Response of Cnidium officinale Makino Plants to Heat Stress and Selection of Superior Clones Using Morphological and Molecular Analysis
Abstract
:1. Introduction
2. Results
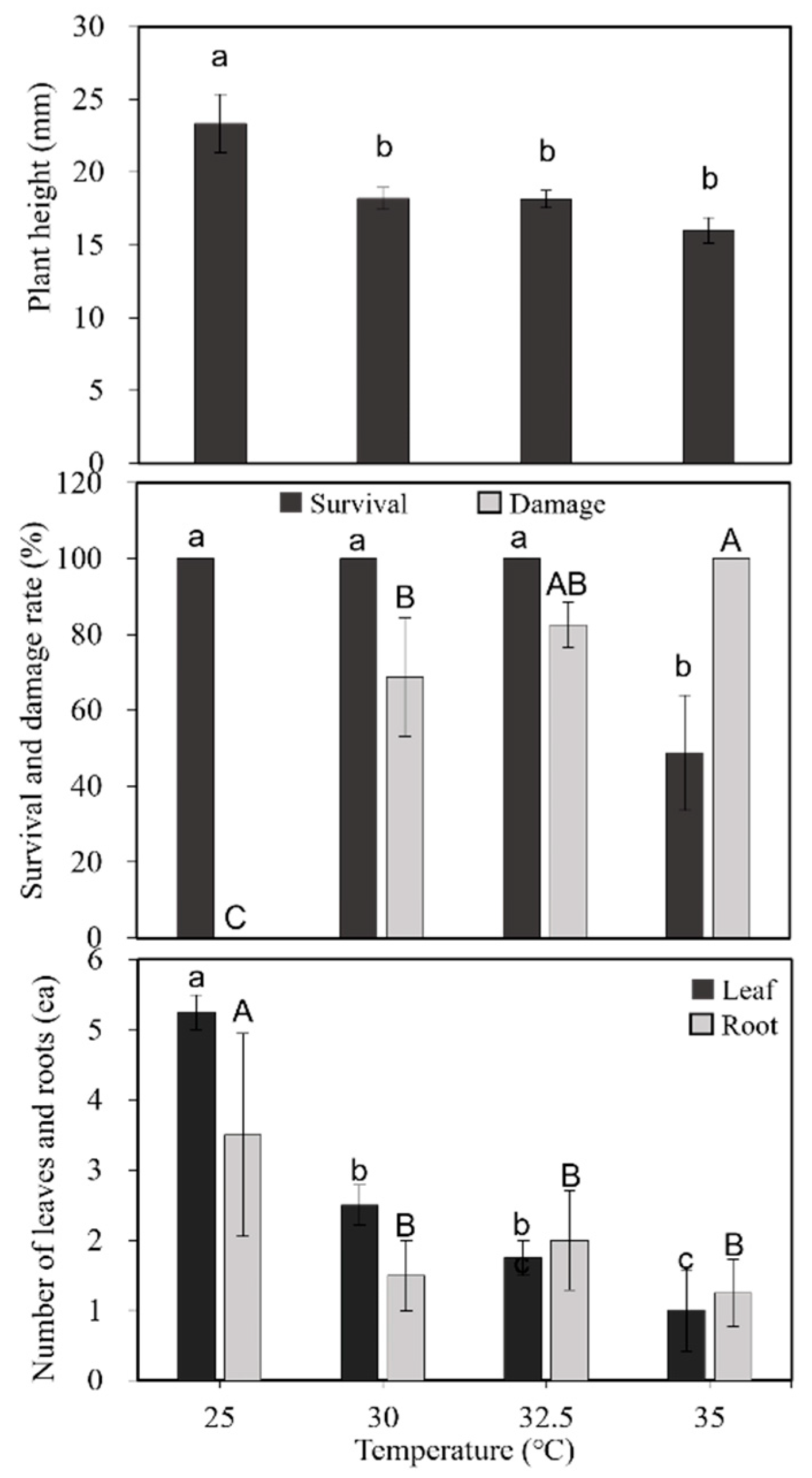
2.1. Morphological Changes Due to Heat Stress
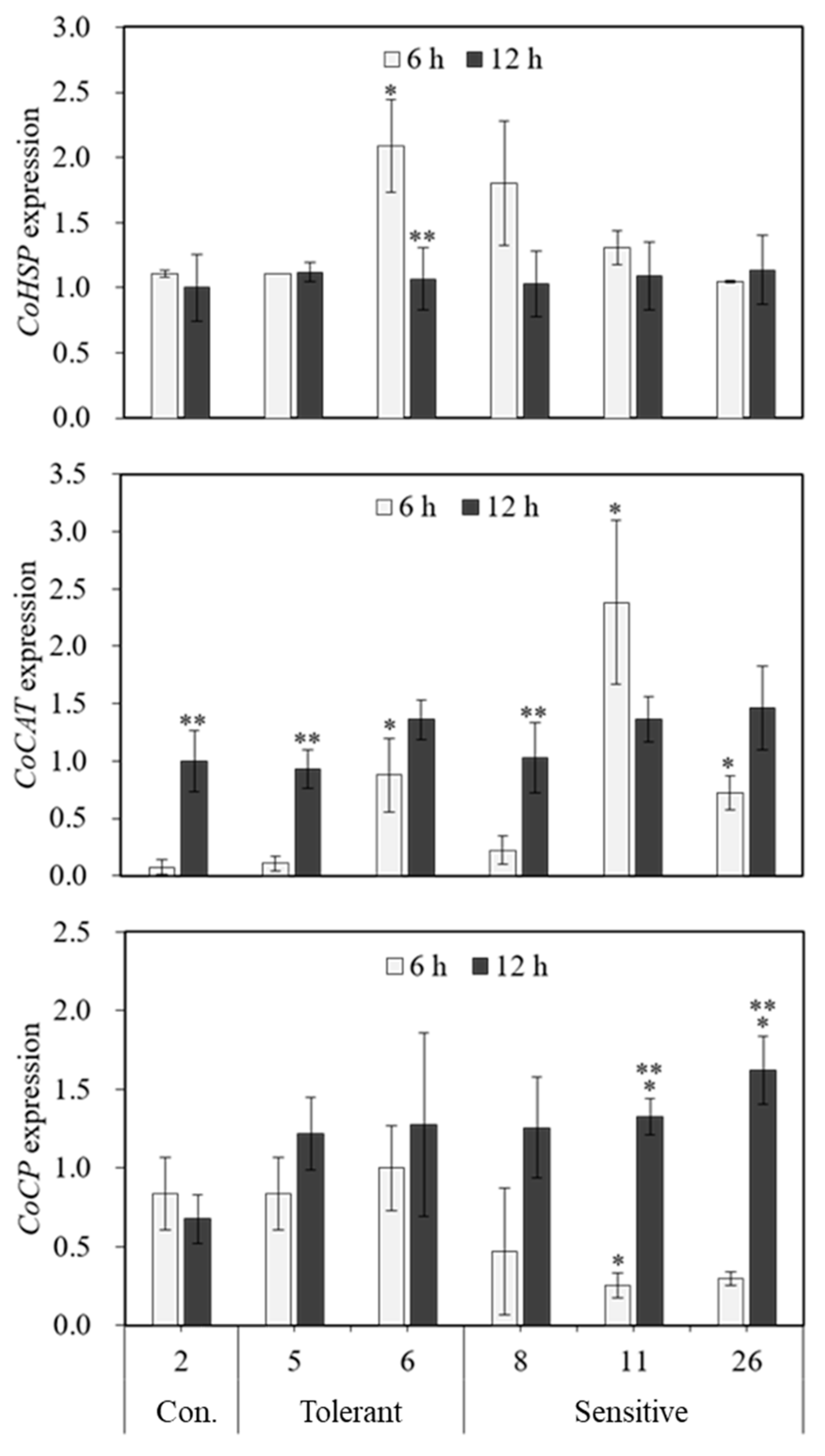
2.2. Expression Levels of Heat Stress-Responsive Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. High-Temperature Treatment and Analysis of Growth Parameters
4.3. Gene Expression Analysis
4.3.1. RNA Isolation and cDNA Synthesis
4.3.2. Quantitative Real-Time PCR (qRT-PCR)
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.Y.; Kim, Y.K.; Kim, Y.S.; Suh, S.Y.; Lee, S.Y.; Park, S.U. Somatic embryogenesis and plant regeneration in Cnidium officinale Makino. J. Med. Plant Res. 2009, 3, 96–100. [Google Scholar]
- Kwak, D.H.; Kim, J.K.; Kim, J.Y.; Jeong, H.Y.; Keum, K.S.; Han, S.H.; Rho, Y.I.; Woo, W.H.; Jung, K.Y.; Choi, B.K.; et al. Anti-angiogenic activities of Cnidium officinale Makino and Tabanus bovinus. J. Ethnopharmacol. 2002, 81, 373–379. [Google Scholar] [CrossRef]
- Lee, K.Y.; Kim, J.H.; Kim, E.Y.; Yeom, M.; Jung, H.S.; Sohn, Y. Water extract of Cnidii Rhizoma suppresses RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting NFATc1/c-Fos signaling and prevents ovariectomized bone loss in SD-rat. BMC Complement. Altern. Med. 2019, 19, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.D.L.; Kim, D.J.; Hwang, S.G. Anti-cancer effects of Cnidium officinale Makino extract mediated through apoptosis and cell cycle arrest in the HT-29 human colorectal cancer cell line. Asian Pac. J. Cancer Prev. 2014, 15, 5117–5121. [Google Scholar] [CrossRef]
- Hong, H.; Cheolan, J.; Curz, J.F.D.L.; Hwang, S.G. Cnidium officinale Makino extract induces apoptosis through activation of caspase-3 and p53 in human liver cancer HepG2 cells. Exp. Ther. Med. 2017, 14, 3191–3197. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.I.; Kwak, D.H.; Lee, S.; Choo, Y.K.; Woo, W.H.; Keum, K.S.; Choi, B.K.; Jung, K.Y. Inhibitory effects of Cnidium officinale Makino and Tabanus fulvas Meigan on the high glucose-induced proliferation of glomerular mesangial cells. Phytomedicine 2005, 12, 648–655. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Oh, E.Y.; Kim, G.Y.; Choi, B.T.; Kim, C.; Choi, Y.H. Ethanol extract of Cnidium officinale exhibits anti-inflammatory effects in BV2 microglial cells by suppressing NF-kB nuclear translocation and activation of the PI3K/Kkt singling pathway. Int. J. Mol. Med. 2013, 32, 876–882. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.K.; Cao, T.Q.; Kim, J.A.; Youn, U.Y.; Kim, S.; Woo, M.H.; Min, B.S. Anti-inflammatory activity of compounds from the rhizome of Cnidium officinale. Arch. Pharm. Res. 2018, 41, 977–985. [Google Scholar] [CrossRef]
