Arbuscular Mycorrhizal Fungi Alleviate Low Phosphorus Stress in Maize Genotypes with Contrasting Root Systems
Abstract
1. Introduction
2. Results
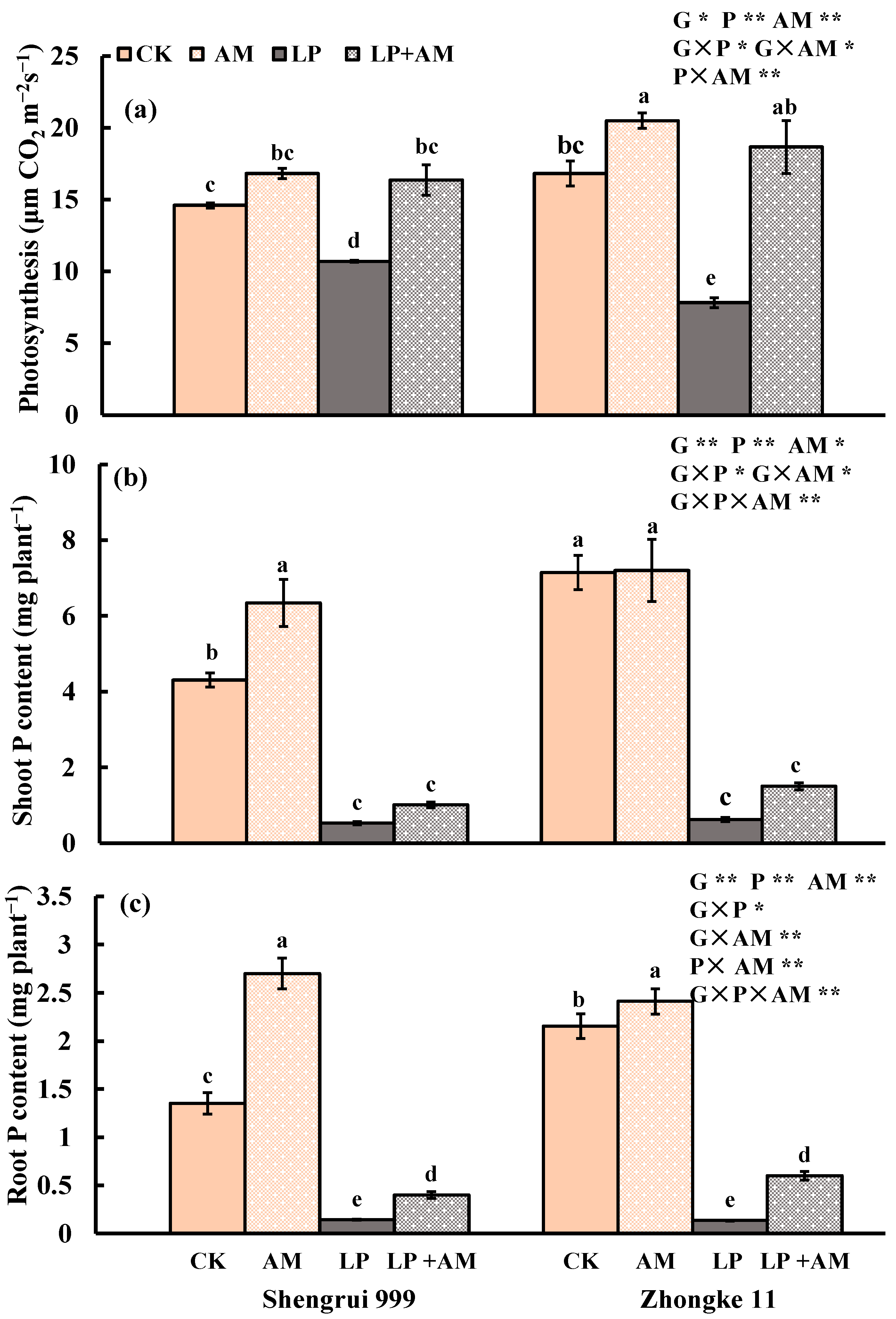
2.1. Effect of Low P Supply on Plant Growth and P Efficiency
2.2. Genotypic Variation in Response to Low P Stress
2.3. Effect of AMF Inoculation on Plant Growth and P Efficiency
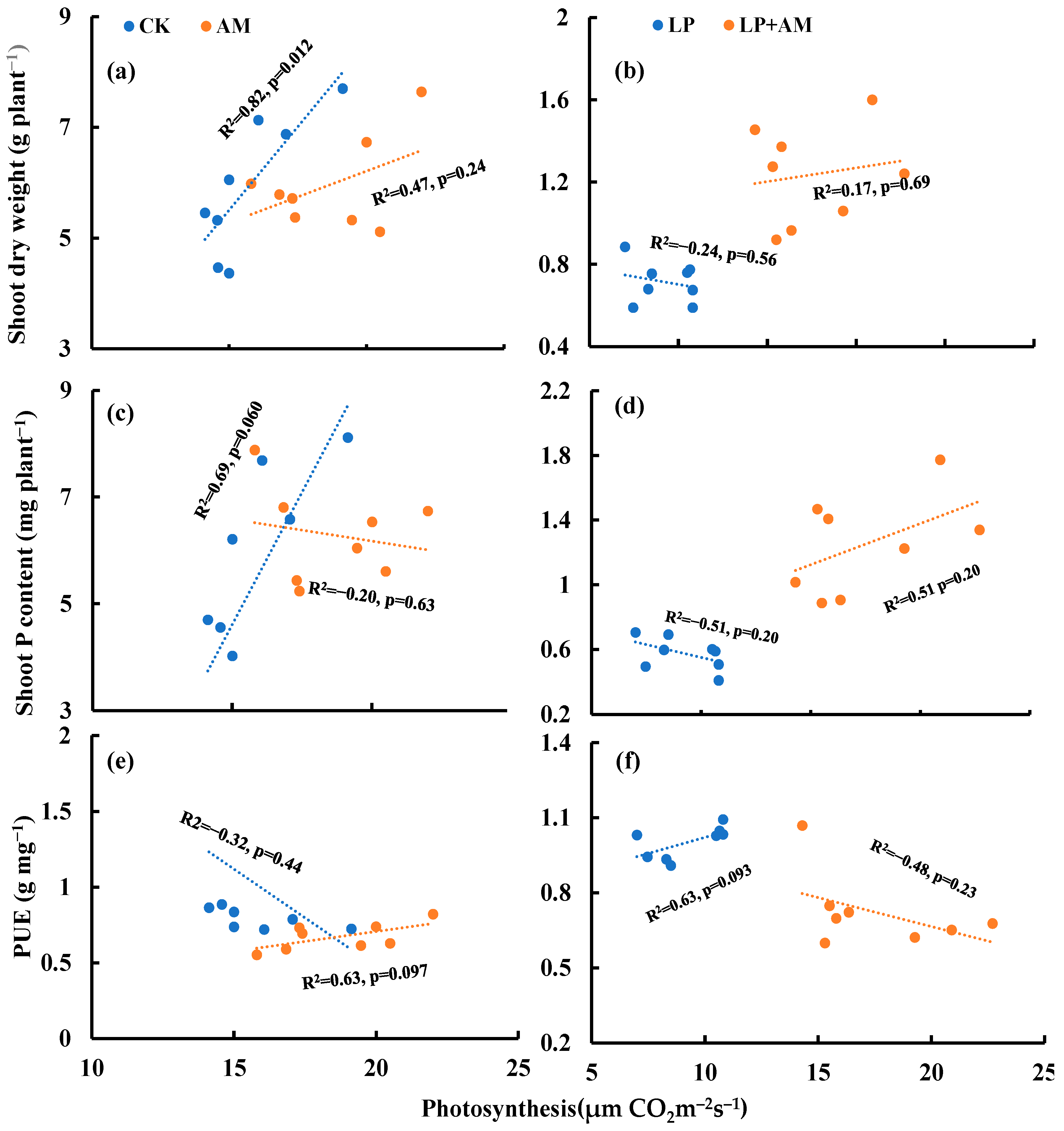
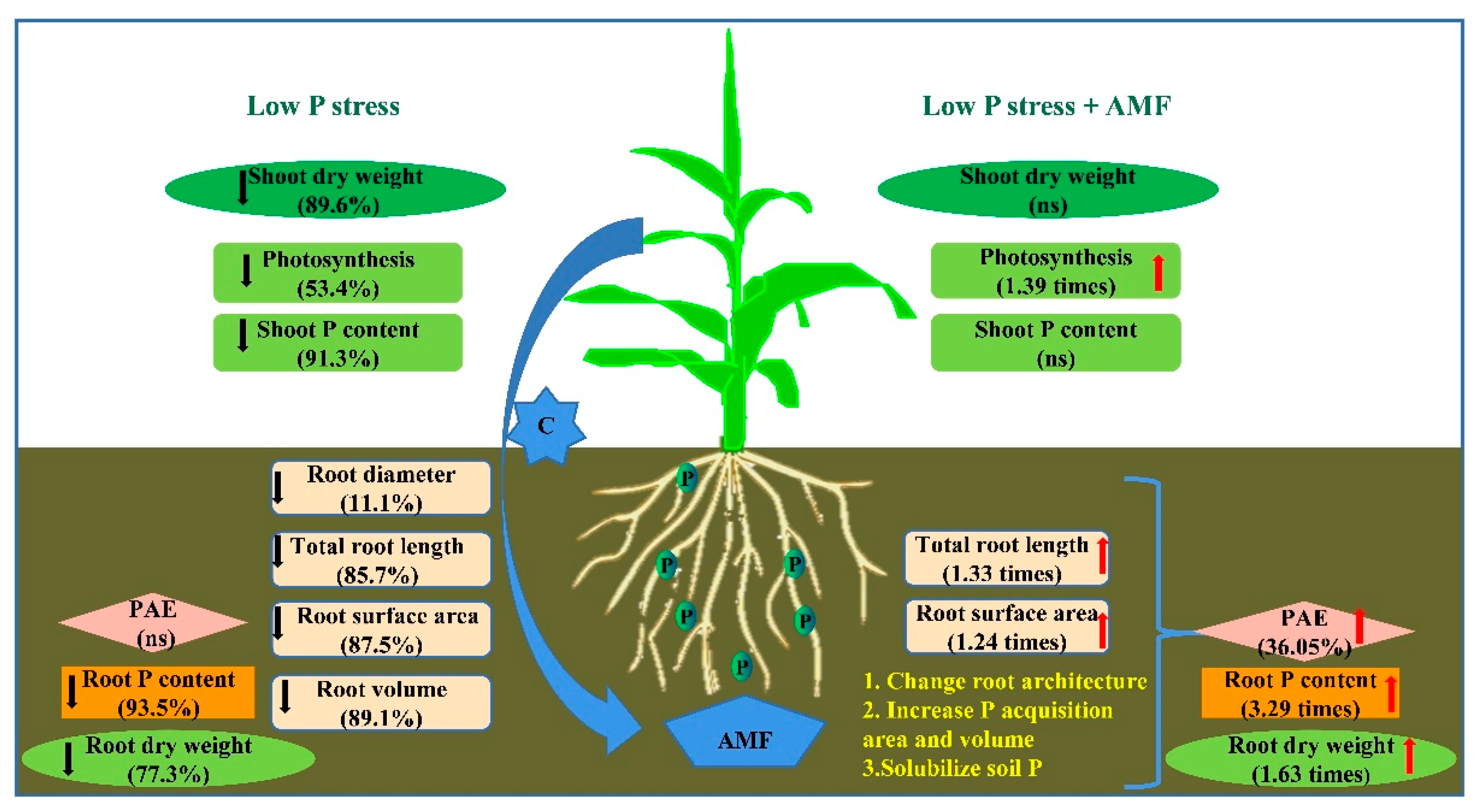
2.4. Alleviative Effect of AMF Inoculation on Plant Growth, Photosynthesis, and P Efficiency under Low P Stress
2.5. Genotypic Variation in Response to AMF Inoculation
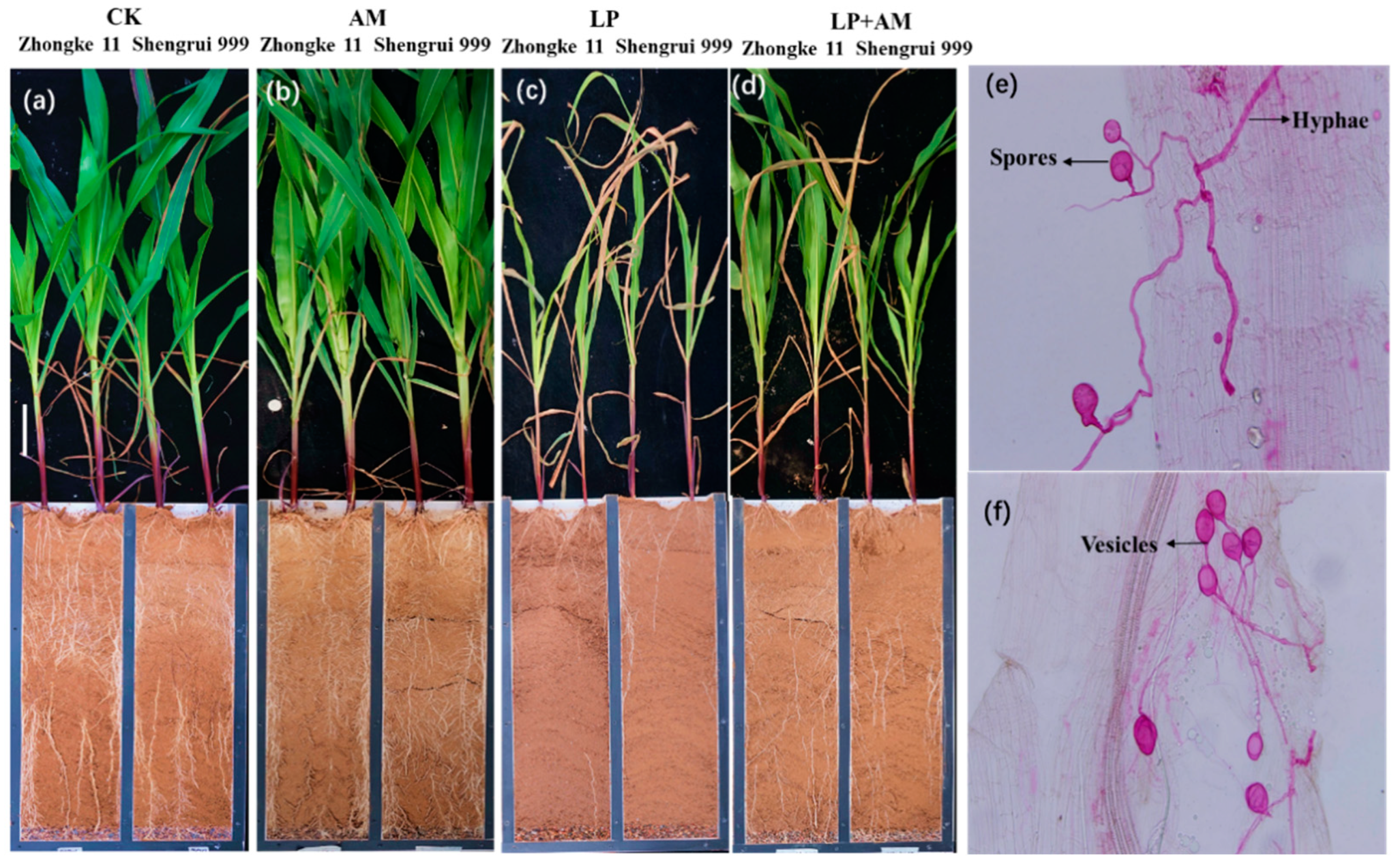
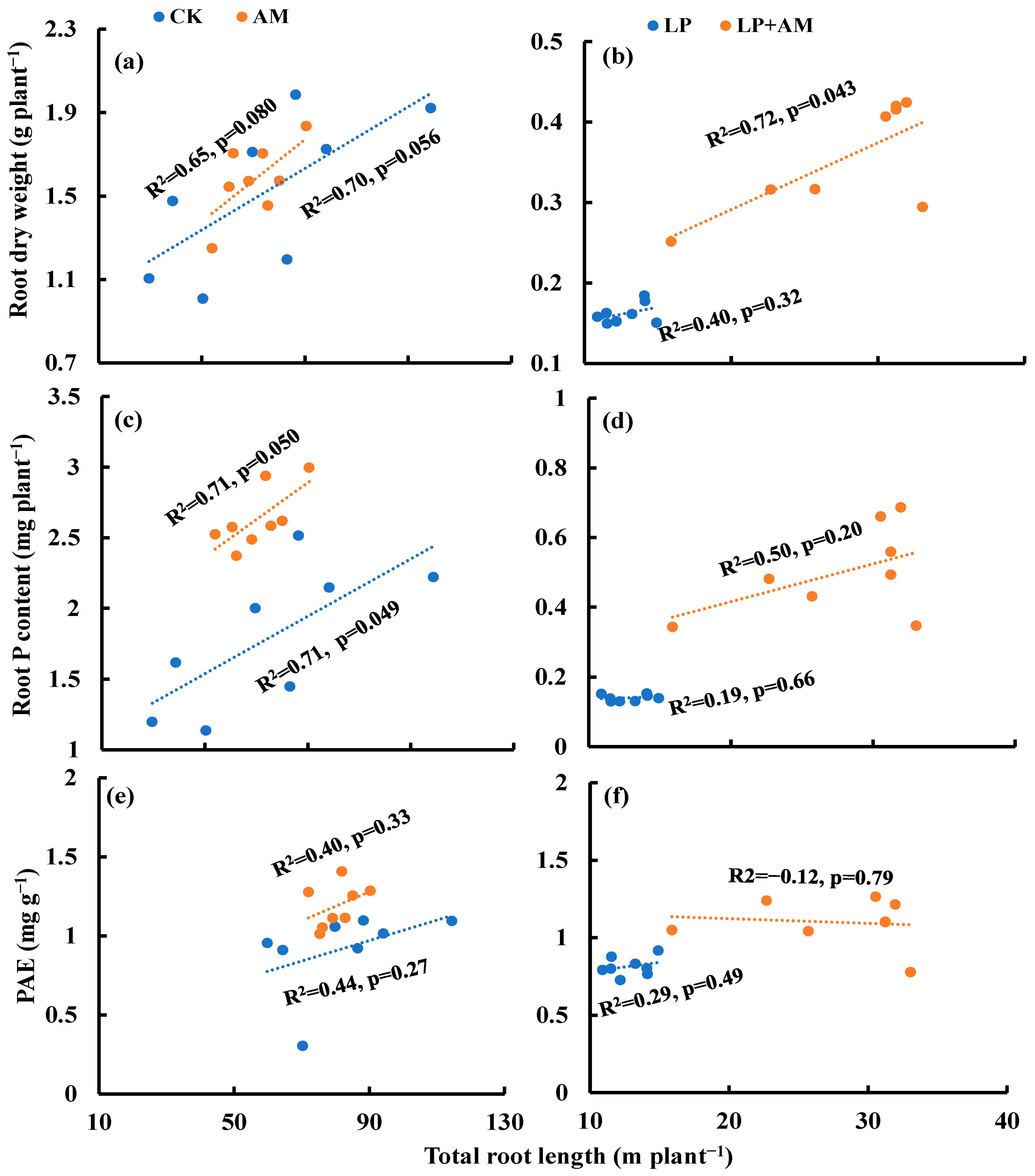
2.6. Accumulated Root Length
3. Discussion
3.1. Root Morphological Traits in Response to Low P Stress
3.2. The Role of AMF in Plant Alleviation under Low P Stress
3.3. Genotypic Variations in Response to Low P Stress and AMF
4. Materials and Methods
4.1. Experimental Design, Plant Materials, and AM Inoculum
4.2. Rhizoboxes, Soil, and Potting
4.3. Seed Germination, Plant Cultivation, and Maintenance
4.4. Plant Harvest and Assessments
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, J.B.; Yuan, L.X.; Zhang, J.L.; Li, H.G.; Bai, Z.H.; Chen, X.P.; Zhang, W.F.; Zhang, F.S. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, L. A study on the potential of phosphorus uptake by different maize varieties under low phosphorus stress. J. Pure Appl. Microbiol. 2013, 7, 801–804. [Google Scholar]
- Shen, Y.; Zhang, Y.Z.; Lin, H.J.; Gao, S.B.; Pan, G.T. Effect of low phosphorus stress on endogenous hormone levels of different maize genotypes in seedling stage. Int. J. Biol. Sci. 2012, 12, 308–314. [Google Scholar] [CrossRef][Green Version]
- Cozzolino, V.; Di Meo, V.; Piccolo, A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013, 129, 40–44. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hartmann, T.E.; Wang, X.H.; Cui, Z.L.; Hou, Y.; Meng, F.L.; Yu, X.C.; Wu, J.C.; Zhang, F.S. Phosphorus flow analysis in the maize-based food-feed-energy systems in China. Environ. Res. 2020, 184, 109319. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 11–126. [Google Scholar] [CrossRef]
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; et al. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 2011, 349, 89–120. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X.; Ma, C. What are the key factors affecting maize yield response to and agronomic efficiency of phosphorus fertilizer in China? Field Crop Res. 2021, 270, 108221. [Google Scholar] [CrossRef]
