, L.; Addo-Danso, S.D.; Ding, G.; Sun, M.; Wu, S.; Lin, S. Nitrogen supply enhances the physiological resistance of Chinese fir plantlets under polyethylene glycol (PEG)-induced drought stress. Sci. Rep. 2020, 10, 7509. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, W.; Broughton, K.; Smith, R.; Sharwood, R.; Payton, P.; Bange, M.; Tissue, D.T. Impacts of growth temperature, water deficit and heatwaves on carbon assimilation and growth of cotton plants (Gossypium hirsutum L.). Environ. Exp. Bot. 2020, 179, 104204. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Arraes, F.B.M.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.R.; Albuquerque, E.V.S.; Marin, S.R.R.; Purgatto, E.; Nepomuceno, A.L.; Grossi-de-Sa, M.F. Implications of ethylene biosynthesis and signaling in soybean drought stress tolerance. BMC Plant Biol. 2015, 15, 213. [Google Scholar] [CrossRef]
- Brunetti, C.; Savi, T.; Nardini, A.; Loreto, F.; Gori, A.; Centritto, M. Changes in abscisic acid content during and after drought are related to carbohydrate mobilization and hydraulic recovery in poplar stems. Tree Physiol. 2020, 40, 1043–1057. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, M.; Guo, C. Crop pollen development under drought: From the phenotype to the mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, N.; Ilyas, N.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol. Biochem. 2021, 166, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.-D.; Liu, H.-X.; Wang, Y.-P.; Guo, J.-H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Linderholm, H.W.; Song, Y.; Wang, F.; Liu, Y.; Tian, J.; Xu, J.; Song, Y.; Ren, G. Impacts of drought on maize and soybean production in northeast China during the past five decades. Int. J. Environ. Res. Public Health 2020, 17, 2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef]
- Bao, G.; Ashraf, U.; Wang, C.; He, L.; Wei, X.; Zheng, A.; Mo, Z.; Tang, X. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 2018, 133, 149–157. [Google Scholar] [CrossRef]
- Suárez, R.; Wong, A.; Ramírez, M.; Barraza, A.; Orozco, M.D.C.; Cevallos, M.A.; Lara, M.; Hernández, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant-Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef] [Green Version]
- Vílchez, J.I.; García-Fontana, C.; Román-Naranjo, D.; González-López, J.; Manzanera, M. Plant drought tolerance enhancement by trehalose production of desiccation-tolerant microorganisms. Front. Microbiol. 2016, 7, 1577. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009, 181, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Durán, P.; Acuña, J.; Armada, E.; López-Castillo, O.; Cornejo, P.; Mora, M.; Azcón, R. Inoculation with selenobacteria and arbuscular mycorrhizal fungi to enhance selenium content in lettuce plants and improve tolerance against drought stress. J. Soil Sci. Plant Nutr. 2016, 16, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Campos-García, J.; López-Bucio, J. Pseudomonas putida and Pseudomonas fluorescens influence Arabidopsis root system architecture through an auxin response mediated by bioactive cyclodipeptides. J. Plant Growth Regul. 2020, 39, 254–265. [Google Scholar] [CrossRef]
- Singh, T.B.; Sahai, V.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Screening and evaluation of PGPR strains having multiple PGP traits from hilly terrain. J. Appl. Biol. Biotechnol. 2020, 8, 3–4. [Google Scholar]
- Karimi, N.; Goltapeh, E.M.; Amini, J.; Mehnaz, S.; Zarea, M.J. Effect of Azospirillum zeae and seed priming with zinc, manganese and auxin on growth and yield parameters of wheat, under dryland farming. Agric. Res. 2021, 10, 44–55. [Google Scholar] [CrossRef]
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a Bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM 196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Satlewal, A.; Varma, A. Characterization of plant growth-promoting rhizobacteria (PGPR): A perspective of conventional versus recent techniques. In Heavy Metal Contamination of Soils; Soil Biology; Sherameti, I., Varma, A., Eds.; Springer: Cham, Switzerland, 2015; Volume 44. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A.; Ali, S.; Babar, M. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Kang, S.-M.; Waqas, M.; Khan, A.L.; Lee, I.-J. Plant-growth-promoting rhizobacteria: Potential candidates for gibberellins production and crop growth promotion. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; Volume 1, pp. 1–19. [Google Scholar]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in arabidopsis plants. Plant Growth Regul. 2008, 54, 97–103. [Google Scholar] [CrossRef]
- Zaheer, M.S. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Cytokinins on the Growth and Yield of Wheat (Triticum aestivum L.) under Drought. Doctoral Dissertation, Islamia University, Bahawalpur, Pakistan, 2019. Available online: http://173.208.131.244:9060/xmlui/handle/123456789/2261 (accessed on 23 October 2022).
