The Suppressive Effects of Biochar on Above- and Belowground Plant Pathogens and Pests: A Review
Abstract
1. Introduction
2. Results
2.1. Research Efforts over the Last Seven Years: Pathosystem, Feedstock Type, and Pyrolysis Conditions
2.2. Disease Suppression by Biochar
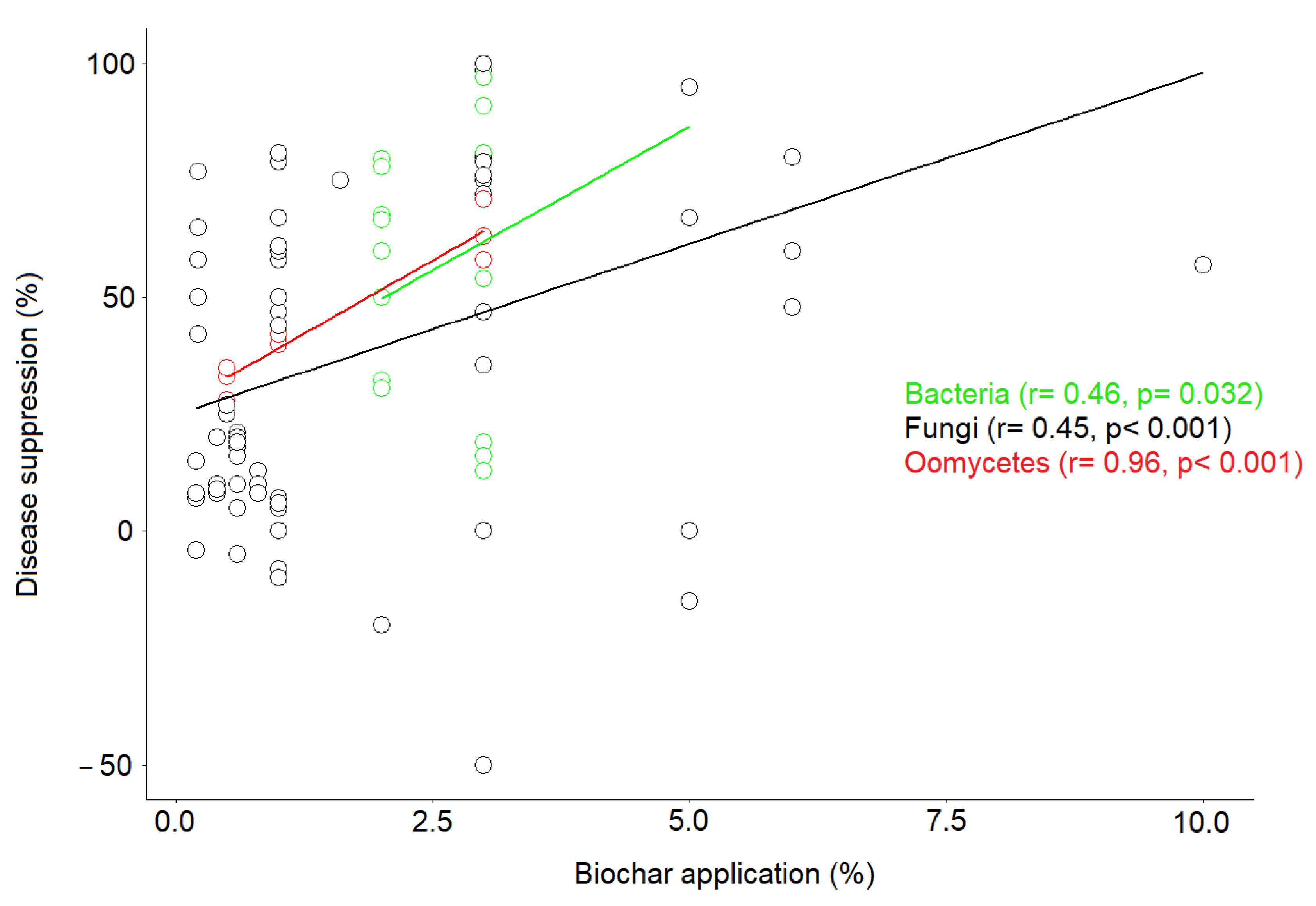
2.3. Application Rate
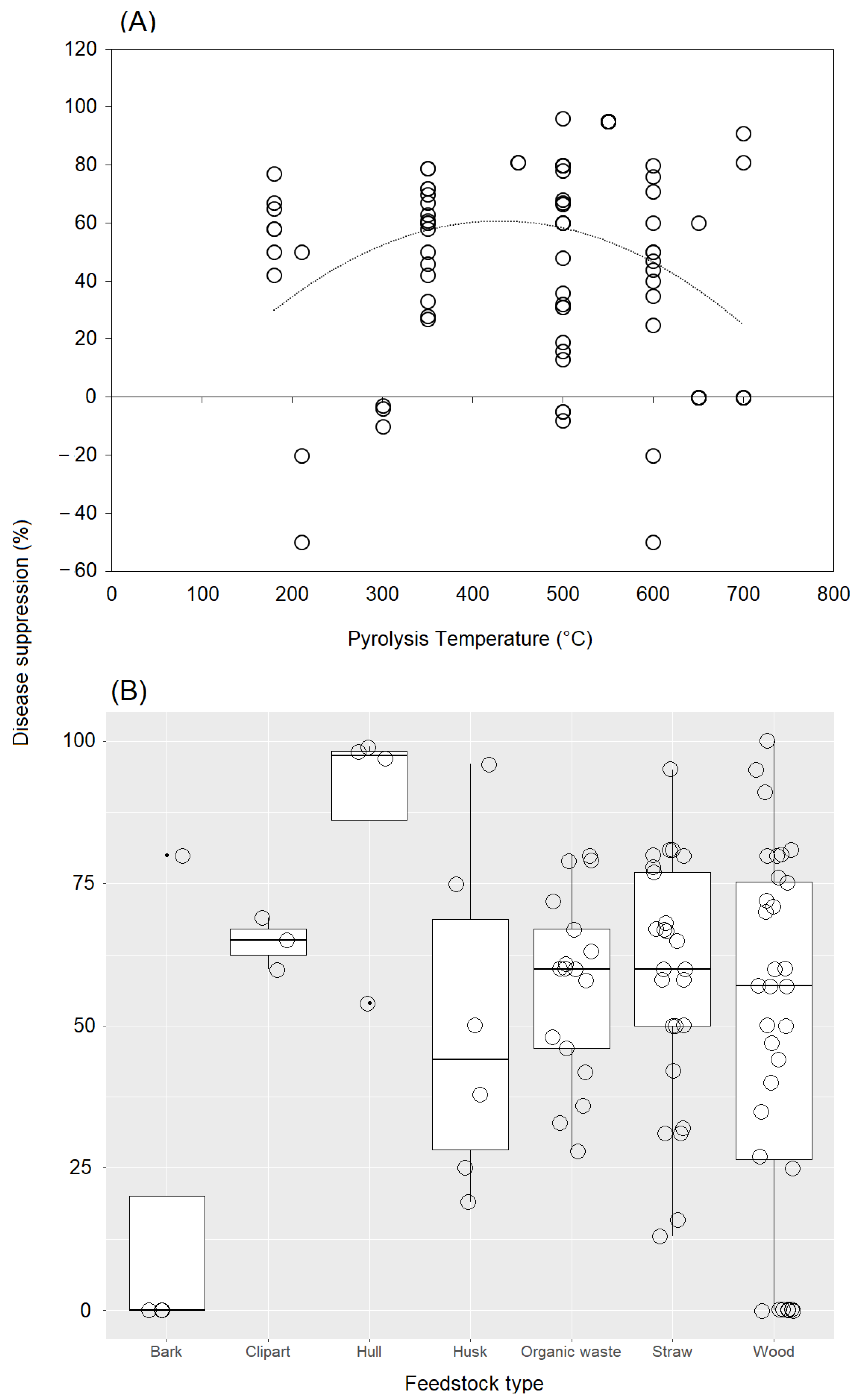
2.4. Feedstock Type and Pyrolysis Temperature
2.5. Disease-Suppression Putative Causal Mechanisms
3. Discussion
3.1. Fungi and Oomycetes
3.2. Bacteria
3.3. Viruses
3.4. Nematodes and Insects
3.5. Parasitic Plants
4. Materials and Methods
Data Collection and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Biochar Initiative. Standardized Product Definition and Product Testing Guidelines for Biochar that Is Used in Soil; IBI Biochar Standards: Canandaigua, NY, USA, 2012. [Google Scholar]
- Lehmann, D.J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan Books Ltd.: London, UK, 2009. [Google Scholar]
- Duku, M.H.; Gu, S.; Hagan, E.B. Biochar production potential in Ghana—A review. Renew. Sustian. Energy Rev. 2011, 15, 3539–3551. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Zech, W. Slash and char: An alternative to slash and burn practiced in the Amazon basin. In Amazonian Dark Earth: Exploration in Space and Time; Glaser, B., Woods, W.I., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004. [Google Scholar]
- Tollefson, J. Footprints in the forest. Nature 2013, 502, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Rycaj, M.; Lehmann, J.; Cornelissen, G. Influence of activated carbon and biochar on phytotoxicity of air-dried sewage sludges to Lepidium sativum. Ecotoxicol. Environ. Saf. 2012, 80, 321–326. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil and concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O. Fire-derived charcoal causes loss of forest humus. Science 2008, 320, 629–630. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into microbial biomass estimated by 14C labelling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Organ. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Scala, F. A “black” future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J. Plant Pathol. 2015, 97, 223–234. [Google Scholar]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an electron shuttle between bacteria and Fe (III) minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Baiamonte, G.; De Pasquale, C.; Marsala, V.; Cimò, G.; Alonzo, G.; Crescimanno, G.; Conte, P. Structure alteration of a sandy-clay soil by biochar amendments. J. Soils Sediments 2015, 15, 816–824. [Google Scholar] [CrossRef]