- Ha, S.; Tran, L.S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Lang, M.; Zhang, C.Y.; Su, W.H.; Chen, X.X.; Zou, C.Q.; Chen, X.P. Long-term P fertilization significantly altered the diversity.; composition and mycorrhizal traits of arbuscular mycorrhizal fungal communities in a wheat-maize rotation. Appl. Soil Ecol. 2022, 170, 104261. [Google Scholar] [CrossRef]
- Furuya, M.; Shin, M.; Masumoto, H.; Takata, S.; Takano, J.; Matsumura, A. Root response of soybean genotypes to low phosphorus availability from juvenile to adult vegetative stages. Soil Sci. Plant Nutr. 2022, 68, 361–373. [Google Scholar] [CrossRef]
- Liu, D. Root developmental responses to phosphorus nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef]
- Amtmann, A.; Bennett, M.J.; Henry, A. Root phenotypes for the future. Plant Cell Environ. 2022, 45, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Liu, P.; Zhao, B.; Zhang, J.W.; Ren, B.Z.; Li, Z.; Wang, Z.Q. Root physiological adaptations that enhance the grain yield and nutrient use efficiency of maize (Zea mays L.) and their dependency on phosphorus placement depth. Field Crops Res. 2022, 276, 10837. [Google Scholar]
- Ferrol, N.; Azcon-Aguilar, C.; Perez-Tienda, J. Review: Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef]
- Smith, S.E. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; Mccormack, M.L.; Ma, Z.; Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 2017, 216, 1140–1150. [Google Scholar] [CrossRef]
- Shane, M.W.; Lambers, H. Cluster roots: A curiosity in context. Plant Soil 2005, 274, 101–125. [Google Scholar] [CrossRef]
- Frew, A. Arbuscular mycorrhizal fungal diversity increases growth and phosphorus uptake in C3 and C4 crop plants. Soil Boil Biochem. 2019, 135, 248–250. [Google Scholar] [CrossRef]
- Javot, H.; Pumplin, N.; Harrison, M.J. Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles. Plant Cell Environ. 2007, 30, 310–322. [Google Scholar] [CrossRef]
- Qiao, S.; Fang, Y.; Wu, A.J.; Xu, B.C.; Zhang, S.Q.; Deng, X.P.; Djalovic, I.; Siddique, K.H.M.; Chen, Y.L. Dissecting root trait variability in maize genotypes using the semi-hydroponic phenotyping platform. Plant Soil 2019, 439, 75–90. [Google Scholar] [CrossRef]
- Chen, Y.L.; Rengel, Z.; Palta, J.; Siddique, K.H.M. Efficient root systems for enhancing tolerance of crops to water and phosphorus limitation. Indian J. Plant Physiol. 2018, 23, 689–696. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 2001, 236, 243–250. [Google Scholar] [CrossRef]
- Smith, S.; De, S.I. Root system architecture: Insights from Arabidopsis and cereal crops. Philos. Trans. R. Soc. Lond. 2012, 367, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Dunbabin, V.M.; Postma, J.A.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Modelling root plasticity and response of narrow-leafed lupin to heterogeneous phosphorus supply. Plant Soil 2013, 372, 319–337. [Google Scholar] [CrossRef]
