Asymmetric Reciprocal Crossing Behavior of an Andean Blueberry (V. meridionale) × Lingonberry (V. vitis-idaea) Hybrid
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Pollinations
4.3. Seed Extraction and Germination
4.4. Ploidy Determinations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luby, J.J.; Ballington, J.R.; Draper, A.D.; Pliszka, K.; Austin, M.E. Blueberries and cranberries (Vaccinium). Acta Hort. 1991, 290, 391–456. [Google Scholar] [CrossRef]
- Gaviria, C.A.; Ochoa, C.I.; Sánchez, N.Y.; Medina, C.I.; Lobo, M.; Galeano, P.L.; Mosquera, A.J.; Tamayo, A.; Lopera, Y.E.; Rojano, B.A. Propiedades antioxidantes de los frutos de agraz o mortiño (Vaccinium meridionale Swartz). In Perspectivas de Cultivo de Agraz o Mortiño (Vaccinium meridionale Swartz); Ligaretto, G.A., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2009; pp. 93–112. [Google Scholar]
- Ehlenfeldt, M.K.; Ballington, J.R. Prolific triploid production in intersectional crosses of 4x Vaccinium corymbodendron Dunal (section Pyxothamnus) by 2x section Cyanococcus species. Euphytica 2017, 213, 238–246. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J.; Rowland, L.J.; Ogden, E.; Luteyn, J.L. Fertile intersectional hybrids of 4x Andean blueberry (Vaccinium meridionale) and 2x lingonberry (V. vitis-idaea). HortScience 2022, 57, 525–531. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Luteyn, J.L. Fertile intersectional F1 hybrids of 4x Vaccinium meridionale (section Pyxothamnus) and highbush blueberry, V. corymbosum (section Cyanococcus). HortScience 2021, 56, 318–323. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J.; Vorsa, N.; Zalapa, J.; de la Torre, F.; Luteyn, J.L. Fertile intersectional F1 hybrids of 4x Andean blueberry (Vaccinium meridionale) and 4x American cranberry (V. macrocarpon). HortScience, 2023; accepted. [Google Scholar]
- Cabezas, D.; de Bem Oliveira, I.; Acker, M.; Lyrene, P.; Munoz, P.R. Evaluating wild germplasm introgression into autotetraploid blueberry. Agronomy 2021, 11, 614. [Google Scholar] [CrossRef]
- Lobos, G.A.; Hancock, J.F. Breeding blueberries for a changing global environment: A review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef]
- Ballington, J.R. The role of interspecific hybridization in blueberry improvement. Acta Hort. 2008, 810, 49–60. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- De Wet, J.M.J. Polyploidy and evolution in plants. Taxon 1971, 20, 29–35. [Google Scholar] [CrossRef]
