Pectin Characteristics Affect Root Growth in Spinach under Salinity
Abstract
:1. Introduction
2. Results
2.1. Root Growth
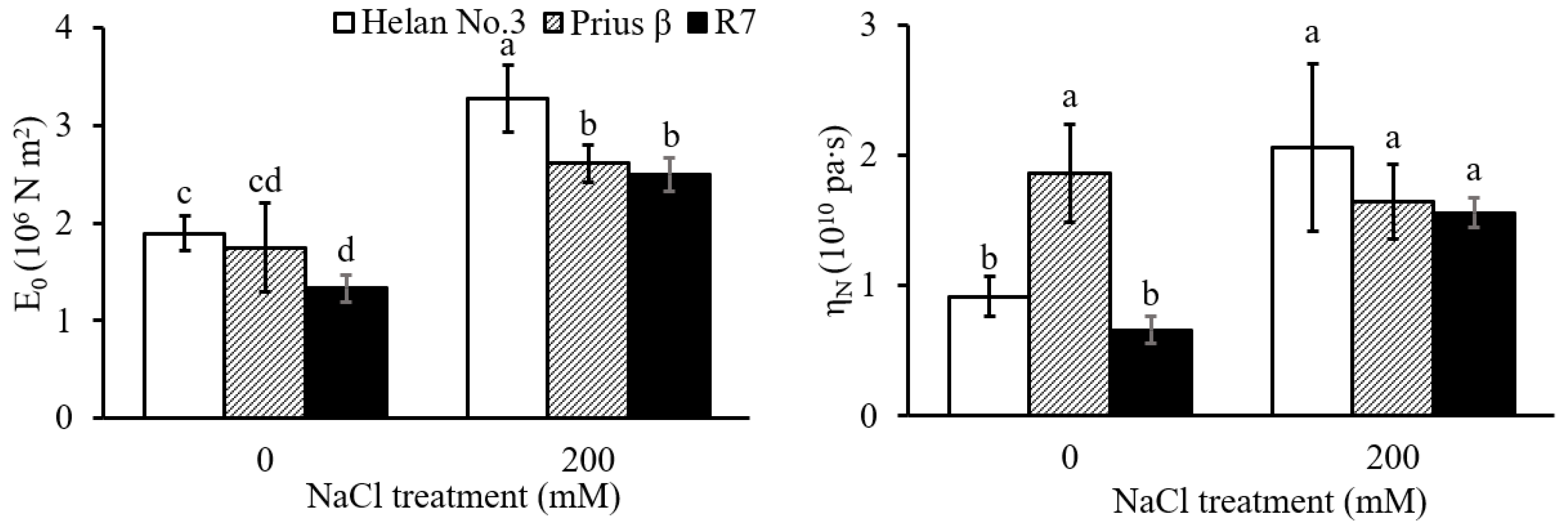
2.2. Root Cell Wall Extensibility and Viscosity
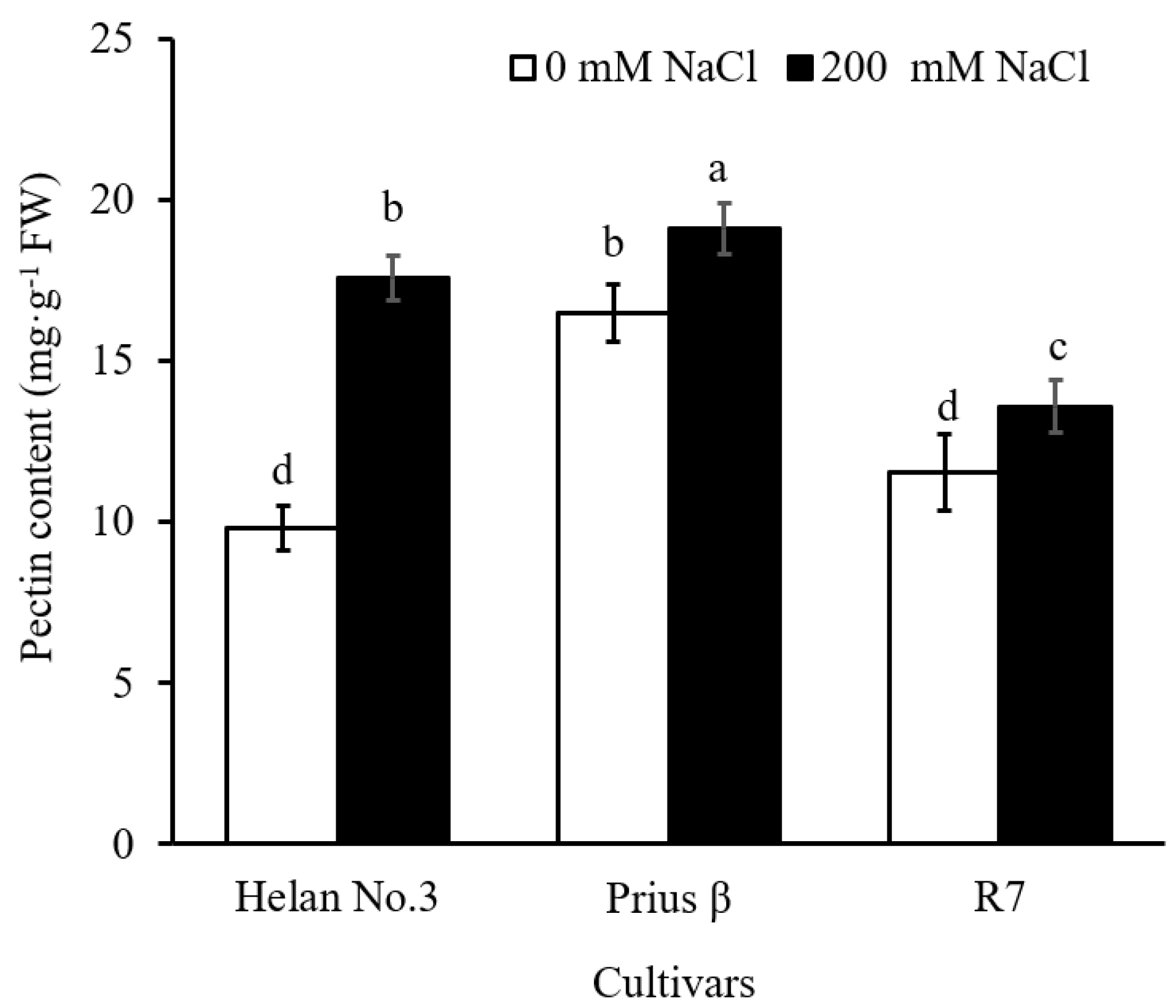
2.3. Chemical Composition of Root Cell Wall
3. Discussion
3.1. Root Growth
3.2. Root Cell Wall Extensibility and Viscosity
3.3. Chemical Composition of Pectin
4. Materials and Methods
4.1. Plant Materials
4.2. Mechanical Parameters of the Root Cell Wall
4.3. Extraction of Cell Wall Fractions
4.4. Characterization of the Extracts
4.4.1. Sugar Composition
4.4.2. Determination of Pectin Methyl-Esterification
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negrão, S.; Schmöckel, S.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ors, S.; Suarez, D. Salt tolerance of spinach as related to seasonal climate. Hortic. Sci. 2016, 43, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Scudiero, E.; Corwin, D.; Anderson, R.; Yemoto, K.; Clary, W.; Wang, Z.; Skaggs, T. Remote sensing is a viable tool for mapping soil salinity in agricultural lands. Calif. Agric. 2017, 71, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [Green Version]
- Richter, J.; Ploderer, M.; Mongelard, G.; Gutierrez, L.; Hauser, M.-T. Role of Cr RLK1L Cell Wall Sensors HERCULES1 and 2, THESEUS1, and FERONIA in Growth Adaptation Triggered by Heavy Metals and Trace Elements. Front. Plant Sci. 2017, 8, 1554. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Li, X.; Zheng, Y.; Matsuura, A.; Abe, J.; Eneji, A.; Tanimoto, E.; Inanaga, S. Effects of NaCl on root growth and cell wall composition of two soya bean cultivars with contrasting salt tolerance. J. Agron. Crop Sci. 2014, 200, 212–218. [Google Scholar] [CrossRef]
- Shao, Y.; An, P.; Feng, X.; Muhammad, I.; Otie, V.; Li, W.; Zheng, Y.; Qiman, Y. Differential responses of roots for varying tolerance to salinity stress in wheat with special reference to elasticity. Plant Growth Regul. 2021, 94, 183–193. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Albersheim, P.; Darvill, A.G.; O’Neill, M.A.; Schols, H.A.; Voragen, A.G.J. An hypothesis: The same six polysaccharides are components of the primary cell walls of all higher plants. In Progress in Biotechnology; Visser, J., Voragen, A.G.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 47–55. [Google Scholar]