- Liu, W.; Kuang, X.; Wang, M.; Li, D. Genetic study and molecular breeding for high phosphorus use efficiency in maize. Front. Agric. Sci. Eng. 2019, 6, 366–379. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Ma, Q.H.; Zhang, F.S.; Rengel, Z.; Shen, J.B. Localized application of NH4+-N plus P at the seedling and later growth stages enhances nutrient uptake and maize yield by inducing lateral root proliferation. Plant Soil 2013, 372, 65–80. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. Lond. 2012, 367, 1598–1604. [Google Scholar] [CrossRef]
- Lynch, J.P.; Wojciechowski, T. Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J. Exp. Bot. 2015, 66, 2199–2210. [Google Scholar] [CrossRef]
- Gu, R.; Chen, F.; Long, L.; Cai, H.; Liu, Z.; Yang, J.; Wang, L.; Li, H.; Li, J.; Liu, W.; et al. Enhancing phosphorus uptake efficiency through QTL-based selection for root system architecture in maize. J. Genet. Genom. 2016, 43, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.K.; Chen, J.; Yan, Y.; Lucas, W.J. Insights into plant phosphate sensing and signaling. Curr. Opin. Biotechnol. 2018, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, D.; McCully, M.; Wenzel, C. The nodal roots of Zea: Their development in relation to structural features of the stem. Can. J. Bot. 2011, 64, 2524–2537. [Google Scholar] [CrossRef]
- Sun, B.; Gao, Y.; Lynch, J.P. Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol. 2018, 177, 90–104. [Google Scholar] [CrossRef]
- Moraisde Sousa, S.; Clark, R.T.; Mendes, F.V.F.; Carlos de Oliveira, A.; de Vasconcelos, M.J.V.; Parentoni, S.N.; Kochian, L.V.; Guimarães, C.T.; Magalhães, J.V. A role for root morphology and related candidate genes in P acquisition efficiency in maize. Funct. Plant Biol. 2012, 39, 925–935. [Google Scholar] [CrossRef]
- Thudi, M.; Chen, Y.; Pang, J.; Kalavikatte, D.; Bajaj, P.; Roorkiwal, M.; Chitikineni, A.; Ryan, M.H.; Lambers, H.; Siddique, K.H.M.; et al. Novel genes and genetic loci associated with root morphological traits.; phosphorus-acquisition efficiency and phosphorus-use efficiency in chickpea. Front. Plant Sci. 2021, 12, 636973. [Google Scholar] [CrossRef]
- Wada, Y.; Kusano, H.; Tsuge, T.; Aoyama, T. Phosphatidylinositol phosphate 5-kinase genes respond to phosphate deficiency for root hair elongation in Arabidopsis thaliana. Plant J. 2015, 81, 426–437. [Google Scholar] [CrossRef]
- Miura, K.; Rus, A.; Sharkhuu, A.; Yokoi, S.; Karthikeyan, A.S.; Raghothama, K.G. The arabidopsis SUMO E3 ligase SIZ1 controls phosphate defificiency responses. Proc. Natl. Acad. Sci. USA 2005, 102, 7760–7765. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X. Role of OsPHR2 on phosphorus homoestasis and root hairs development in rice (Oryza sativa L). Plant Signal Behav. 2008, 3, 674–675. [Google Scholar] [CrossRef]