- Laverty, T.; Vorsa, N. Fertility of aneuploids between the 5x and 6x levels in blueberry: The potential for gene transfer from 4x to 6x levels. J. Am. Soc. Hortic. Sci. 1991, 116, 330–335. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Vorsa, N.; Jelenkovic, G.; Draper, A.D.; Welker, W.V. Fertility of 4x × 5x and 5x × 4x progenies derived from Vaccinium ashei/corymbosum pentaploid hybrids. J. Am. Soc. Hortic. Sci. 1987, 112, 993–997. [Google Scholar] [CrossRef]
- Johnston, S.A.; Hanneman, R.E., Jr. Manipulations of Endosperm Balance Number overcome crossing barriers between diploid Solanum species. Science 1982, 217, 446–448. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Hanneman, R.E., Jr. Genetic control of Endosperm Balance Number (EBN): Three additive loci in a threshold-like system. Theor. Appl. Genet. 1988, 75, 825–832. [Google Scholar] [CrossRef]
- Cooper, D.C.; Brink, R.A. Seed collapse following matings between diploid and tetraploid races of Lycopersicon pimpinellifolium. Genetics 1945, 30, 376–401. [Google Scholar] [CrossRef]
- Haig, D.; Westoby, M. Genomic imprinting in endosperm: Its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philos. Trans. R. Soc. B Biol. Sci. 1991, 333, 1–13. [Google Scholar]
- Johnston, S.A.; Hanneman, R.E., Jr. Support of the endosperm balance number hypothesis utilizing some tuber-bearing Solanum species. Am. Potato J. 1980, 57, 7–14. [Google Scholar] [CrossRef]
- Hancock, J.F.; Siefker, J.H. Levels of inbreeding in highbush blueberry cultivars. HortScience 1982, 17, 363–366. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K. The genetic composition and tetrasomic inbreeding coefficients of highbush blueberry cultivars. HortScience 1994, 29, 1342–1345. [Google Scholar] [CrossRef]
- Brevis, P.A.; Bassil, N.V.; Ballington, J.R.; Hancock, J.F. Impact of wide hybridization on highbush blueberry breeding. J. Am. Soc. Hortic. Sci. 2008, 133, 427–437. [Google Scholar] [CrossRef]
- NeSmith, D.S.; Draper, A.D. ‘Camellia’ southern highbush blueberry. J. Amer. Pom. Soc. 2007, 61, 34–37. [Google Scholar]
- Ehlenfeldt, M.K.; Stretch, A.W.; Vorsa, N.; Draper, A.D. ‘Cara’s Choice’ blueberry. HortScience 2005, 40, 1556–1557. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture—Agricultural Research Service. Notice to Nurserymen of the Naming and Release for Propagation of Dixieblue a New Southern Highbush Blueberry Cultivar; Cultivar Release Notice; U.S. Department of Agriculture—Agricultural Research Service: Washington, DC, USA, 2005. [Google Scholar]