- Corrêa-Ferreira, M.L.; Viudes, E.B.; de Magalhães, P.M.; de Santana Filho, A.P.; Sassaki, G.L.; Pacheco, A.C.; de Oliveira Petkowicz, C.L. Changes in the composition and structure of cell wall polysaccharides from Artemisia annua in response to salt stress. Carbohydr. Res. 2019, 483, 107753. [Google Scholar] [CrossRef]
- Huang, J.-H.; Kortstee, A.; Dees, D.C.; Trindade, L.M.; Visser, R.G.; Gruppen, H.; Schols, H.A. Evaluation of both targeted and non-targeted cell wall polysaccharides in transgenic potatoes. Carbohydr. Polym. 2017, 156, 312–321. [Google Scholar] [CrossRef]
- Huang, J.-H.; Kortstee, A.; Dees, D.C.T.; Trindade, L.M.; Schols, H.A.; Gruppen, H. Modification of potato cell wall pectin by the introduction of rhamnogalacturonan lyase and β-galactosidase transgenes and their side effects. Carbohydr. Polym. 2016, 144, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef]
- Liu, J.; Shao, Y.; Feng, X.; Otie, V.; Matsuura, A.; Irshad, M.; Zheng, Y.; An, P. Cell Wall Components and Extensibility Regulate Root Growth in Suaeda salsa and Spinacia oleracea under Salinity. Plants 2022, 11, 900. [Google Scholar] [CrossRef]
- Kim, B.M.; Lee, H.J.; Song, Y.H.; Kim, H.J. Effect of salt stress on the growth, mineral contents, and metabolite profiles of spinach. J. Sci. Food Agric. 2021, 101, 3787–3794. [Google Scholar] [CrossRef]
- Turhan, A.; Kuşçu, H.; Şeniz, V. Effects of different salt concentrations (NaCl) on germination of some spinach cultivars. Uludağ Üniversitesi Ziraat Fakültesi Derg. 2011, 25, 65–77. [Google Scholar]
- Xu, C.; Mou, B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-Induced Changes in Cell Wall Mechanics Underlie Organ Initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskowiak, J.; Kwasniewska, J.; Milewska-Hendel, A.; Kurczynska, E.U.; Szurman-Zubrzycka, M.; Szarejko, I. Aluminum Alters the Histology and Pectin Cell Wall Composition of Barley Roots. Int. J. Mol. Sci. 2019, 20, 3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Feng, X.; Nakahara, H.; Irshad, M.; Eneji, A.E.; Zheng, Y.; Fujimaki, H.; An, P. Apical-root apoplastic acidification affects cell wall extensibility in wheat under salinity stress. Physiol. Plant. 2021, 173, 1850–1861. [Google Scholar] [CrossRef]