- Schroeder, M.S.; Janos, D.P. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 2005, 15, 203–216. [Google Scholar] [CrossRef]
- Liu, A.; Hamel, C.; Hamilton, R.I.; Smith, D.L. Mycorrhizae formation and nutrient uptake of new corn (Zea mays L) hybrids with extreme canopy and leaf architecture as influenced by soil N and P levels. Plant Soil 2000, 221, 157–166. [Google Scholar] [CrossRef]
- Londono, D.M.M.; Meyer, E.; Gonzalez, D.; Hernandez, A.G.; Soares, C.; Lovato, P.E. Landrace maize varieties differ from conventional and genetically modified hybrid maize in response to inoculation with arbuscular mycorrhizal fungi. Mycorrhiza 2019, 29, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2015, 207, 1–22. [Google Scholar] [CrossRef]
- Smith, S.E.; Manjarrez, M.; Stonor, R.; McNeill, A.; Smith, F.A. Indigenous arbuscular mycorrhizal (AM) fungi contribute to wheat phosphate uptake in a semi-arid field environment.; shown by tracking with radioactive phosphorus. Appl. Soil Ecol. 2015, 96, 68–74. [Google Scholar]
- Saboor, A.; Ali, M.A.; Husain, S.; Tahir, M.S.; Irfan, M.; Bilal, M.; Baig, K.S.; Datta, R.; Ahmed, N.; Danish, S.; et al. Regulation of phosphorus and zinc uptake in relation to arbuscular mycorrhizal fungi for better maize growth. Agronomy 2021, 11, 2322. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature. 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Wen, Z.H.; Li, H.B.; Shen, Q.; Tang, X.M.; Shen, J.B. Tradeoffs among root morphology.; exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Zhu, B.; Koide, R.T.; Eissenstat, D.M.; Guo, D. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytol. 2015, 208, 125–136. [Google Scholar] [CrossRef]
- Mccormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Li, Y.F.; Luo, A.C.; Wei, X.H.; Yao, X.G. Genotypic Variation of rice in phosphorus acquisition from iron phosphate: Contributions of root morphology and phosphorus uptake kinetics. Russ. J. Plant Physiol. 2007, 54, 230–236. [Google Scholar] [CrossRef]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological interactions between symbionts in vesicular-arbuscular mycorrhizal plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobialand arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Verlinden, M.S.; Abdelgawad, H.; Ven, A.; Verryckt, L.T.; Wieneke, S.; Janssens, I.A. Phosphorus stress strongly reduced plant physiological activity.; but only temporarily.; in a mesocosm experiment with Zea mays colonized by arbuscular mycorrhizal fungi. Biogeosciences 2021, 19, 2353–2364. [Google Scholar] [CrossRef]