- Draper, A.; Galletta, G.; Jelenkovic, G.; Vorsa, N. ‘Duke’ highbush blueberry. HortScience 1987, 22, 320. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Notice to Nurserymen of the Naming and Release for Propagation of Pearl River, Magnolia, and Jubilee, Three New Southern Highbush Blueberry Cultivars; Cultivar Release Notice; U.S. Department of Agriculture: Washington, DC, USA, 1994. [Google Scholar]
- Sharpe, R.H.; Sherman, W.B. ‘Sharpblue’ blueberry. HortScience 1976, 11, 65. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Notice of Release of Sweetheart Highbush Blueberry; Cultivar Release Notice; U.S. Department of Agriculture: Washington, DC, USA, 2010. [Google Scholar]
- Ehlenfeldt, M.K. ‘Talisman’ northern highbush blueberry: A productive late-midseason cultivar. HortScience 2020, 56, 101–103. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J. Highly fertile intersectional blueberry hybrids of Vaccinium padifolium section Hemimyrtillus and V. corymbosum section Cyanococcus. J. Amer. Soc. Hort. Sci. 2014, 139, 30–38. [Google Scholar] [CrossRef]
- Austin, M.E.; Draper, A.D. ‘Baldwin’ rabbiteye blueberry. HortScience 1985, 20, 454. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and University of Georgia College of Agriculture. Notice to Fruit Growers and Nurserymen Relative to the Introduction of New Rabbiteye Blueberry Varieties—Southland, Briteblue, and Delite; Cultivar Release Notice; U.S. Department of Agriculture and University of Georgia College of Agriculture: Washington, DC, USA, 1969. [Google Scholar]
- Lyrene, P.L. Blueberry Plant Called ‘Florida Rose’. U.S. Plant Patent 14,485, 27 January 2004. [Google Scholar]
- Ehlenfeldt, M.K.; Martin, R.B.; Rowland, L.J. ‘Nocturne’ hybrid blueberry: A winter-hardy, mixed-species hexaploid with ornamental landscape interest and novel fruit quality. HortScience 2015, 50, 1825–1827. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Finn, C.E. G-435 and ARS 96-138, Pink-fruited blueberry selections. HortScience 2007, 42, 172–173. [Google Scholar] [CrossRef]
- North Carolina Agricultural Experiment Station; U.S. Department of Agriculture. Notice to Blueberry Growers and Nurserymen Relative to the Naming and Release of Premier, Powderblue, and Centurion, Three New Rabbiteye Blueberries; Cultivar Release Notice; North Carolina Agricultural Experiment Station: Raleigh, NC, USA; U.S. Department of Agriculture: Washington, DC, USA, 1975. [Google Scholar]
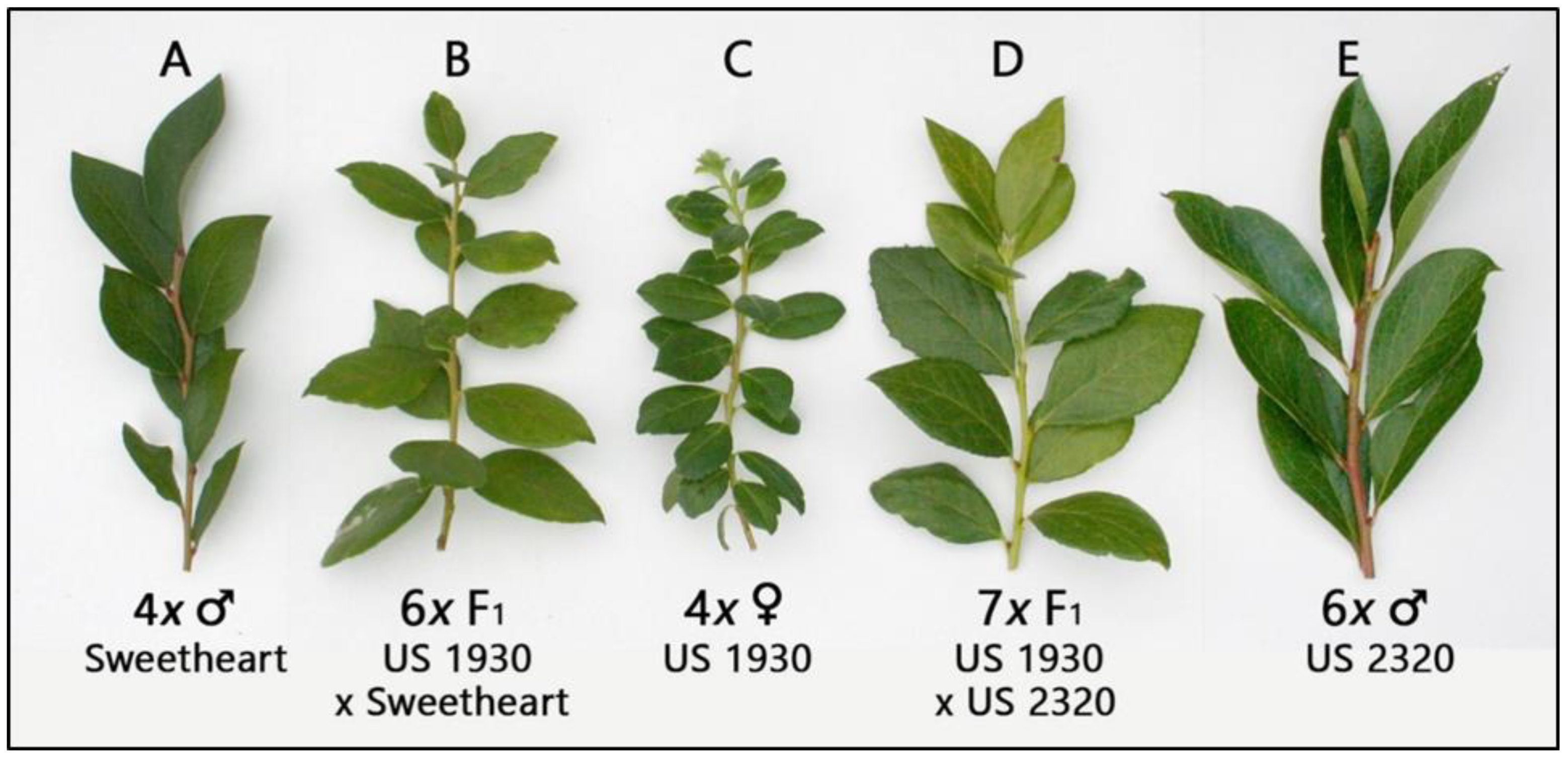
| Seed Quality | Viable | No. of | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poll. | Fruit | Good | g-f | Fair | f-p | Poor | Seed z | s/poll y | s/f x | Plants | Ploidy | Notes | |||
| 4x F1 Hybrids × 4x V. corymbosum and Other 4x Selections | |||||||||||||||