- Cohen, A.C.; Bottini, R.; Piccoli, P. Role of abscisic acid producing PGPR in sustainable agriculture. In Bacterial Metabolites in Sustainable Agroecosystem; Maheshwari, D.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 259–282. [Google Scholar]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Brunetti, C. Abscisic acid biosynthesis and signaling in plants: Key targets to improve water use efficiency and drought tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Salomon, M.V.; Bottini, R.; de Souza Filho, G.A.; Cohen, A.C.; Moreno, D.; Gil, M.; Piccoli, P. Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, induce abscisic acid accumulation and synthesis of defense-related terpenes in in vitro cultured grapevine. Physiol. Plant. 2014, 151, 359–374. [Google Scholar] [CrossRef]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef]
- Shakir, M.A.; Asghari, B.; Muhammad, A. Rhizosphere bacteria containing ACC-deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ. 2012, 31, 108–112. [Google Scholar]
- Vandana, U.K.; Singha, B.; Gulzar, A.; Mazumder, P. Molecular mechanisms in plant growth promoting bacteria (PGPR) to resist environmental stress in plants. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Sharma, V., Salwan, R., Khalil, L., Al-Ani, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–233. [Google Scholar]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Chapin, L.J.; Taylor, C.G.; Jones, M.L. Identification of Pseudomonas Spp. that increase ornamental crop quality during abiotic stress. Front. Plant Sci. 2020, 10, 1754. [Google Scholar] [CrossRef] [Green Version]
- Arshad, M.; Shaharoona, B.; Mahmood, T. Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere 2008, 18, 611–620. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004, 166, 525–530. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, S.-D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013, 29, 201. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regul. 2003, 39, 137–141. [Google Scholar] [CrossRef]
- Bandurska, H. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 2005, 27, 379–386. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Growth and nutrition of cowpea (Vigna unguiculata) under water deficit as influenced by microbial inoculation via seed coating. J. Agron. Crop Sci. 2019, 205, 447–459. [Google Scholar] [CrossRef]
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Guo, J.; Zhu, A.; Zhang, L.; Zhang, M. Exogenous jasmonic acid can enhance tolerance of wheat seedlings to salt stress. Ecotoxicol. Environ. Saf. 2014, 104, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-M.; Kim, Y.H.; Anderson, A.J.; Kim, Y.C. Nitric oxide and hydrogen peroxide production are involved in systemic drought tolerance induced by 2R, 3R-butanediol in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 427. [Google Scholar] [CrossRef] [Green Version]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.; Khan, W.; Enshasy, H.A.E.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G.; De-Bashan, L.E. Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances (1997–2003). Can. J. Microbiol. 2004, 50, 521–577. [Google Scholar] [CrossRef] [Green Version]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen Sulfur Species in Plants: Production, Metabolism, Signaling Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley & Sons: New York, NY, USA, 2019; pp. 311–336. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Thangappan, S.; Uthandi, S. Plant growth-promoting Bacillus sp. cahoots moisture stress alleviation in rice genotypes by triggering antioxidant defense system. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Kordi, S. Hardening pretreatment by drought and low temperature enhanced chilling stress tolerance of cucumber seedlings. Acta Sci. Pol. Hortorum Cultus 2019, 18, 29–37. [Google Scholar] [CrossRef]
- Yaseen, R.; Aziz, O.; Saleem, M.H.; Riaz, M.; Zafar-ul-Hye, M.; Rehman, M.; Ali, S.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A. Ameliorating the drought stress for wheat growth through application of ACC-deaminase containing rhizobacteria along with biogas slurry. Sustainability 2020, 12, 6022. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, N. Microbial amelioration of salinity stress in HD 2967 wheat cultivar by up-regulating antioxidant defense. Commun. Integr. Biol. 2021, 14, 136–150. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; DaCosta, M.; Jiang, Y. Research advances in mechanisms of turfgrass tolerance to abiotic stresses: From physiology to molecular biology. Crit. Rev. Plant Sci. 2014, 33, 141–189. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants; Ahmad, P., Ed.; John Wiley & Sons: New York, NY, USA, 2016; Volume 1, pp. 24–40. [Google Scholar] [CrossRef]