- Ajayi, A.E.; Holthusen, D.; Horn, R. Changes in microstructural behaviour and hydraulic functions of biochar amended soils. Soil Tillage Res. 2016, 155, 166–175. [Google Scholar] [CrossRef]
- Noble, R.; Coventry, E. Suppression of soil-borne plant diseases with composts: A review. Biocontrol Sci. Technol. 2005, 15, 3–20. [Google Scholar] [CrossRef]
- Elad, Y.; David, D.R.; Harel, Y.M.; Borenshtein, M.; Kalifa, H.B.; Silber, A.; Graber, E.R. Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Tian, J.; Rao, S.; Gao, Y.; Lu, Y.; Cai, K. Wheat straw biochar amendment suppresses tomato bacterial wilt caused by Ralstonia solanacearum: Potential effects of rhizosphere organic acids and amino acids. J. Integr. Agric. 2021, 20, 2450–2462. [Google Scholar] [CrossRef]
- Rasool, M.; Akhter, A.; Soja, G.; Haider, M.S. Role of biochar, compost and plant growth promoting rhizobacteria in the management of tomato early blight disease. Sci. Rep. 2021, 11, 6092. [Google Scholar] [CrossRef] [PubMed]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2016, 213, 1393–1404. [Google Scholar] [CrossRef]
- De Tender, C.; Haegeman, A.; Vandecasteele, B.; Clement, L.; Cremelie, P.; Dawyndt, P.; Maes, M.; Debode, J. Dynamics in the strawberry rhizosphere microbiome in response to biochar and Botrytis cinerea leaf infection. Front. Microbiol. 2016, 7, 2062. [Google Scholar] [CrossRef]
- Kumar, A.; Elad, Y.; Tsechansky, L.; Abrol, V.; Lew, B.; Offenbach, R.; Graber, E.R. Biochar potential in intensive cultivation of Capsicum annuum L. (sweet pepper): Crop yield and plant protection. J. Sci. Food Agric. 2018, 98, 495–503. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.J.; Tafti, N.D.; Hollier, C.A.; Myers, G.; Wang, X. Effect of alkali-enhanced biochar on silicon uptake and suppression of gray leaf spot development in perennial ryegrass. Crop Prot. 2019, 119, 9–16. [Google Scholar] [CrossRef]
- Ahmed, F.; Islam, M.S.; Iqbal, M.T. Biochar amendment improves soil fertility and productivity of mulberry plant. Eur. J. Soil Sci. 2017, 6, 37–43. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Frenkel, O.; Tsechansky, L.; Ela, Y.; Graber, E.R. Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: A possible mechanism involved in soilborne disease suppression. Soil Biol. Biochem. 2018, 121, 59–66. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Y.; Huang, H.C.; Ye, C.; Guo, C.W.; Xu, Y.G.; Wang, W.; He, X.; Liu, Y.; Zhu, S. Steaming combined with biochar application eliminates negative plant-soil feedback for sanqi cultivation. Soil Tillage Res. 2019, 189, 189–198. [Google Scholar] [CrossRef]