| F1 US 1930 | × | 4x V. corymbosum ARS 99-72 | 43 | 8 | 10 | 9 | 1 | 4 | 1 | 20 | 0.5 | 2.5 | - | ||
| “ ” | × | ‘Cara’s Choice’ | 47 | 28 | 6 | 3 | 9 | 5 | 1 | 18 | 0.4 | 0.6 | 2 | 6x | low vigor |
| “ ” | × | ‘Dixieblue’ | 45 | 31 | 12 | 12 | 9 | 8 | 190 | 33 | 0.7 | 1.1 | 3 | 5x, 5.75x, 5x | “ |
| “ ” | × | ‘Magnolia’ | 17 | 15 | 8 | 14 | 9 | 4 | - | 31 | 1.8 | 2.1 | - | ||
| “ ” | × | 4x F1 US 1896 | 32 | 27 | 14 | 24 | 9 | 16 | 207 | 47 | 1.5 | 1.7 | 3 | 6x | low vigor |
| “ ” | × | 4x V. corymbosum US 2117 | 15 | * | * | * | * | * | * | - | - | - | 1 | 6x | “ |
| “ ” | × | 4x V. padifolium US 908 | 40 | 16 | 23 | 8 | 19 | 28 | 27 | 50 | 1.3 | 3.1 | 1 | 6x | US 2532, low vigor |
| “ ” | × | ‘Sweetheart’ | * | * | * | * | * | * | * | - | - | - | 1 | 6x | low vigor |
| F1 US 1933 | × | ‘Camellia’ | 16 | 8 | 10 | 1 | - | - | - | 11 | 0.7 | 1.4 | 4 | 6x | “ |
| “ ” | × | ‘Sweetheart’ | 30 | * | * | * | * | * | * | - | - | - | 1 | 6x | “ |
| F1 US 1993 | × | ‘Sweetheart’ | 9 | * | * | * | * | * | * | - | - | - | 1 | 6x | US 2386, low vigor |
| Total | 294 | 133 | 210 | avg. | avg. | 17 | |||||||||
| 0.71 | 1.6 | ||||||||||||||
| 2nd generation 6x F1 × 4x V. corymbosum | |||||||||||||||
| US 2386 | × | ‘Camellia’ | 19 | 18 | 249 | 3 | 10 | - | - | 262 | 13.8 | 14.6 | <170 | 20 sampled @ 5x | medium vigor |
| “ | × | ‘Chandler’ | 19 | 16 | 119 | - | 1 | - | 2 | 120 | 6.3 | 7.5 | not planted | - | |
| Total | 38 | 34 | 382 | avg. | avg. | ||||||||||
| 10.05 | 11.2 | ||||||||||||||
| 2nd generation 6x F1 × 6x V. virgatum | |||||||||||||||
| US 2386 | × | ARS 07-97 | 4 | 1 | 28 | - | - | 1 | - | 28 | 7.0 | 28.0 | 16 | not determined | good vigor |
| Seed Quality | Viable | No. of | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poll. | Fruit | Good | g-f | Fair | f-p | Poor | Seed z | s/poll y | s/f x | Plants | Ploidy | Notes | |||
| 4x F1 Hybrid × 6x V. virgatum and Other 6x Selections | |||||||||||||||
| F1 US 1930 | × | ARS 07-97 | 36 | 36 | 11 | - | 1 | - | - | 12 | 0.3 | 0.3 | - | ||
| “ ” | × | ARS 16-57 | 23 | 1 | 2 | - | - | - | - | 2 | 0.1 | 2.0 | - | ||
| “ ” | × | ‘Baldwin’ | 27 | 21 | 14 | 1 | - | - | 8 | 15 | 0.6 | 0.7 | 2 | 7x | US 2522-# |
| “ ” | × | ‘Delite’ | 14 | 12 | 4 | 1 | - | - | - | 5 | 0.4 | 0.4 | - | ||
| “ ” | × | ‘Florida Rose’ | 29 | 26 | 8 | 1 | 2 | 1 | 2 | 11 | 0.4 | 0.4 | - | ||
| “ ” | × | ‘Pink Lemonade’ | 34 | 24 | 16 | 2 | 4 | 1 | - | 22 | 0.6 | 0.9 | - | ||
| “ ” | × | ‘Powderblue’ | 26 | 8 | 3 | - | 6 | - | - | 9 | 0.3 | 1.1 | - | ||
| “ ” | × | US 2320 | 58 | 30 | 14 | - | 1 | - | - | 15 | 0.3 | 0.5 | 6 | 7x | US 2521-# |