- Neumann, P.; Azaizeh, H.; Leon, D. Hardening of root cell walls: A growth inhibitory response to salinity stress. Plant Cell Environ. 1994, 17, 303–309. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- Tanimoto, E.; Fujii, S.; Yamamoto, R.; Inanaga, S. Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L. Plant Soil 2000, 226, 21–28. [Google Scholar] [CrossRef]
- Hu, J.-q.; Qi, Q.; Zhao, Y.-l.; Tian, X.-m.; Lu, H.; Gai, Y.; Jiang, X.-n. Unraveling the impact of Pto4CL1 regulation on the cell wall components and wood properties of perennial transgenic Populus tomentosa. Plant Physiol. Biochem. 2019, 139, 672–680. [Google Scholar] [CrossRef]
- Novaković, L.; Guo, T.; Bacic, A.; Sampathkumar, A.; Johnson, K.L. Hitting the wall—Sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L. Plant cell expansion: Regulation of cell wall mechanical properties. Annu. Rev. Plant Physiol. 1984, 35, 585–657. [Google Scholar] [CrossRef]
- Reboul, R.; Geserick, C.; Pabst, M.; Frey, B.; Wittmann, D.; Lütz-Meindl, U.; Léonard, R.; Tenhaken, R. Down-regulation of UDP-glucuronic acid biosynthesis leads to swollen plant cell walls and severe developmental defects associated with changes in pectic polysaccharides. J. Biol. Chem. 2011, 286, 39982–39992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdunek, A.; Kozioł, A.; Cybulska, J.; Lekka, M.; Pieczywek, P.M. The stiffening of the cell walls observed during physiological softening of pears. Planta 2016, 243, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Moelants, K.; Jolie, R.P.; Palmers, S.K.; Cardinaels, R.; Christiaens, S.; Van Buggenhout, S.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. The effects of process-induced pectin changes on the viscosity of carrot and tomato sera. Food Bioprocess Technol. 2013, 6, 2870–2883. [Google Scholar] [CrossRef]