- Řezáčová, V.R.; Slavíková, L.; Zemková, T.; Konvalinková, V.; Procházková, V.; Šťovíček, H.; Hršelová, O.; Beskid, M.; Hujslová, H.; Gryndlerová, M.; et al. Mycorrhizal symbiosis induces plant carbon reallocation differently in C3 and C4 Panicum grasses. Plant Soil 2018, 425, 441–456. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Gronlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Bucher, M. Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol. 2007, 173, 11–26. [Google Scholar] [CrossRef]
- Liu, J.; Versaw, W.K.; Pumplin, N.; Gomez, S.K.; Blaylock, L.A.; Harrison, M.J. Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. J. Biol. Chem. 2008, 283, 24673–24681. [Google Scholar] [CrossRef]
- Rausch, C.; Daram, P.; Brunner, S.; Jansa, J.; Laloi, M.; Leggewie, G.; Amrhein, N.; Bucher, M. A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 2001, 414, 462–470. [Google Scholar] [CrossRef]
- Yang, S.Y.; Grønlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.J.; Jiang, H.H.; Jiang, C.S.; Du, Y.B.; Cheng, G.; Wang, W.; Zhu, S.W.; Han, G.M.; Cheng, B.Q. Systematic identification.; evolution and expression analysis of the Zea mays pht1 gene family reveals several new members involved in root colonization by arbuscular mycorrhizal fungi. Int. J. Mol. Sci. 2016, 17, 930. [Google Scholar] [CrossRef]
- Sergio, S.; Vito, R.; Paolo, R.; Rosa, A.M.; Francesco, S.; Dario, G.; Frenda, A.S.; Federico, M. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar]
- Sawers, R.J.H.; Svane, S.F.; Quan, C.; Grønlund, M.; Wozniak, B.; González-Muñoz, E.; Chávez Montes, R.A.; Baxter, I.; Goudet, J.; Jakobsen, I.; et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding pht1 phosphate transporters. New Phytol. 2017, 214, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.; Shen, W.; Kuzyakov, Y. Phosphatase activity and acidification in lupine and maize rhizosphere depend on phosphorus availability and root properties: Coupling zymography with planar optodes. Appl. Soil Ecol. 2021, 167, 104029. [Google Scholar] [CrossRef]
- Péret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, G.; Shi, L.; Feng, J.; Xu, F.; Meng, J. Quantitative trait loci for Root morphology in response to low phosphorus stress in Brassic anapus. Theor. Appl. Genet. 2010, 121, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Bayuelo-Jimenez, J.S.; Gallardo-Valdez, M.; Perez-Decelis, V.A.; Magdaleno-Armas, L.; Ochoa, I.; Lynch, J.P. Genotypic variation for root traits of maize (Zea mays L) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crop Res. 2011, 121, 350–362. [Google Scholar] [CrossRef]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef]