| “ ” | × | US 2321 | 50 | 22 | 7 | 1 | 2 | - | - | 10 | 0.2 | 0.5 | - | ||
| F1 US 1993 | × | ‘Powderblue’ | 36 | 1 | 1 | - | - | - | - | 1 | 0.03 | 1.0 | 1 | 7x | US 2523 |
| Total | 333 | 181 | 102 | avg. | avg. | 9 | |||||||||
| 0.31 | 0.6 | ||||||||||||||
| 2nd generation 7x (=4x F1 hybrid × 6x V. virgatum) × 6x V. virgatum | |||||||||||||||
| 7x US 2521-A | × | ‘Florida Rose’ | 8 | 3 | 3 | 3 | 0 | 0 | 0 | 6 | 0.8 | 2.0 | |||
| “ ” | × | US 2320 | 8 | 6 | 47 | 0 | 2 | 2 | 0 | 49 | 6.1 | 8.2 | |||
| “ ” | × | US 2328 | 9 | 9 | 27 | 0 | 1 | 2 | 0 | 28 | 3.1 | 3.1 | |||
| Total | 25 | 18 | 83 | avg. | avg. | ||||||||||
| 3.3 | 4.6 | ||||||||||||||
| Seed Quality | Viable | No. of | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poll. | Fruit | Good | g-f | Fair | f-p | Poor | Seed z | s/poll y | s/f x | Plants | Ploidy | Notes | |||
| 4x V. corymbosum Materials × 4x F1 Hybrids | |||||||||||||||
| ‘Camellia’ | × | F1 US 1930 | 24 | 22 | 2 | 3 | - | - | - | 5 | 0.2 | 0.2 | 0 | - | |
| ‘Cara’s Choice’ | × | “ ” | 11 | 11 | 2 | 2 | 2 | 3 | 15 | 6 | 0.5 | 0.5 | 21 | 4x | medium vigor |
| ‘Sharpblue’ | × | “ ” | 12 | 6 | 2 | 33 | 5 | 1 | 14 | 40 | 3.3 | 6.7 | 24 | 4x | US 2537-A, medium vigor |
| US 2117 | × | “ ” | 7 | 6 | 11 | 10 | 2 | - | - | 23 | 3.3 | 3.8 | 12 | not determined | |
| “ | × | F1 US 1933 | 25 | 22 | 2 | 10 | 23 | 5 | - | 35 | 1.4 | 1.6 | 12 | not determined | |
| Total | 54 | 45 | 109 | avg. | avg. | 69 | |||||||||
| 2.02 | 2.4 | ||||||||||||||
| 6x V. virgatum materials × 4x F1 hybrids | |||||||||||||||
| ‘Nocturne’ | × | F1 US 1930 | 72 | 30 | 40 | - | - | 1 | 1 | 40 | 0.6 | 1.3 | 0 | ||
| “ | × | F1 US 1933 | 7 | 3 | 9 | - | - | - | 1 | 9 | 1.3 | 3.0 | 2 | 5x | vigorous |
| US 2320 | × | F1 US 1930 | 17 | 11 | 3 | - | - | - | - | 3 | 0.2 | 0.3 | 0 | ||
| Total | 89 | 41 | 52 | avg. | avg. | 2 | |||||||||
| 0.58 | 1.3 | ||||||||||||||
| 2nd generation (4x V. corymbosum × F1 hybrid) × 4x V. corymbosum | |||||||||||||||
| US 2537-A | × | ‘Duke’ | 8 | 8 | 81 | 6 | 7 | 7 | 8 | 94 | 11.8 | 11.8 | germinating | ||
| “ | × | ‘Magnolia’ | 9 | 7 | 133 | 18 | 13 | 4 | 21 | 164 | 18.2 | 23.4 | germinating | ||
| “ | × | ‘Talisman’ | 20 | 11 | 34 | 2 | 4 | 2 | 21 | 40 | 2.3 | 3.9 | germinating | ||
| Total | 37 | 26 | 298 | avg. | avg. | ||||||||||
| 8.05 | 11.5 | ||||||||||||||
| Species and Genotype | Species Composition (%) z,y and Reference | |
|---|---|---|
| 4x V. meridionale-V. vitis-idaea S1 hybrids | ||
| US 1930, US 1933, US 1993 | 50 merid, 50 v-i [4] | |
| 4x V. corymbosum cultivars and other 4x germplasm | ||