- Tomoda, M.; Ohara, N.; Gonda, R.; Shimizu, N.; Takada, K.; Satoh, Y.; Shirai, S. An acidic polysaccharide having immunological activities from the rhizome of Cnidium officinale. Chem. Pharm. Bull. 1992, 40, 3025–3029. [Google Scholar] [CrossRef] [Green Version]
- Lim, E.Y.; Kim, J.G.; Lee, J.; Lee, C.; Shim, J.; Kim, Y.T. Analgesic effect of Cnidium officinale extracts on postoperative, neuropathic, and menopausal pain in rat models. Evid.-Based Complement. Altern. Med. 2019, 2019, 9698727. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Shim, Y.S.; An, S.Y.; Kang, M.K. Surface characterization, biocompatibility and antifungal efficacy of a denature-lining material containing Cnidium officinale extracts. Molecules 2021, 26, 1440. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kang, M.K. Analysis of the antimicrobial, cytotoxic, and antioxidant activities of Cnidium officinale extracts. Plants 2020, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jung, D.H.; Lee, H.W. Therapeutic effect of Cnidium officinale Makino extract on ovariectomized hand-limb ischemic mice. Int. Med. Res. 2019, 8, 107–115. [Google Scholar]
- Kim, Y.J. Inhibition effect of Cnidium officinale Makino extracts on MMP1 expression in human dermal fibroblasts. Asian J. Beauty Cosmetol. 2018, 16, 131–138. [Google Scholar] [CrossRef]
- Cha, H.J. Cnidium officinale Makino extracts inhibit α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Asian J. Beauty Cosmetol. 2018, 16, 122–130. [Google Scholar] [CrossRef]
- Tomoda, M.; Ohara, N.; Shimizu, N.; Gonda, R. Characterization of a novel glucan, which exhibits reticuloendothelial system potentiating and anti-complementary activities, from the rhizome of Cnidium officinale. Chem. Pharm. Bull. 1994, 42, 630–633. [Google Scholar] [CrossRef] [Green Version]
- Tomoda, M.; Ohara, N.; Shimizu, N.; Gonda, R. Characterization of a novel heteroglucan from the rhizome of Cnidium officinale exhibiting high reticuloendothelial system-potentiating and anti-complementary activities. Biol. Pharm. Bull. 1994, 17, 973–976. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Fujita, M.; Mitsuhashi, H. Components of Cnidium officinale Makino: Occurrence of pregneolone, coniferyl ferulate, and hydoxyphthlides. Chem. Pharm. Bull. 1984, 32, 3770–3773. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Kim, M.S.L.; Sawamura, M. Constituents of the essential oil of Cnidium officinale Makino, a Korean medicinal plant. Flavour Frag. J. 2001, 17, 49–53. [Google Scholar] [CrossRef]