- Zhu, J.M.; Zhang, C.C.; Lynch, J.P. The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct. Plant Biol. 2010, 37, 313–322. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Ye, X.; Xu, F. Genotypic variation in phosphorus acquisition from sparingly soluble P sources is related to root morphology and root exudates in Brassica napus. Sci. China Life Sci. 2011, 54, 1134–1142. [Google Scholar]
- Hochholdinger, F.; Tuberosa, R. Genetic and genomic dissection of maize root development and architecture. Curr. Opin. Plant Biol. 2009, 12, 1–6. [Google Scholar] [CrossRef]
- Lyu, Y.; Tang, H.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Shen, J.B. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front. Plant Sci. 2016, 7, 1939. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Phosphorus starvation boosts carboxylate secretion in P-deficient genotypes of Lupinus angustifolius with contrasting root structure. Crop Pasture 2013, 64, 588–599. [Google Scholar] [CrossRef]
- Wu, A.; Fang, Y.; Liu, S.; Wang, H.; Xu, B.C.; Zhang, S.Q.; Palta, J.A.; Siddique, K.H.M.; Chen, Y.L. Root morphology and rhizosheath acid phosphatase activity in legume and graminoid species respond differently to low phosphorus supply. Rhizosphere 2021, 19, 100391. [Google Scholar] [CrossRef]
- Tang, H.; Chen, X.; Gao, Y.; Hong, L.; Chen, Y.L. Alteration in root morphological and physiological traits of two maize cultivars in response to phosphorus deficiency. Rhizosphere 2020, 14, 100201. [Google Scholar] [CrossRef]
- Han, Y.; Hong, W.; Xiong, C.; Lambers, H.; Sun, Y.; Xu, Z.; Schulze, W.X.; Cheng, L. Combining analyses of metabolite profiles and phosphorus fractions to explore high phosphorus utilization efficiency in maize. J. Exp. Bot. 2022, 73, 4184–4203. [Google Scholar] [CrossRef]
- Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Parke, J.L.; Mueller, S.M.; Senior, L.; Stuber, C.; Tracy, W.F. Variation among maize Inbred Lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 2000, 40, 358–364. [Google Scholar] [CrossRef]
- Li, X.; Quan, X.; Mang, M.; Neumann, G.; Melchinger, A.; Ludewig, U. Flint maize root mycorrhization and organic acid exudates under phosphorus deficiency: Trends in breeding lines and doubled haploid lines from landraces. J. Plant Nutr. Soil Sci. 2021, 184, 346–359. [Google Scholar] [CrossRef]
- Roger, A.; Colard, A.; Angelard, C.; Sanders, I.R. Relatedness among arbuscular mycorrhizal fungi drives plant growth and intraspecific fungal coexistence. ISME J. 2013, 7, 2137–2146. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Effects of phosphate-solubilizing bacteria; rock phosphate and chemical fertilizers on maize–wheat cropping cycle and economics. Pedosphere 2015, 25, 428–437. [Google Scholar] [CrossRef]