- Shariati, J.V.; Malboobi, M.A.; Tabrizi, Z.; Tavakol, E.; Owlia, P.; Safari, M. Comprehensive genomic analysis of a plant growth-promoting rhizobacterium Pantoea agglomerans strain P5. Sci. Rep. 2017, 7, 15610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defez, R.; Andreozzi, A.; Bianco, C. The overproduction of indole-3-acetic acid (IAA) in endophytes upregulates nitrogen fixation in both bacterial cultures and inoculated rice plants. Microb. Ecol. 2017, 74, 441–452. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Chang, W.; van de Mortel, M.; Nielsen, L.; de Guzman, G.; Li, X.; Halverson, L.J. Alginate production by Pseudomonas putida creates a hydrated microenvironment and contributes to biofilm architecture and stress tolerance under water-limiting conditions. J. Bacteriol. 2007, 189, 8290–8299. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Ghosh, D.; Mohapatra, S. Modulation of polyamine biosynthesis in Arabidopsis thaliana by a drought mitigating Pseudomonas putida strain. Plant Physiol. Biochem. 2018, 129, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Sonbarse, P.P.; Kiran, K.; Sharma, P.; Parvatam, G. Biochemical and molecular insights of PGPR application for the augmentation of carotenoids, tocopherols, and folate in the foliage of Moringa oleifera. Phytochemistry 2020, 179, 112506. [Google Scholar] [CrossRef]
- Kakar, K.; Ren, X.L.; Nawaz, Z.; Cui, Z.Q.; Li, B.; Xie, G.L.; Hassan, M.; Ali, E.; Sun, G.C. A consortium of rhizobacterial strains and biochemical growth elicitors improve cold and drought stress tolerance in rice (Oryza sativa L.). Plant Biol. 2016, 18, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Nosheen, A.; Naz, R.; Bano, A.; Keyani, R. L-tryptophan-assisted PGPR-mediated induction of drought tolerance in maize (Zea mays L.). J. Plant Interact. 2017, 12, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Galván, A.E.; Cortés-Patiño, S.; Romero-Perdomo, F.; Uribe-Vélez, D.; Bashan, Y.; Bonilla, R.R. Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Appl. Soil Ecol. 2020, 147, 103367. [Google Scholar] [CrossRef]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef]
- Yasmin, H.; Bano, A.; Wilson, N.L.; Nosheen, A.; Naz, R.; Hassan, M.N.; Ilyas, N.; Saleem, M.H.; Noureldeen, A.; Ahmad, P. Drought-tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiol. Plant 2022, 174, e13497. [Google Scholar] [CrossRef]
- Luziatelli, F.; Gatti, L.; Ficca, A.G.; Medori, G.; Silvestri, C.; Melini, F.; Muleo, R.; Ruzzi, M. Metabolites secreted by a plant-growth-promoting Pantoea agglomerans strain improved rooting of Pyrus communis L. cv Dar Gazi cuttings. Front. Microbiol. 2020, 11, 539359. [Google Scholar] [CrossRef]
- Belimov, A.; Dodd, I.; Safronova, V.; Shaposhnikov, A.; Azarova, T.; Makarova, N.; Davies, W.; Tikhonovich, I. Rhizobacteria that produce auxins and contain 1-amino-cyclopropane-1-carboxylic acid deaminase decrease amino acid concentrations in the rhizosphere and improve growth and yield of well-watered and water-limited potato (Solanum tuberosum). Ann. Appl. Biol. 2015, 167, 11–25. [Google Scholar] [CrossRef]
- Pourbabaee, A.; Bahmani, E.; Alikhani, H.; Emami, S. Promotion of wheat growth under salt stress by halotolerant bacteria containing ACC deaminase. JAST 2016, 18, 855–864. [Google Scholar]
- García, J.E.; Maroniche, G.; Creus, C.; Suárez-Rodríguez, R.; Ramirez-Trujillo, J.A.; Groppa, M.D. In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2017, 202, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Silva, R.; Filgueiras, L.; Santos, B.; Coelho, M.; Silva, M.; Estrada-Bonilla, G.; Vidal, M.; Baldani, J.I.; Meneses, C. Gluconacetobacter diazotrophicus changes the molecular mechanisms of root development in Oryza sativa L. growing under water stress. Int. J. Mol. Sci. 2020, 21, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlauch, K.A.; Grimplet, J.; Cushman, J.; Cramer, G.R. Transcriptomics Analysis Methods: Microarray Data Processing, Analysis and Visualization Using the Affymetrix Genechip® Vitis Vinifera Genome Array. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. MBio 2015, 6, e00615–e00621. [Google Scholar] [CrossRef] [Green Version]