- De Araujo, A.S.; Bassay Blum, L.E.; Vinícius Nunes Andrade, D.; Batista da Silva Júnior, P.; De Figueiredo, C.C. Sewage Sludge Biochar Effects on Phytopathogenic Fungi and Beneficial Microorganisms. Braz. Arch. Biol. Technol. 2021, 64, e21210266. [Google Scholar] [CrossRef]
- Elmer, W.H. Effect of leaf mold mulch, biochar, and earth worms on mycorrhizal colonization and yield of asparagus affected by Fusarium crown and root rot. Plant Dis. 2016, 100, 2507–2512. [Google Scholar] [CrossRef]
- Akanmu, A.O.; Sobowale, A.A.; Abiala, M.A.; Olawuyi, O.J.; Odebode, A.C. Efficacy of biochar in the management of fusarium verticillioides Sacc. causing ear rot in Zea mays L. Biotechnol. Rep. 2020, 26, e00474. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Wang, X.; Sun, Q.; Dong, H.; Wang, G.; Chen, X.; Yin, C.; Han, Z.; Mao, Z. Effects of biochar on the growth of apple seedlings, soil enzyme activities and fungal communities in replant disease soil. Sci. Hortic. 2019, 256, 108641. [Google Scholar] [CrossRef]
- Ogundeji, A.O.; Li, Y.; Liu, X.; Meng, L.; Sang, P.; Mu, Y.; Wu, H.; Ma, Z.; Hou, J.; Li, S. Eggplant by grafting enhanced with biochar recruits specific microbes for disease suppression of Verticillium wilt. Appl. Soil Ecol. 2021, 163, 103912. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 4th ed.; Academic: Amsterdam, The Netherlands, 2005; p. 635. [Google Scholar]
- Lammirato, C.; Miltner, A.; Kaestner, M. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol. Biochem. 2011, 43, 1936–1942. [Google Scholar] [CrossRef]
- Graber, E.R.; Tschansky, L.; Cohen, E. Reducing capacity of water extracts of biochars and their solubilization of soil Mn and Fe. Eur. J. Soil Sci. 2014, 65, 162–172. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Alkan, N.; Elad, Y.; Sela, N.; Philosoph, A.M.; Graber, E.R.; Frenkel, O. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci. Rep. 2020, 10, 13934. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Brussaard, L. Biodiversity and ecosystem functioning in soil. AMBIO 1997, 26, 563–570. [Google Scholar]