- Fitter, A. Costs and benefits of mycorrhizas: Implications for functioning under natural conditions. Experientia 1991, 47, 350–355. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 94–407. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield; nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, H.; Feng, G.; Christie, P.; Zhang, J.; Li, X.; Gai, J. Soil phosphorus availability modifies the relationship between AM fungal diversity and mycorrhizal benefits to maize in an agricultural soil. Soil Biol. Biochem. 2020, 144, 107790. [Google Scholar] [CrossRef]
- Hetrick, B.A.D.; Wilson, G.W.T.; Cox, T.S. Mycorrhizal ficial evolutionary forces of a plant breeding program dependence of modern wheat varieties, landraces, and ancestors are likely to be substantially different than evolutionary. Can. J. Bot. 1992, 70, 2032–2040. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Liu, R.J.; Chen, Y.L. Mycorrhizology; Science Press: Beijing, China, 2007; pp. 376–393. ISBN 978-7-03-017290-7. [Google Scholar]
- Li, C.; Wang, J.; Zhang, Y.C. Root growth and phosphorus efficiency among sweet potato genotypes under low phosphorus. J. Plant Nutr. 2020, 43, 1320–1330. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]


| Genotype | Treatment | Shoot Height (cm) | Root Surface Area (cm2 Plant−1) | Root Volume (cm3 plant−1) | Root Diameter (mm Plant−1) | Root to Shoot Dry Mass Ratio | Shoot P Concentration (mg g−1) | Root P Concentration (mg g−1) | PAE (mg g−1) | PUE (g mg−1) | AM Infection Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shengrui 999 | CK | 61.3 b | 787 b | 9.55 b | 0.46 a | 0.24 b | 0.88 b | 1.13 c | 0.77 c | 1.32 a | 0 a |
| AM | 69.8 a (13.9) | 1076 a (36.7) | 11.5 a (20.4) | 0.41 b (−10.8) | 0.30 a (25.0) | 1.11 a (25.4) | 1.58 a (40.1) | 1.21 a (57.1) | 0.64 b (−51.5) | 33.8 b | |
| LP | 41.3 d (−32.6) | 150 d (−80.9) | 1.58 c (−83.5) | 0.43 b (−6.7) | 0.23 b (−0.04) | 0.75 b (−15.1) | 0.88 d (−22.5) | 0.77 c (0) | 1.05 a (−20.5) | 0 a | |
| LP + AM | 45.3 c (−26.1) | 295 c (−62.5) | 2.86 c (−70.1) | 0.39 c (−14.8) | 0.28 ab (0.17) | 0.94 ab (6.6) | 1.36 b (20.2) | 1.03 ab (33.8) | 0.79 b (−40.2) | 37.4 b | |
| Zhongke 11 | CK | 69.5 b | 1350 a | 15.6 a | 0.46 a | 0.27 ab | 1.03 b | 1.17 c | 1.07 ab | 0.74 b | 0 a |
| AM | 71.4 a (2.7) | 1024 b (−24.1) | 10.8 b (−30.8) | 0.41 b (−10.8) | 0.24 b (−0.11) | 1.16 a (12.3) | 1.66 a (41.2) | 1.17 a (9.34) | 0.70 b (−5.40) | 32.7 b | |
| LP | 37.8 d (−45.6) | 169 d (−87.5) | 1.70 c (−89.1) | 0.41 b (−11.1) | 0.23 b (−0.15) | 0.86 c (−16.7) | 0.84 d (−28.5) | 0.86 bc (−19.6) | 0.95 ab (28.4) | 0 a | |