| ARS 99-72 (=‘Elliott’ × ‘Bluegold’) | 90 cor, 7 ang, 3 unk, USDA sel’n. | |
| ‘Camellia’ | 72 cor, 20 dar, 4 vir, 2 ang, 3 unk [23] | |
| ‘Cara’s Choice’ | 48 cor, 20 dar, 15 vir, 15 con, 2 ang [24] | |
| ‘Dixieblue’ | 71 cor, 25 dar, 4 ang [25] | |
| ‘Duke’ | 96 cor, 4 ang [26] | |
| ‘Magnolia’ | 77 cor, 10 dar, 8 vir, 6 ang, 1 ten [27] | |
| ‘Sharpblue’ | 42 cor, 29 dar, 15 vir, 2 ang, 13 unk [28] | |
| ‘Sweetheart’ | 67 cor, 16 dar, 12 ang, 5 unk, <1 ten [29] | |
| ‘Talisman’ | 88 cor, 5 dar, 3 ang, 4 vir [30] | |
| US 908 (4x V. padifolium) | 100 pad [31] | |
| US 1896 (=US 908 × US 1825) | 50 pad, 30 cor, 12 dar, 3 vir, 2 ang, 3 unk [31] | |
| US 2117 (=‘Cara’s Choice’ × US 1116) | 67 cor, 11 dar, 8 vir, 8 con, 3 ang, 4 unk, <1 ten, USDA sel’n. | |
| US 2537-A (=‘Sharpblue’ × US 1930) | see parents, USDA breeding selection | |
| 6x V. virgatum cultivars and other 6x germplasm | ||
| ARS 07-97 (=T 451 × ‘Nocturne’) | 75 vir, 13 con, 13 cor, <1 dar/ten/ang/unk, USDA sel’n. | |
| ARS 16-57 (=T 451 × ARS 08-176) | 75 vir, 25 con, USDA sel’n. | |
| ‘Baldwin’ | 100 vir [32] | |
| ‘Delite’ | 100 vir [33] | |
| ‘Florida Rose’ | 100 vir [34] | |
| × ‘Nocturne’ | 50 vir, 25 con, 25 cor, <1 dar/ten/ang/unk [35] | |
| × ‘Pink Lemonade’ | 50 vir, 50 cor, <1 dar/ten/ang/unk [36] | |
| ‘Powderblue’ | 100 vir [37] | |
| US 2320 (=‘Nocturne’ × ‘Florida Rose’) | see parents, USDA breeding selection | |
| US 2321 (=‘Nocturne’ × ‘Florida Rose’) | see parents, USDA breeding selection | |
| US 2386 (=US 1993 × ‘Sweetheart’) | see parents, USDA breeding selection | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlenfeldt, M.K.; Ogden, E.; Rowland, L.J. Asymmetric Reciprocal Crossing Behavior of an Andean Blueberry (V. meridionale) × Lingonberry (V. vitis-idaea) Hybrid. Plants 2022, 11, 3152. https://doi.org/10.3390/plants11223152
Ehlenfeldt MK, Ogden E, Rowland LJ. Asymmetric Reciprocal Crossing Behavior of an Andean Blueberry (V. meridionale) × Lingonberry (V. vitis-idaea) Hybrid. Plants. 2022; 11(22):3152. https://doi.org/10.3390/plants11223152
Chicago/Turabian StyleEhlenfeldt, Mark K., Elizabeth Ogden, and Lisa J. Rowland. 2022. "Asymmetric Reciprocal Crossing Behavior of an Andean Blueberry (V. meridionale) × Lingonberry (V. vitis-idaea) Hybrid" Plants 11, no. 22: 3152. https://doi.org/10.3390/plants11223152
APA StyleEhlenfeldt, M. K., Ogden, E., & Rowland, L. J. (2022). Asymmetric Reciprocal Crossing Behavior of an Andean Blueberry (V. meridionale) × Lingonberry (V. vitis-idaea) Hybrid. Plants, 11(22), 3152. https://doi.org/10.3390/plants11223152