- Lindow, S.E.; Leveau, J.H.J. Phyllosphere microbiology. Curr. Opin. Biotechnol. 2002, 13, 238–243. [Google Scholar] [CrossRef]
- Lu, Y.; Rao, S.; Huang, F.; Cai, Y.; Wang, G.; Cai, K. Effects of biochar amendment on tomato bacterial wilt resistance and soil microbial amount and activity. Int. J. Agron. 2016, 2016, 2938282. [Google Scholar] [CrossRef]
- Bonanomi, G.; Alioto, D.; Minutolo, M.; Marra, R.; Cesarano, G.; Vinale, F. Organic amendments modulate soil microbiota and reduce virus disease incidence in the TSWV-tomato pathosystem. Pathogens 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Van Bockhaven, J.; De Vleesschauwer, D.; Hofte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Khattab, H.I.; Emam, M.A.; Emam, M.M.; Helal, N.M.; Mohamed, M.R. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol. Plantar. 2014, 58, 265–273. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691. [Google Scholar] [CrossRef]
- Chen, C.; Ma, T.; Shang, Y.; Gao, B.; Jin, B.; Dan, H.; Li, Q.; Yue, Q.; Li, Y.; Wang, Y.; et al. In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: Considering the intrinsic N/Fe in Enteromorpha and non-radical reaction. Appl. Catal. B 2019, 250, 382–395. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Bian, R.; Chen, D.; Qu, J.; Kibue, G.W.; Pan, G.; Zhang, X.; Zheng, J.; Zheng, J. Effect of biochar amendment on soil-silicon availability and rice uptake. J. Plant Nutr. Soil Sci. 2014, 177, 91–96. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Keeoing, M.G.; Meyer, J.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Qiang Han, Y.; Hui Wen, J.; Pu Peng, Z.; Yong Zhang, D.; Lin Hou, M. Effects of silicon amendment on the occurrence of rice insect pests and diseases in a field test. J. Integr. Agric. 2018, 17, 2172–2181. [Google Scholar]
- Iacomino, G.; Sarker, T.C.; Ippolito, F.; Bonanomi, G.; Vinale, F.; Staropoli, A.; Idbella, M. Biochar and Compost Application either Alone or in Combination Affects Vegetable Yield in a Volcanic Mediterranean Soil. Agronomy 2022, 12, 1996. [Google Scholar] [CrossRef]
- Tippe, D.E.; Bastiaans, L.; van Ast, A.; Dieng, I.; Cissoko, M.; Kayeke, J.; Makokha, D.W.; Rodenburg, J. Fertilisers differentially affect facultative and obligate parasitic weeds of rice and only occasionally improve yields in infested fields. Field Crops Res. 2020, 254, 107845. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Potential of Fusarium wilt-inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil 2016, 406, 425–440. [Google Scholar] [CrossRef]