- Mierczyńska, J.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Effect of storage on rheology of water-soluble, chelate-soluble and diluted alkali-soluble pectin in carrot cell walls. Food Bioprocess Technol. 2015, 8, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Pieczywek, P.M.; Kozioł, A.; Konopacka, D.; Cybulska, J.; Zdunek, A. Changes in cell wall stiffness and microstructure in ultrasonically treated apple. J. Food Eng. 2017, 197, 1–8. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Zhang, H.; Wu, D.; Ye, X.; Linardt, R.J.; Chen, S. Gelling mechanism of RG-I enriched citrus pectin: Role of arabinose side-chains in cation-and acid-induced gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- John, J.; Ray, D.; Aswal, V.K.; Deshpande, A.P.; Varughese, S. Dissipation and strain-stiffening behavior of pectin–Ca gels under LAOS. Soft Matter 2019, 15, 6852–6866. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Šturcová, A.; Jarvis, M.C.; Wess, T.J. Hydration effects on spacing of primary-wall cellulose microfibrils: A small angle X-ray scattering study. Cellulose 2007, 14, 401–408. [Google Scholar] [CrossRef]
- Kirui, A.; Du, J.; Zhao, W.; Barnes, W.; Kang, X.; Anderson, C.T.; Xiao, C.; Wang, T. A pectin methyltransferase modulates polysaccharide dynamics and interactions in Arabidopsis primary cell walls: Evidence from solid-state NMR. Carbohydr. Polym. 2021, 270, 118370. [Google Scholar] [CrossRef]
- White, P.B.; Wang, T.; Park, Y.B.; Cosgrove, D.J.; Hong, M. Water–polysaccharide interactions in the primary cell wall of Arabidopsis thaliana from polarization transfer solid-state NMR. J. Am. Chem. Soc. 2014, 136, 10399–10409. [Google Scholar] [CrossRef] [PubMed]
- Eticha, D.; Staß, A.; Horst, W.J. Localization of aluminium in the maize root apex: Can morin detect cell wall-bound aluminium? J. Exp. Bot. 2005, 56, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, X.; Tao, L.; Yang, Y.; Gao, L.; Xiong, J. Aeration Increases Cadmium (Cd) Retention by Enhancing Iron Plaque Formation and Regulating Pectin Synthesis in the Roots of Rice (Oryza sativa) Seedlings. Rice 2019, 12, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesten, C.; Menna, A.; Sánchez-Rodríguez, C. Regulation of cellulose synthesis in response to stress. Curr. Opin. Plant Biol. 2017, 40, 106–113. [Google Scholar] [CrossRef]
- Vetter, S.; Kunzek, H.; Senge, B. The influence of the pre-treatment of apple cell wall samples on their functional properties. Eur. Food Res. Technol. 2001, 212, 630–635. [Google Scholar] [CrossRef]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef]