- Bae, K.E.; Choi, Y.W.; Kim, S.T.; Kim, Y.K. Components of rhizome extract of Cnidium officinale Makino and their in vitro biological effects. Molecules 2011, 16, 8833–8847. [Google Scholar] [CrossRef] [Green Version]
- Mo, E.J.; Jo, Y.H.; Jeong, J.Y.; Kim, S.B.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Pancreatic lipase inhibitory phthalide derivatives from the rhizome of Cnidium officinale. Rec. Nat. Prod. 2016, 10, 148–153. [Google Scholar]
- Tsukamoto, T.; Ishikawa, Y.; Miyazawa, M. Larvicidal and adulticidal activity of alkylphthalide derivatives from rhizome of Cnidium officinale against Drosophila melanogaster. J. Agric. Food Chem. 2005, 53, 5549–5553. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale suppress LPS-induced NO, PGE2, IL-1β, IL-6, and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Sakai, C.; Ninomiya, K.; Shiotani, M.; Morikawa, T. Phthalides from rhizomes of Cnidium officinale accelerate metabolism of triglyceride in hepatocytes. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Luo, Y.; Li, X.; Liu, T.; Cao, Y.; Zhang, J.; Yaseen, A.; Sun, F.; Zheng, W.; Jiang, Y.; Si, C.L.; et al. Senkyunolide H protects against MPP+-induced apoptosis via the ROS-mediated mitogen-activated protein kinase pathway in PC12 cells. Environ. Toxicol. Pharmacol. 2019, 65, 73–81. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhary, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9543–9684. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s Wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef]
- Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kovacik, J.; Sowa, I.; Latalski, M.; Wojciak, M. High temperature alters secondary metabolites and photosynthetic efficiency in Haracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756. [Google Scholar] [CrossRef]
- Gao, C.H.; Ko, S.M.; Koh, S.; Kim, Y.J.; Bae, H.J. Photosynthesis and environments: Photoinhibition and repair mechanism in plants. J. Plant Biol. 2012, 55, 93–101. [Google Scholar]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [Green Version]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Shinozaki, K.; Michael, A.J. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B. Salinity effects on phenolic content and antioxidant activity of Salvia macrosiphon. Iran. J. Sci. Technol. A Sci. 2017, 41, 295–300. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Yeh, S. Isoprene emission from plants. Annu. Rev. Plant Biol. 2001, 52, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment. Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Nam, H.H.; Seo, Y.J.; Jang, W.C. Effect of shading types and duration on alleviation of high-temperature stress in Cnidium officinale Makino. Korean J. Med. Crop Sci. 2020, 28, 111–118. [Google Scholar] [CrossRef]