- Wu, H.M.; Lin, M.H.; Rensing, C.; Qin, X.J.; Zhang, S.K.; Chen, J.; Wu, L.K.; Zhao, Y.L.; Lin, S.; Lin, W.X. Plant-mediated rhizospheric interactions in intraspecific intercropping alleviate the replanting disease of Radix pseudostellariae. Plant Soil 2020, 454, 411–430. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y.; Chenia, H.Y.; Govinden, R.; Luo, J.; Ren, G. Biochar-mediated control of phytophthora blight of pepper is closely related to the improvement of the rhizosphere fungal community. Front. Microbiol. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Copley, T.; Bayen, S.; Jabaji, S. Biochar amendment modifies expression of soybean and Rhizoctonia solani genes leading to increased severity of Rhizoctonia Foliar Blight. Front. Plant Sci. 2017, 8, 221. [Google Scholar] [CrossRef]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Vinale, F.; Lombardi, N.; Crasto, A.; Woo, G.S.L.; Scala, F. Biochar chemistry defined by 13C-CPMAS NMR explains opposite effects on soilborne microbes and crop plants. Appl. Soil Ecol. 2018, 124, 351–361. [Google Scholar] [CrossRef]
- El-Hafez, O.A.A.; Amer, M.A. The influence of biochar on Common scab disease of potatoes. J. Plant Prot. Pathol. 2021, 12, 373–380. [Google Scholar]
- Gu, Y.; Hou, Y.; Huang, D.; Hao, Z.; Wang, X.; Wei, Z.; Jousset, A.; Tan, S.; Xu, D.; Shen, Q.; et al. Application of biochar reduces Ralstonia solanacearum infection via effects on pathogen chemotaxis, swarming motility, and root exudate adsorption. Plant Soil 2017, 415, 269–281. [Google Scholar] [CrossRef]
- Kawanna, M.; Elbebany, A.; Basyony, A. Impact of Biochar Soil Amendment on Tomato mosaic virus Infection, Growth and Nutrients Uptake of Tomato Plants. Alex. Sci. Exch. J. 2021, 42, 799–807. [Google Scholar] [CrossRef]
- Zeshan, M.A.; Iftikhar, Y.; Ali, S.; Ahmed, N.; Ghani, M.; Kamran, N.; Khan, Q.N. Induction of resistance in tomato plants against Tomato leaf curl virus by using biochar and seed priming. Pak. J. Phytopathol. 2018, 30, 19–25. [Google Scholar] [CrossRef]
- Arshad, U.; Azeem, F.; Mustafa, G.; Bakhsh, A.; Toktay, H.; McGiffen, M.; Amjad Nawaz, M.; Naveed, M.; Amjad Ali, M. Combined application of biochar and biocontrol agents enhances plant growth and activates resistance against Meloidogyne incognita in tomato. Gesunde Pflanz. 2021, 73, 591–601. [Google Scholar] [CrossRef]