- Alavi, P.; Starcher, M.R.; Zachow, C.; Müller, H.; Berg, G. Root-microbe systems: The effect and mode of interaction of stress protecting agent (SPA) Stenotrophomonas rhizophila DSM14405T. Front. Plant Sci. 2013, 4, 141. [Google Scholar] [CrossRef] [Green Version]
- Vargas, L.; de Carvalho, T.L.G.; Ferreira, P.C.G.; Baldani, V.L.D.; Baldani, J.I.; Hemerly, A.S. Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependent on plant and bacterial genotypes. Plant Soil 2012, 356, 127–137. [Google Scholar] [CrossRef]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the potentialities of beneficial endophytes for improved plant growth. Saudi J. Biol. Sci. 2020, 27, 3622–3633. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J. Microbiol. Methods 2020, 170, 105860. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sanchez, C.; Li, H.; Chen, S. Recent advances and challenges in plant phosphoproteomics. Proteomics 2015, 15, 1127–1141. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Kim, Y.-J.; Choi, E.-S.; Koh, S.-C.; Lee, S.-W.; Kim, Y.-J.; Yang, D.C. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiol. Res. 2015, 172, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ghabooli, M.; Khatabi, B.; Ahmadi, F.S.; Sepehri, M.; Mirzaei, M.; Amirkhani, A.; Jorrín-Novo, J.V.; Salekdeh, G.H. Proteomics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J. Proteom. 2013, 94, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Yang, P.; Han, F.; Jing, Y.; Shen, S. Proteomic analysis of rice seedlings infected by Sinorhizobium meliloti 1021. Proteomics 2010, 10, 1861–1874. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A.; Hassen, A.I.; Labuschagne, N. Rhizobacteria-induced systemic tolerance against drought stress in Sorghum bicolor (L.) Moench. Microbiol. Res. 2020, 232, 126388. [Google Scholar] [CrossRef]
- Martínez-Cortés, T.; Pomar, F.; Merino, F.; Novo-Uzal, E. A proteomic approach to Physcomitrella patens rhizoid exudates. J. Plant Physiol. 2014, 171, 1671–1678. [Google Scholar] [CrossRef]
- Luziatelli, F.; Ficca, A.G.; Bonini, P.; Muleo, R.; Gatti, L.; Meneghini, M.; Tronati, M.; Melini, F.; Ruzzi, M. A genetic and metabolomic perspective on the production of indole-3-acetic acid by Pantoea agglomerans and use of their metabolites as biostimulants in plant nurseries. Front. Microbiol. 2020, 1475. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W.; Wang, J.; Ma, J.; Li, C.; Zhou, F.; Zhang, S.; Yu, Y.; Zhang, L.; Li, W. Identification of differentially expressed genes in sunflower (Helianthus annuus) leaves and roots under drought stress by RNA sequencing. Bot. Stud. 2017, 58, 42. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Y.; de Jong, A.; Frenzel, E.; Kuipers, O.P. Comparative transcriptomics of Bacillus mycoides strains in response to potato-root exudates reveals different genetic adaptation of endophytic and soil isolates. Front. Microbiol. 2017, 8, 1487. [Google Scholar] [CrossRef] [PubMed]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.-M.; Kang, B.R.; Kim, Y.C. Transcriptome analysis of induced systemic drought tolerance elicited by Pseudomonas chlororaphis O6 in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 209. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, B.A.; Kantar, M.; Budak, H. Root precursors of microRNAs in wild emmer and modern wheats show major differences in response to drought stress. Funct. Integr. Genom. 2015, 15, 587–598. [Google Scholar] [CrossRef]
- Vargas, L.; Santa Brigida, A.B.; Mota Filho, J.P.; De Carvalho, T.G.; Rojas, C.A.; Vaneechoutte, D.; Van Bel, M.; Farrinelli, L.; Ferreira, P.C.; Vandepoele, K. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [Green Version]
- Ghasemlou, F.; Amiri, H.; Karamian, R.; Mirzaie-asl, A. Alleviation of the effects of on drought stress Verbascum nudicuale by methyl jasmonate and titanium dioxide nanoparticles. Iran. J. Plant Physiol. 2019, 9, 2911–2920. [Google Scholar]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Ullah, I.; Ali, S.; Kang, S.-M.; Lee, I.-J. Alleviation of salt stress response in soybean plants with the endophytic bacterial isolate Curtobacterium sp. SAK1. Ann. Microbiol. 2019, 69, 797–800. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Wang, Y.; Ohara, Y.; Nakayashiki, H.; Tosa, Y.; Mayama, S. Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol. Plant-Microbe Interact. 2005, 18, 385–396. [Google Scholar] [CrossRef]
- Guterman, L. Distortions to Agricultural Incentives in Colombia; Agricultural Distortions Working Paper Series No. 1856–2016–152637; Word Bank: Washington, DC, USA, 2007; Available online: https://ideas.repec.org/p/ags/wbadwp/ (accessed on 1 August 2022).