- Xu, W.F.; Shi, W.M. Mechanisms of salt tolerance in transgenic Arabidopsis thaliana constitutively overexpressing the tomato 14-3-3 protein TFT7. Plant Soil 2007, 301, 17–28. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Nonami, H.; Tanimoto, K.; Tabuchi, A.; Fukuyama, T.; Hashimoto, Y. Salt stress under hydroponic conditions causes changes in cell wall extension during growth. Hydroponics Transpl. Prod. 1994, 396, 91–98. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Zhao, T.; Mao, G.; Feng, W.; Mao, R.; Gu, X.; Li, T.; Li, Q.; Bao, Y.; Yang, L.; Wu, X. Isolation, characterization and antioxidant activity of polysaccharide from Schisandra sphenanthera. Carbohydr. Polym. 2014, 105, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef] [PubMed]

| Cultivar | NaCl (mM) | Mol% | ||||||
|---|---|---|---|---|---|---|---|---|
| Rha | Ara | Xyl | Man | Glc | Gal | UA | ||
| Helan 3 | 0 | 5.9 (0.4) c | 9.2 (0.6) b | 4.3 (0.4) a | 2.4 (0.2) b | 8.1 (1.3) a | 14.7 (0.8) c | 55.5 (2.1) a |
| 200 | 7.5 (0.3) b | 12.1 (0.7) a | 5.1 (0.5) a | 4.0 (0.6) b | 5.1 (1.1) ab | 17.9(1.2) ab | 48.3 (2.3) b | |
| Prius β | 0 | 6.2 (0.7) c | 11.1(1.3) ab | 3.1 (0.5) b | 9.7 (1.2) a | 2.7 (0.5) b | 19.8 (2.1) a | 47.4 (3.9) b |
| 200 | 6.2 (0.4) c | 9.8 (0.6) ab | 2.1 (0.2) bc | 11.2 (0.9) a | 1.7(0.2) b | 17.6(1.2) ab | 51.4(3.1) ab | |
| R7 | 0 | 8.8 (0.8) b | 10.6 (0.9) b | 2.4 (0.3) bc | 1.3 (0.1) b | 4.2 (0.5) b | 14.6 (1.0) c | 58.2 (2.6) a |
| 200 | 10.4 (0.7) a | 12.5 (0.8) a | 1.5 (0.3) c | 2.5 (0.2) b | 5.1 (0.7) ab | 20.9 (1.3) a | 47.0 (2.9) b | |
| Cultivar | NaCl (mM) | PMD (%) | HG:RG-I Ratio | Galactan Side Chain | Arabinangalactan Side Chain |
|---|---|---|---|---|---|
| Helan 3 | 0 | 35.1 (6.7) c | 9.8 (1.1) a | 2.6 (0.3) ab | 4.2 (0.3) b |
| 200 | 31.4 (2.8) c | 6.5(0.7) bc | 2.4 (0.1) bc | 4.0 (0.1) bc | |
| Prius β | 0 | 42.4 (3.5) b | 8.3 (1.5) ab | 3.3 (0.3) a | 5.0 (0.3) a |
| 200 | 38.8 (3.2) bc | 8.6 (1.1) ab | 2.8 (0.1) ab | 4.4 (0.1) ab | |
| R7 | 0 | 59.0 (6.9) a | 7.0 (0.8) ab | 1.7 (0.1) d | 2.9 (0.1) d |
| 200 | 42.5 (2.2) b | 4.7 (0.6) c | 2.0 (0.1) c | 3.2 (0.1) c |
| Root Length | E0 | ηN | Pectin Content | Uronic Acid | HG:RG-I Ratio | PMD | |
|---|---|---|---|---|---|---|---|