- Eche, C.O.; Okafor, O.E. Control potential of some indigenous biochars against Meloidogyne incognita [(Kofoid and White) Chitwood] in tomato (Solanum lycopersicum L.). J. Entomol. Nematol. 2020, 12, 32–38. [Google Scholar]
- Marra, R.; Vinale, F.; Cesarano, G.; Lombardi, N.; D’Errico, G.; Crasto, A.; Mazzei, P.; Piccolo, A.; Incerti, G.; Woo, S.L.; et al. Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS ONE 2018, 13, 6. [Google Scholar] [CrossRef]
- Edenborn, L.; Johnson, L.M.K.; Edenborn, H.M.; Albarran-Jack, M.R.; Demetrion, L.D. Amendment of a hardwood biochar with compost tea: Effects on plant growth, insect damage and the functional diversity of soil microbial communities. Biol. Agric. Hortic. 2018, 34, 88–106. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. Parasitic plants: Parallels and contrasts with herbivores. Oecologia 2002, 131, 479–489. [Google Scholar] [CrossRef]
- Eizenberg, H.; Plakhine, D.; Ziadne, H.; Tsechansky, L.; Graber, E.R. Non-chemical control of root parasitic weeds with biochar. Front. Plant Sci. 2017, 8, 939. [Google Scholar] [CrossRef]
- Saudy, H.S.; Hamed, M.F.; El–Metwally, I.M.; Ramadan, K.A.; Aisal, K.H. Assessing the Effect of Biochar or Compost Application as a Spot Placement on Broomrape Control in Two Cultivars of Faba Bean. J. Soil Sci. Plant Nutr. 2021, 21, 1856–1866. [Google Scholar] [CrossRef]
- Poveda, J.; Martínez-Gómez, Á.; Fenoll, C.; Escobar, C. The use of biochar for plant pathogen control. Phytopathology 2021, 111, 1490–1499. [Google Scholar] [CrossRef]
- Bonanomi, G.; Jesu, G.; Zotti, M.; Idbella, M.; D’Errico, G.; Laudonia, S.; Vinale, F.; Abd-ElGawad, A. Biochar-derived smoke-water exerts biological effects on nematodes, insects, and higher plants but not fungi. Sci. Total Environ. 2021, 750, 142307. [Google Scholar] [CrossRef] [PubMed]
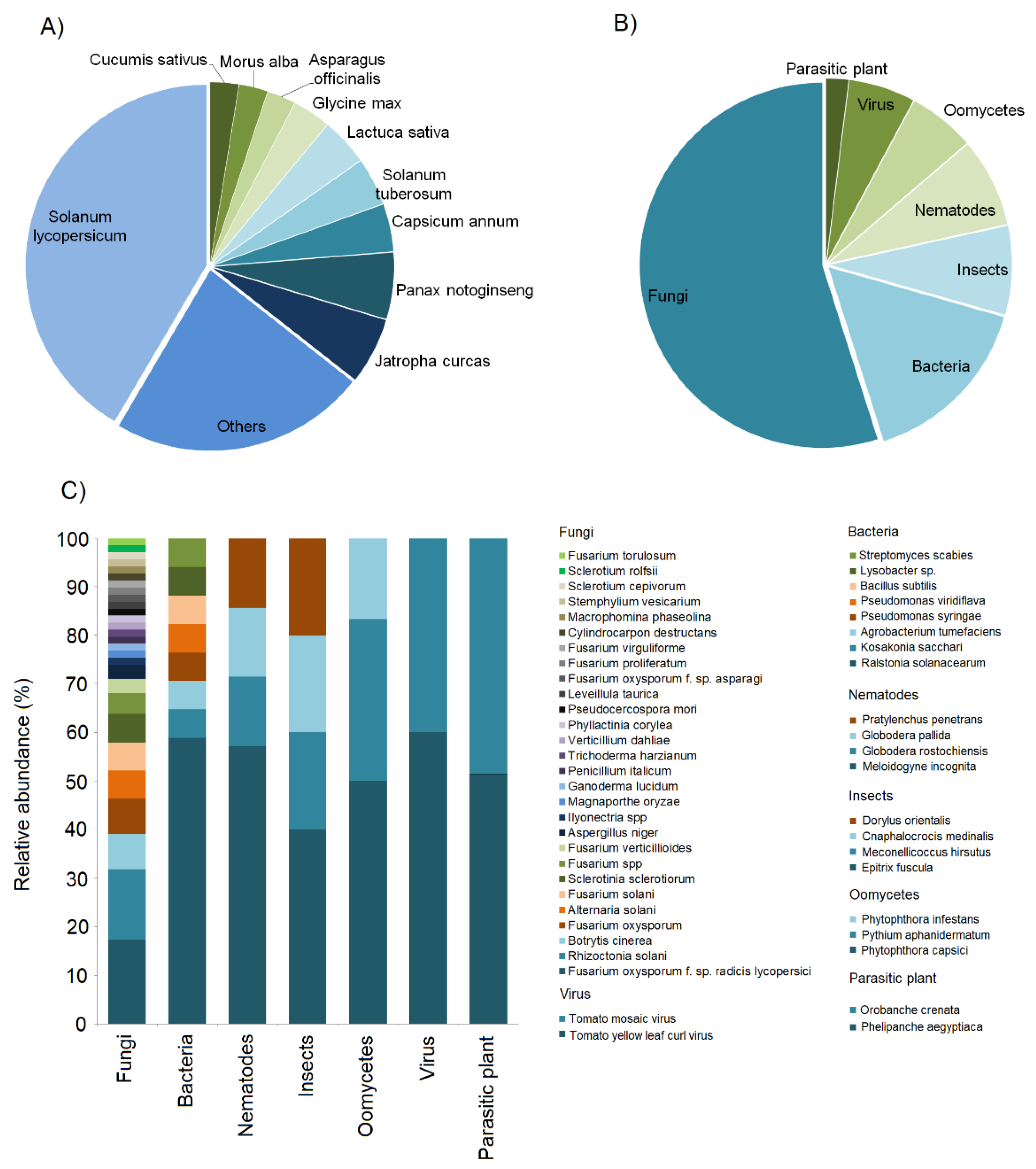


| Pathogen and Pest Type | Adsorption/Deactivation of Virulence Factors | ISR, SAR | Alteration of Soil Microbial Community | Increased Si Content |
|---|---|---|---|---|
| Fungi | 7 | 3 | 50 | 1 |
| Bacteria | 2 | 2 | 13 | 0 |
| Insects | 0 | 0 | 1 | 1 |
| Nematodes | 0 | 4 | 1 | 0 |
| Oomycetes | 0 | 0 | 6 | 0 |
| Viruses | 0 | 3 | 2 | 0 |
| Parasitic plants | 3 | 0 | 0 | 0 |
| Pathogen | Host Plant | Biochar Type and Application Rate | Response | References |
|---|---|---|---|---|
| Fusarium oxysporum f. sp. radicis lycopersici (SB) | Solanum lycopersicum | Wood (1–3%) | Medium–high | [32] |