- Kim, K.S.; Seo, Y.J.; Kim, D.C.; Nam, H.H.; Lee, B.Y.; Kim, J.H. Effect of soil water and shading treatment on chlorophyll fluorescence parameters and photosynthetic capacity in Cnidium officinale Makino. Korean J. Med. Crop Sci. 2020, 28, 413–420. [Google Scholar]
- Seo, Y.J.; Kim, K.S.; Kim, D.C.; Nam, H.H.; Kim, J.H.; Lee, B.Y. Lysimetric analysis for transpiration and carbon assimilation of Cnidium officinale Makino in hot weather conditions. Korean J. Med. Crop Sci. 2020, 28, 463–470. [Google Scholar] [CrossRef]
- Kim, H.E.; Han, J.E.; Lee, H.; Kim, J.H.; Kim, H.H.; Lee, K.Y.; Shin, J.H.; Kim, H.K.; Park, S.Y. Tetraploidization increases the contents of functional metabolites in Cnidium officinale. Agronomy 2021, 11, 1561. [Google Scholar] [CrossRef]
- Chaudhary, S.; Devi, P.; Bhardwaj, A.; Jha, U.C.; Sharma, K.D.; Prasad, P.V.V.; Siddique, K.H.M.; Bindumadhava, H.; Kumar, S.; Nayyar, H. Identification and characterization of contrasting genotypes/cultivars for developing heart tolerance in agricultural crops: Current status and prospects. Front. Plant Sci. 2020, 11, 587264. [Google Scholar] [CrossRef]
- Maleki, M.; Shojaeiyan, A.; Mokhtassi-Bidgoli, A. Genotypic variation in biochemical and physiological responses of fenugreek (Trigonella foenum-graecum L.) landraces to prolonged drought stress and subsequent rewatering. Sci. Hortic. 2021, 287, 110114. [Google Scholar] [CrossRef]
- Misic, D.; Siller, B.; Filipovic, B.; Popovic, Z.; Zivkovic, S.; Cvetic, T.; Mijovic, A. Rapid in vitro selection of salt-tolerant genotypes of the potentially medicinal plant Centaurium maritimum (L.) Fritsch. Arch. Biol. Sci. 2009, 61, 57–69. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, biochemical, and agronomic trait responses of Nigella sativa genotypes to water stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Khodadadi, E.; Fakheri, B.A.; Ahrizad, S.; Emamjomeh, A.; Norouzi, M.; Komatsu, S. Leaf proteomics of drought-sensitive and -tolerant genotypes of fennel. BBA-Proteins Proteom. 2017, 1865, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Cornea-Cipcigan, M.; Cordea, M.I.; Margaon, R.; Pamfil, D. Exogenously applied GA3 enhances morphological parameters of tolerant and sensitive Cyclamen persicum genotypes under ambient temperature and heat stress conditions. Plants 2022, 11, 1868. [Google Scholar] [CrossRef]