- Wang, D.; Pan, Y.; Zhao, X.; Zhu, L.; Fu, B.; Li, Z. Genome-wide temporal-spatial gene expression profiling of drought responsiveness in rice. BMC Genom. 2011, 12, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Ma, L.; Liu, Q.; Vestergård, M.; Topalovic, O.; Wang, Q.; Zhou, Q.; Huang, L.; Yang, X.; Feng, Y. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard. Mater. 2020, 395, 122661. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Arora, N.K. Bioformulations for plant growth promotion and combating phytopathogens: A Sustainable Approach. In Bioformulations: For Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 3–33. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H. Seed coating with arbuscular mycorrhizal fungi as an ecotechnologicalapproach for sustainable agricultural production of common wheat (Triticum aestivum L.). J. Toxicol. Environ. Health Part A 2016, 79, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadhave, K.R.; Devlin, P.F.; Ebertz, A.; Ross, A.; Gange, A.C. Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microb. Ecol. 2018, 76, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, A.; Turgay, O.C.; Farooq, M.; Hayat, R. Seed biopriming with plant growth promoting rhizobacteria: A review. FEMS Microbiol. Ecol. 2016, 92, fiw112. [Google Scholar] [CrossRef] [Green Version]
- Hafez, E.M.; Alsohim, A.S.; Farig, M.; Omara, A.E.-D.; Rashwan, E.; Kamara, M.M. Synergistic effect of biochar and plant growth promoting rhizobacteria on alleviation of water deficit in rice plants under salt-affected soil. Agronomy 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Vosátka, M.; Látr, A.; Gianinazzi, S.; Albrechtová, J. Development of arbuscular mycorrhizal biotechnology and industry: Current achievements and bottlenecks. Symbiosis 2012, 58, 29–37. [Google Scholar] [CrossRef]
- Azmat, A.; Yasmin, H.; Hassan, M.N.; Nosheen, A.; Naz, R.; Sajjad, M.; Ilyas, N.; Akhtar, M.N. Co-application of bio-fertilizer and salicylic acid improves growth, photosynthetic pigments and stress tolerance in wheat under drought stress. PeerJ 2020, 8, e9960. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Anis, M.; Zaki, M.J.; Dawar, S. Development of a Na-alginate-based bioformulation and its use in the management of charcoal rot of sunflower (Helianthus annuus L.). Pak. J. Bot. 2012, 44, 1167–1170. [Google Scholar]
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of endophytic Pseudomonas fluorescens and a bacterial consortium to Brassica napus can increase plant height and biomass under greenhouse and field conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar based inoculants improve soybean growth and nodulation. Agric. Sci. 2017, 8, 1048–1064. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-de-La-Cruz, G.; Aguirre-Mancilla, C.L.; Godínez-Garrido, N.A.; Osornio-Flores, N.M.; Torres-Castillo, J.A. Chitosan mixed with beneficial fungal conidia or fungicide for bean (Phaseolus vulgaris L.) seed coating. Interciencia 2017, 42, 307–312. [Google Scholar]
- Khan, N.; Mishra, A.; Chauhan, P.; Nautiyal, C. Induction of Paenibacillus lentimorbus biofilm by sodium alginate and CaCl2 alleviates drought stress in chickpea. Ann. Appl. Biol. 2011, 159, 372–386. [Google Scholar] [CrossRef]
- Quastel, J. ‘Krilium’ and synthetic soil conditioners. Nature 1953, 171, 7–10. [Google Scholar] [CrossRef]
- Leinauer, B.; Serena, M.; Singh, D. Seed coating and seeding rate effects on turfgrass germination and establishment. HortTechnology 2010, 20, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Gorim, L.; Asch, F. Effects of composition and share of seed coatings on the mobilization efficiency of cereal seeds during germination. J. Agron. Crop Sci. 2012, 198, 81–91. [Google Scholar] [CrossRef]