| LP + AM | 41.5 c (−40.3) | 379 c (−71.9) | 3.65 c (−76.6) | 0.39 b (−14.8) | 0.31 a (0.15) | 1.09 ab (6.2) | 1.44 b (22.6) | 1.17 a (9.34) | 0.66 b (1.08%) | 33.0 b | |
| ANOVA | G | ns | ** | ** | ns | ns | ** | ns | * | ns | ns |
| P | ** | ** | ** | ** | ns | ** | ** | ns | ns | ns | |
| AM | ** | * | ns | ** | ** | ** | ** | ** | * | ** | |
| G × P | ** | ** | * | ns | ns | ns | ns | ns | ns | ns | |
| G × AM | ** | ** | ** | ns | ns | ns | ns | ns | ns | ns | |
| P × AM | ns | ** | ** | ns | ns | ns | ns | ns | ns | ns | |
| G × P × AM | ** | ** | ** | ns | * | ns | ns | ns | ns | ns |
| Trait | LP | LP + AM | Response (%) | ANOVA |
|---|---|---|---|---|
| Shoot height (cm) | 79.1 | 86.8 | 9.73 | ** |
| Shoot dry weight (g plant−1) | 1.43 | 2.47 | 72.7 | ** |
| Root dry weight (g plant−1) | 0.32 | 0.71 | 122 | ** |
| Total root length (m plant−1) | 25.6 | 55.5 | 117 | ** |
| Root diameter (mm plant−1) | 0.84 | 0.78 | −7.14 | ** |
| Root surface area (cm2 plant−1) | 319 | 674 | 111 | ** |
| Root volume (cm3 plant−1) | 3.28 | 6.51 | 98.5 | ** |
| Root to shoot dry mass ratio | 0.46 | 0.59 | 28.3 | ** |
| Photosynthesis (μm CO2m−2s−1) | 18.53 | 35.1 | 89.4 | ** |
| Shoot P content (mg plant−1) | 1.15 | 2.51 | 118 | ** |
| Root P content (mg plant−1) | 0.28 | 1 | 257 | ** |
| Shoot P concentration (mg g−1) | 1.61 | 2.03 | 26.1 | ** |
| Root P concentration (mg g−1) | 1.72 | 2.8 | 62.8 | ** |
| PAE (mg g−1) | 1.63 | 2.2 | 35.0 | ** |
| PUE (g mg−1) | 2 | 1.45 | −27.5 | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Liu, B.; Huang, D.; Kuang, Q.; An, T.; Liu, S.; Liu, R.; Xu, B.; Zhang, S.; Deng, X.; et al. Arbuscular Mycorrhizal Fungi Alleviate Low Phosphorus Stress in Maize Genotypes with Contrasting Root Systems. Plants 2022, 11, 3105. https://doi.org/10.3390/plants11223105
Liang L, Liu B, Huang D, Kuang Q, An T, Liu S, Liu R, Xu B, Zhang S, Deng X, et al. Arbuscular Mycorrhizal Fungi Alleviate Low Phosphorus Stress in Maize Genotypes with Contrasting Root Systems. Plants. 2022; 11(22):3105. https://doi.org/10.3390/plants11223105
Chicago/Turabian StyleLiang, Liyan, Baoxing Liu, Di Huang, Qiqiang Kuang, Tingting An, Shuo Liu, Runjin Liu, Bingcheng Xu, Suiqi Zhang, Xiping Deng, and et al. 2022. "Arbuscular Mycorrhizal Fungi Alleviate Low Phosphorus Stress in Maize Genotypes with Contrasting Root Systems" Plants 11, no. 22: 3105. https://doi.org/10.3390/plants11223105
APA StyleLiang, L., Liu, B., Huang, D., Kuang, Q., An, T., Liu, S., Liu, R., Xu, B., Zhang, S., Deng, X., Macrae, A., & Chen, Y. (2022). Arbuscular Mycorrhizal Fungi Alleviate Low Phosphorus Stress in Maize Genotypes with Contrasting Root Systems. Plants, 11(22), 3105. https://doi.org/10.3390/plants11223105