- Kim, H.E.; Han, J.E.; Lee, H.; Murthy, H.N.; Kwon, H.J.; Lee, G.M.; Park, S.Y. Establishment of an efficient in vitro propagation of Cnidium officinale Makino and selection of superior clones through flow cytometric assessment of DNA content. Genes 2022, 13, 1815. [Google Scholar] [CrossRef]
- Kokknti, R.R.; Hindu, V.; Latha, P.; Vasanti, R.P.; Sudhakar, P.; Usha, R. Assessment of genetic variability and molecular characterization of heat stress tolerant genes in Arachis hypogea L. through qRT-PCR. Biocatal. Agric. Biotechnol. 2019, 20, 101242. [Google Scholar] [CrossRef]
- Srikanthababu, V.; Ganeshlumar; Krishnaprasad, B.T.; Gopalakrishna, R.; Savitha, M.; Udayakumar, M. Identification of pea genotypes with enhanced thermo-tolerance using temperature induction response (TIIR) technique. J. Plant Physiol. 2002, 59, 535–545. [Google Scholar] [CrossRef]
- Vijayalakshmi, D.; Sirvidhya, S.; Vivitha, P.; Raveendran, M. Temperature induction response (TIR) as a rapid screening protocol to dissect the genetic variability in acquired thermotolerance in rice and to identify novel donors for high-temperature stress tolerance. Indian J. Plant Physiol. 2015, 20, 368–374. [Google Scholar] [CrossRef]
- Rekah Rani, K.; Chanundeswari, K.; Usha, R. Screening of thermotolerant groundnut genotypes using temperature induction response—A novel approach to assess genetic variability. Int. J. Pharm. Biol. Sci. 2018, 8, 360–364. [Google Scholar]
- Papdorf, K.; Richter, K. Protein folding, misfolding, and quality control: The role of molecular chaperones. Essays Biochem. 2014, 56, 53–68. [Google Scholar]
- Waters, E.R.; Lee, E.J.; Vierling, E. Evolution, structure and function of the small heat shock proteins in plants. J. Exp. Bot. 1996, 47, 325–338. [Google Scholar] [CrossRef]
- Scandalios, J.G.; Guan, L.; Polidoros, A.N. Catalases in plants: Gene structure, properties, regulation, and expression. Cold Spring Harb. Protoc. 1997, 34, 343–406. [Google Scholar]
- Kindric, M.; Kos, J.; Sabotic, J. Proteases, and their endogenous inhibitors in the plant response to abiotic stress. Bot. Serbica 2014, 38, 139–158. [Google Scholar]
- Aldubai, A.A.; Alsadon, A.A.; Migdadi, H.H.; Alghamdi, S.S.; Al-Faifi, S.A.; Afzal, M. Response of tomato (Solanum lycopersicum L.) genotypes to heat stress using morphological and expression study. Plants 2022, 11, 615. [Google Scholar] [CrossRef]