| Alternaria solani (A) | Solanum lycopersicum | Wood—green wastes (3–6%) | high | [26] |
| Fusarium oxysporum f. sp. radicis lycopersici (SB) | Solanum lycopersicum | Wood—wastes (1–3%) | Medium–high | [32] |
| Fusarium oxysporum f. sp. radicis lycopersici (SB) | Solanum lycopersicum | Wood—wastes (3%) | high | [58] |
| Botrytis cinerea (A) | Solanum lycopersicum | Wood (1%) | high | [27] |
| Fusarium oxysporum f. sp. radicis lycopersici (SB) | Solanum lycopersicum | Green house wastes (1–3%) | high | [42] |
| Alternaria solani (A) | Solanum lycopersicum | Wood—green wastes (3–6%) | Medium–low | [26] |
| Fusarium oxysporum f. sp. lycopersici (SB) | Solanum lycopersicum | Wood (0.5–3%) | Medium–high | [32] |
| Fusarium verticillioides (SB) | Zea mays | Wastes (1–3%) | Medium | [36] |
| Fusarium oxysporum (SB) | Radix pseudostellariae | Hull (3%) | High | [59] |
| Botrytis cinerea (A) | Fragaria x ananassa | Organic matter (1–3%) | High | [28] |
| Fusarium spp. (SB) | Panax notoginseng | Wood (8 g L−1) | Medium | [33] |
| Magnaporthe oryzae (A) | Lolium perenne L. | Straw (0.22–1%) | Medium–high | [37] |
| Fusarium solani (SB) | Malus | Husk (5–80 g kg−1) | Medium | [37] |
| F.oxysporum f. sp. radicis-lycopersici (SB), Botrytis cinerea (A), Fusarium oxysporum (SB), Ganodema lucidum (SB), Penicillium italicum (A), Rhizoctonia solani (SB), Sclerotinia sclerotiorum (SB) | Solanum lycopersicum | Medicago-Mays—organic wastes (5%) | High | [62] |
| Verticillium dahliae (SB) | Solanum melongena | Husk (10 t/ha biochar) | High | [38] |
| Phyllactinia corylea (A), Pseudocercospora mori (A) | Morus alba | Husk | Medium | [31] |
| Leveillula taurica (A) | Capiscum annum L. | Green house wastes—wood | High | [29] |
| Fusarium oxysporum (SB), Fusarium oxysporum f.sp. asparagi (SB), Fusarium proliferatum (SB) | Asparagus officinalis L. | Wood (10%) | Medium–high | [35] |
| Rhizoctonia solani (SB) | Glycine max | Wood (1–5%) | Negative effect | [61] |
| Fusarium oxysporum f. sp. radicis-lycopersici (SB), Fusarium oxysporum (SB), Rhizoctonia solani (SB), Sclerotinia sclerotiorum (SB), Macrophomina phaseolina (SB), Sclerotium cepivorum (SB), Sclerotium rolfsii (SB) | Jatropha curcas L. | Sewage sludge (0.2–1%) | Medium–high | [34] |
| Fusarium torulosum (SB), Fusarium solani (SB) | Panax ginseng | Straw (1%) | High | [25] |
| Pathogen | Host Plant | Biochar Type and Application Rate | Response | References |
|---|---|---|---|---|