- Dangi, S.R.; Tirado-Corbalá, R.; Gerik, J.; Hanson, B.D. Effect of long-term continuous fumigation on soil microbial communities. Agronomy 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Finkel, O.M.; Salas-González, I.; Castrillo, G.; Conway, J.M.; Law, T.F.; Teixeira, P.J.P.L.; Wilson, E.D.; Fitzpatrick, C.R.; Jones, C.D.; Dangl, J.L. A single bacterial genus maintains root growth in a complex microbiome. Nature 2020, 587, 103–108. [Google Scholar] [CrossRef]
- Cardinale, M.; Ratering, S.; Suarez, C.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 2015, 181, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrar, K.; Bryant, D.; Cope-Selby, N. Understanding and engineering beneficial plant–microbe interactions: Plant growth promotion in energy crops. Plant Biotechnol. J. 2014, 12, 1193–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Cui, J.; Zhai, J.; Li, J.; Han, L.; Meng, J. High-throughput sequencing reveals differential expression of miRNAs in tomato inoculated with Phytophthora infestans. Planta 2015, 241, 1405–1416. [Google Scholar] [CrossRef]
- Fernie, A.R.; Yan, J. De novo domestication: An alternative route toward new crops for the future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Plant growth promoting Rhizobacteria-an efficient tool for agriculture promotion. Adv. Plants Agric. Res. 2016, 4, 426–434. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 16, 127137. [Google Scholar] [CrossRef]
- Kumar, P.; Kundu, A.; Kumar, M.; Solanki, R.; Kapur, M.K. Exploitation of potential bioactive compounds from two soil derived actinomycetes, Streptomyces sp. strain 196 and RI. 24. Microbiol. Res. 2019, 229, 126312. [Google Scholar] [CrossRef]
- Smyth, E.; McCarthy, J.; Nevin, R.; Khan, M.; Dow, J.; O’gara, F.; Doohan, F. In vitro analyses are not reliable predictors of the plant growth promotion capability of bacteria; a Pseudomonas fluorescens strain that promotes the growth and yield of wheat. J. Appl. Microbiol. 2011, 111, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, B.; Khan, A.; Tariq, M.; Ramzan, M.; Khan, M.S.I.; Shahid, N.; Aaliya, K. Bottlenecks in commercialisation and future prospects of PGPR. Appl. Soil Ecol. 2017, 121, 102–117. [Google Scholar] [CrossRef]
- Mutturi, S.; Sahai, V.; Sharma, S.; Bisaria, V. Strategies for high-density cultivation of bioinoculants in submerged culture with special reference to pseudomonads. In Microbial Inoculants in Sustainable Agricultural Productivity: 1: Research Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 181–196. [Google Scholar]

| Plants | Strains/Consortium | Impacts of PGPR | Experimental Type | References |
|---|---|---|---|---|
| Oryza sativa | B. laterosporus B4 and B. amyloliquefaciens Bk7 | Increased chlorophyll content, antioxidants, and leaf proline | Laboratory experiment | Kakar, et al. [107] |
| Zea mays | Proteus sp. and Pseudomonas sp. | Increased gibberellic acid, and IAA | Pot experiment | Yasmin, et al. [108] |
| S. italica | P. fluorescens DR7 | Increased seedling growth, and seed germination | Laboratory experiment | Niu, Song, Xiao and Ge [80] |
| P. Sativum and V. mungo | Pseudomonas sp. RJ15, B. subtilis RJ46, and O. pseudogrignonense RJ12. | Increment in ROS-quenching enzymes, and osmolytes | Pot experiment | Saikia, Sarma, Dhandia, Yadav, Bharali, Gupta and Saikia [14] |
| M. piperita | B. amyloliquefaciens GB03 and P. fluorescens WCS417 | Improved total phenolic content and increased status of antioxidant of the plant | Laboratory experiment | Chiappero, del Rosario Cappellari, Alderete, Palermo and Banchio [15] |
| M. maximus | Bacillus spp. | Reduced activity of glutathione reductase and increment in proline accumulation | Pot experiment | Moreno-Galván, et al. [109] |