- Jha, S.; Singh, J.; Chouhan, C.; Singh, O.; Srivastava, R.K. Evaluation of multiple salinity tolerance indices for screening and comparative biochemical and molecular analysis of pearl millet [Pinnisetum glaucum (L.) R. Br.] genotypes. J. Plant Growth Regul. 2022, 41, 1820–1834. [Google Scholar] [CrossRef]
- Zhang, Y.; Rouf Mian, M.S.; Chekhovskiy, K.; So, S.; Kupfer, D.; Lai, H.; Roe, B.A. Differential gene expression in Festuca under heat stress conditions. J. Exp. Bot. 2005, 56, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]




| Clone Number | Place of Collection |
|---|---|
| Clone 1 | Daegwallyeong, Republic of Korea |
| Clone 2 | Daegwallyeong, Republic of Korea |
| Clone 5 | Daegwallyeong, Republic of Korea |
| Clone 6 | Daegwallyeong, Republic of Korea |
| Clone 8 | Daegwallyeong, Republic of Korea |
| Clone 11 | Bonghwa, Republic of Korea |
| Clone 14 | Bonghwa, Republic of Korea |
| Clone 15 | Bonghwa, Republic of Korea |
| Clone 22 | Yengyang, Republic of Korea |
| Clone 26 | Yengyang, Republic of Korea |
| Genes | Primer Sequence (5′ → 3′) | |
|---|---|---|
| CoHSP (Heat shock protein) | Forward | CGGAGAAGCAACGTGTTCGA |
| Reverse | ACGCTGCAGTCTCTTTTCCG | |
| CoCP (Cysteine protease) | Forward | CAGGTAGTTGCTGGGCATTT |
| Reverse | TCAACGAGCTCTTGCTCAGA | |
| CoCAT (Catalase) | Forward | TGTCTTCTTTGTGCGTGACG |
| Reverse | CATAATGTGCCTTCCCAGCC | |
| CoActin (Actin) | Forward | GGAAGCAGCAGGAATACACG |
| Reverse | CGCTGTGATTTCCTTGCTCA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-E.; Han, J.-E.; Murthy, H.N.; Kwon, H.-J.; Lee, G.-M.; Park, S.-Y. Response of Cnidium officinale Makino Plants to Heat Stress and Selection of Superior Clones Using Morphological and Molecular Analysis. Plants 2022, 11, 3119. https://doi.org/10.3390/plants11223119
Kim H-E, Han J-E, Murthy HN, Kwon H-J, Lee G-M, Park S-Y. Response of Cnidium officinale Makino Plants to Heat Stress and Selection of Superior Clones Using Morphological and Molecular Analysis. Plants. 2022; 11(22):3119. https://doi.org/10.3390/plants11223119
Chicago/Turabian StyleKim, Hyung-Eun, Jong-Eun Han, Hosakatte Niranjana Murthy, Hyuk-Joon Kwon, Gun-Myung Lee, and So-Young Park. 2022. "Response of Cnidium officinale Makino Plants to Heat Stress and Selection of Superior Clones Using Morphological and Molecular Analysis" Plants 11, no. 22: 3119. https://doi.org/10.3390/plants11223119
APA StyleKim, H.-E., Han, J.-E., Murthy, H. N., Kwon, H.-J., Lee, G.-M., & Park, S.-Y. (2022). Response of Cnidium officinale Makino Plants to Heat Stress and Selection of Superior Clones Using Morphological and Molecular Analysis. Plants, 11(22), 3119. https://doi.org/10.3390/plants11223119