| Helan 3 | |||||||
| E0 | −0.875 ** | ||||||
| ηN | −0.868 ** | 0.780 ** | |||||
| Pectin content | −0.885 ** | 0.685 * | 0.825 ** | ||||
| Uronic acid | 0.601 * | −0.656 * | −0.623 * | −0.268 | |||
| HG:RG-I ratio | 0.621 * | −0.556 | −0.638 * | −0.640 * | 0.325 | ||
| PMD | 0.247 | 0.006 | −0.355 | −0.395 | 0.063 | 0.352 | |
| Root diameter | −0.889 ** | 0.591 * | 0.740 ** | 0.927 ** | −0.344 | −0.579 * | −0.412 |
| Prius β | |||||||
| E0 | −0.628 * | ||||||
| ηN | 0.275 | −0.264 | |||||
| Pectin content | −0.712 ** | 0.384 | 0.180 | ||||
| Uronic acid | −0.175 | 0.114 | −0.869 ** | −0.291 | |||
| HG:RG-I ratio | 0.013 | 0.098 | −0.838 ** | −0.408 | 0.964 ** | ||
| PMD | 0.227 | 0.057 | −0.185 | −0.235 | 0.056 | 0.056 | |
| Root diameter | −0.659 * | 0.286 | −0.611 * | 0.586 * | 0.363 | 0.363 | −0.086 |
| R7 | |||||||
| E0 | −0.816 ** | ||||||
| ηN | −0.821 ** | 0.879 ** | |||||
| Pectin content | −0.737 ** | 0.689 * | 0.741 ** | ||||
| Uronic acid | 0.708 ** | −0.611 * | −0.666 * | −0.557 | |||
| HG:RG-I ratio | 0.615 * | −0.633 * | −0.657 * | −0.512 | 0.979 ** | ||
| PMD | 0.807 ** | −0.657 * | −0.651 * | −0.581 * | 0.450 | 0.351 | |
| Root diameter | −0.914 ** | 0.779 ** | 0.786 ** | 0.823 ** | −0.656 * | −0.585 * | −0.687 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Otie, V.; Matsuura, A.; Junichi, K.; Irshad, M.; Zheng, Y.; Fujimaki, H.; An, P. Pectin Characteristics Affect Root Growth in Spinach under Salinity. Plants 2022, 11, 3130. https://doi.org/10.3390/plants11223130
Liu J, Otie V, Matsuura A, Junichi K, Irshad M, Zheng Y, Fujimaki H, An P. Pectin Characteristics Affect Root Growth in Spinach under Salinity. Plants. 2022; 11(22):3130. https://doi.org/10.3390/plants11223130
Chicago/Turabian StyleLiu, Jia, Victoria Otie, Asana Matsuura, Kashiwagi Junichi, Muhammad Irshad, Yuanrun Zheng, Haruyuki Fujimaki, and Ping An. 2022. "Pectin Characteristics Affect Root Growth in Spinach under Salinity" Plants 11, no. 22: 3130. https://doi.org/10.3390/plants11223130
APA StyleLiu, J., Otie, V., Matsuura, A., Junichi, K., Irshad, M., Zheng, Y., Fujimaki, H., & An, P. (2022). Pectin Characteristics Affect Root Growth in Spinach under Salinity. Plants, 11(22), 3130. https://doi.org/10.3390/plants11223130