| Zea mays | E. cloacae and P. aeruginosa, L. adecarboxylata+ Biochar and A. xylosoxidans | Improvement in the yield of grain yield and content of chlorophyll | Pot experiment | Danish, Zafar-ul-Hye, Mohsin and Hussain [16] |
| T. aestivum | A. brasilense and B. subtilis | Increment in the production of EPS, antioxidants and osmolytes | Pot experiment | Ilyas, Mumtaz, Akhtar, Yasmin, Sayyed, Khan, Enshasy, Dailin, Elsayed and Ali [89] |
| Petunia | P. fluorescens 90F12-2 and P. poae 29G9 | Greater shoot biomass, higher leaf nutrient content | Greenhouse experiment | Nordstedt, Chapin, Taylor and Jones [75] |
| Z. mays | P. pseudoalcaligenes | Stimulated production of VOC and increment in the photosynthetic pigments and phytohormones | Laboratory experiment | Yasmin, et al. [110] |
| T. aestivum | A. zeae | Increased content of P and N and Improvement in the yield of grain | Field experiment | Karimi, Goltapeh, Amini, Mehnaz and Zarea [56] |
| T. aestivum | B. megaterium (MU2) | RWC improvement, increment in carotenoid, and chlorophyll a,b | Pot experiment | Rashid, et al. [111] |
| A. thaliana | Pseudomonas sp. | RWC improvement, increment in biomass, proline and chlorophyll contents | Laboratory experiment | Yasmin, et al. [112] |
| S. lycoperciscum | P. agglomerans | Induces a direct and earlier emergence of roots from stem tissues and determines modifications of root morphological parameters and root architecture | Invitro and ex vitro acclimatization | Luziatelli, et al. [113] |
| Solanum tuberosum | A. xylosoxidans, P. oryzihabitans, and V. paradoxus | Increased auxin and ACC deaminsae production, decreased concentration of ethylene and enhanced tuber yield and root biomass improvement | Pot experiment | Belimov, et al. [114] |
| Helianthus annus | Enterobacter sp., B. sporothernoduran and Pseudomonas sp. | Production of Siderophore, improvement of chlorophyll content, plant biomass and availability of nutrients such as iron and nitrogen | Pot experiment | Pourbabaee, et al. [115] |
| Zea mays | Azospirillium sp. | Increment of proline content, shoot enhancement, dry weight, and improvement of the seedling growth rate of germination | Pot experiment | García, et al. [116] |
| Foxmillet | P. fluorescens | Enhancement of the rate of seed germination, and root improvement. adhering soil to root tissue dry mass ratio | Pot experiment | Niu, Song, Xiao and Ge [80] |
| Wheat | P. azotoformans | Improvement of the rate of seed germination, shoot length and root length | Pot experiment | Ansari, et al. [117] |
| Zea mays | Bacillus sp. | Reduction in the activities of peroxidase, glutathione reductase and ascorbate activities, improved content of proline content, and nutrient uptake | Pot experiment | Silva, et al. [118] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadiji, A.E.; Orozco-Mosqueda, M.d.C.; Santos-Villalobos, S.d.l.; Santoyo, G.; Babalola, O.O. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants 2022, 11, 3090. https://doi.org/10.3390/plants11223090
Fadiji AE, Orozco-Mosqueda MdC, Santos-Villalobos Sdl, Santoyo G, Babalola OO. Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants. 2022; 11(22):3090. https://doi.org/10.3390/plants11223090
Chicago/Turabian StyleFadiji, Ayomide Emmanuel, Ma. del Carmen Orozco-Mosqueda, Sergio de los Santos-Villalobos, Gustavo Santoyo, and Olubukola Oluranti Babalola. 2022. "Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation" Plants 11, no. 22: 3090. https://doi.org/10.3390/plants11223090
APA StyleFadiji, A. E., Orozco-Mosqueda, M. d. C., Santos-Villalobos, S. d. l., Santoyo, G., & Babalola, O. O. (2022). Recent Developments in the Application of Plant Growth-Promoting Drought Adaptive Rhizobacteria for Drought Mitigation. Plants, 11(22), 3090. https://doi.org/10.3390/plants11223090