| Ralstonia solanacearum (SB) | Solanum lycopersicum | Straw (2%) | High | [25] |
| Ralstonia solanacearum (SB) | Solanum lycopersicum | Straw (2%) | High | [46] |
| Ralstonia solanacearum (SB) | Solanum lycopersicum | Straw (2%) | Medium–high | [64] |
| Kosakonia sacchari (SB) | Radix pseudostellariae | Hull (3%) | High | [59] |
| Agrobacterium tumefaciens (SB) | Lactuca sativa | Medicago, wood, organic wastes, maize (5%) | High | [62] |
| Pseudomonas syringae (A) | Solanum lycopersicum | Medicago, wood, organic wastes, maize (5%) | High | [62] |
| Pseudomonas viridiflava (A) | Solanum lycopersicum | Medicago, wood, organic wastes, maize (5%) | High | [62] |
| Lysobacter sp. (SB) | Solanum lycopersicum | Medicago, wood, organic wastes, maize (5%) | High | [62] |
| Ralstonia solanacearum (SB) | Nicotiana Tabacum | Hull (7.5–45 ton/ ha) | High | [51] |
| Ralstonia solanacearum (SB) | Solanum lycopersicum | Wood (3%) | High | [64] |
| Streptomyces scabies (SB) | Solanum tuberosum | Different agricultural wastes (0.5 ton/ha) | Medium | [63] |
| Pathogen | Host Plant | Biochar Type and Application Rate | Response | References |
|---|---|---|---|---|
| Curl virus (SB) | Solanum lycopersicum | Maize (1–3%) | High | [66] |
| Tomato Mosaic virus (SB) | Solanum lycopersicum | Husk (0.5–1.5%) | Medium | [65] |
| Tomato spotted wilt virus (TSWV) (SB) | Solanum lycopersicum | Wood | High | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacomino, G.; Idbella, M.; Laudonia, S.; Vinale, F.; Bonanomi, G. The Suppressive Effects of Biochar on Above- and Belowground Plant Pathogens and Pests: A Review. Plants 2022, 11, 3144. https://doi.org/10.3390/plants11223144
Iacomino G, Idbella M, Laudonia S, Vinale F, Bonanomi G. The Suppressive Effects of Biochar on Above- and Belowground Plant Pathogens and Pests: A Review. Plants. 2022; 11(22):3144. https://doi.org/10.3390/plants11223144
Chicago/Turabian StyleIacomino, Giuseppina, Mohamed Idbella, Stefania Laudonia, Francesco Vinale, and Giuliano Bonanomi. 2022. "The Suppressive Effects of Biochar on Above- and Belowground Plant Pathogens and Pests: A Review" Plants 11, no. 22: 3144. https://doi.org/10.3390/plants11223144
APA StyleIacomino, G., Idbella, M., Laudonia, S., Vinale, F., & Bonanomi, G. (2022). The Suppressive Effects of Biochar on Above- and Belowground Plant Pathogens and Pests: A Review. Plants, 11(22), 3144. https://doi.org/10.3390/plants11223144











