Phytochemical Screening and Isolation of New Ent-Clerodane Diterpenoids from Croton guatemalensis Lotsy
Abstract
1. Introduction
2. Results and Discussion
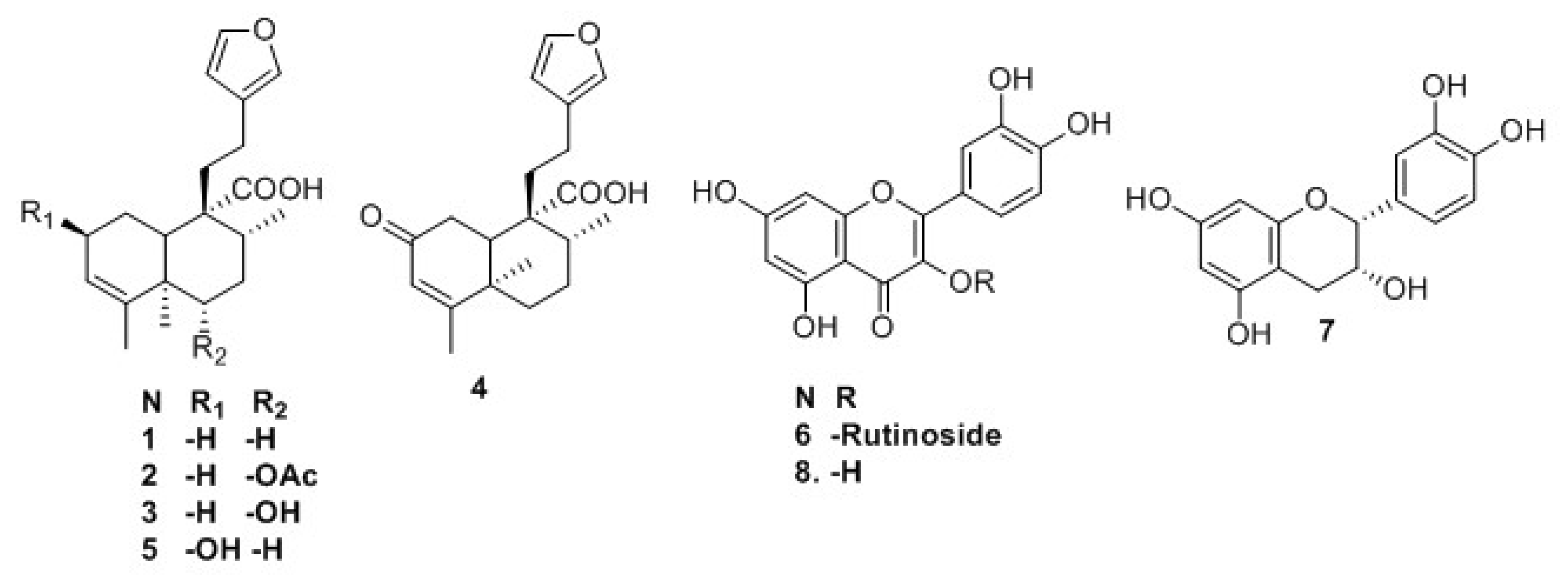
2.1. Isolation and Identification of Previously Undescribed Compounds
2.2. HPLC Phytochemical Profiling
2.3. Quantification of Rutin (6) in C. guatemalensis Extract
2.4. Affinity-Directed Fractionation
2.5. Molecular Docking
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedure
4.2. Plant Material and Extracts
4.3. Isolation Compounds
4.3.1. 6(s)-Acetoxy-15,16-diepoxy-ent-cleroda-3,13(16),14-trien-20-oic Acid (Crotoguatenoic Acid A; 2)
4.3.2. 6(s)-Hydroxy-15,16-diepoxy-ent-cleroda-3,13(16),14-trien-20-oic Acid (Crotoguatenoic Acid B; 3)
4.4. HPLC Analysis
4.5. HPLC Method Validation
4.6. Affinity-Directed Fractionation
4.7. Molecular Docking
4.8. Computational Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.H.; Liu, W.Y.; Liang, Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules 2018, 23, 2333. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Faria Salatino, M.L.; Negri, G. Traditional Uses, Chemistry and Pharmacology of Croton Species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Cruz, E.C.; Andrade-Cetto, A. Ethnopharmacological Field Study of the Plants Used to Treat Type 2 Diabetes among the Cakchiquels in Guatemala. J. Ethnopharmacol. 2015, 159, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Okokon, J.E.; Nwafor, P.A. Antiplasmodial Activity of Root Extract and Fractions of Croton zambesicus. J. Ethnopharmacol. 2009, 121, 74–78. [Google Scholar] [CrossRef]
- Jünior, S.F.P.; Conserva, L.M.; Filho, J.M.B. Clerodane Diterpenes from Croton Species: Distribution and a Compilation of Their 13C NMR Spectral Data. Nat. Prod. Commun. 2006, 1, 1934578X0600100410. [Google Scholar] [CrossRef]
- Gomes, K.K.; MacEdo, G.E.; Rodrigues, N.R.; Ziech, C.C.; Martins, I.K.; Rodrigues, J.F.; De Brum Vieira, P.; Boligon, A.A.; De Brito Junior, F.E.; De Menezes, I.R.A.; et al. Croton campestris A. St.-Hill Methanolic Fraction in a Chlorpyrifos-Induced Toxicity Model in Drosophila melanogaster: Protective Role of Gallic Acid. Oxid. Med. Cell Longev. 2020, 2020, 3960170. [Google Scholar] [CrossRef]
- Ortiz, A.P.; Puebla, P.; Guerrero, M.F. Vascular Interactions of Croton Schiedeanus Major Flavonoids in Isolated Aortic Rings from Wistar Rats. Vitae 2021, 28, 1–14. [Google Scholar] [CrossRef]
- Cheila, C.B.; dos Anjos, G.L.; Nóbrega, R.S.A.; da Magaton, A.; de Miranda, F.M.; de Dias, F. Greener Ultrasound-Assisted Extraction of Bioactive Phenolic Compounds in Croton heliotropiifolius Kunth Leaves. Microchem. J. 2020, 159, 105525. [Google Scholar] [CrossRef]
- Oliani, J.; Ferreira, M.J.P.; Salatino, A.; Salatino, M.L.F. Leaf Flavonoids from Croton Urucurana and C. floribundus (Euphorbiaceae). Biochem. Syst. Ecol. 2021, 94, 104217. [Google Scholar] [CrossRef]
- Turiel, N.A.; Ribeiro, A.F.; Carvalho, E.E.N.; Domingos, V.D.; Lucas, F.C.A.; Carreira, L.M.M.; Andrade, E.H.A.; Maia, J.G.S. Essential Oils Composition of Croton Species from the Amazon. Nat. Prod. Commun. 2013, 8, 1471–1772. [Google Scholar] [CrossRef]
- Morais, S.M.; Cossolosso, D.S.; Silva, A.A.S.; de Moraes Filho, M.O.; Teixeira, M.J.; Campello, C.C.; Bonilla, O.H.; de Paula, V.F.; Vila-Nova, N.S. Essential Oils from Croton Species: Chemical Composition, in Vitro and in Silico Antileishmanial Evaluation, Antioxidant and Cytotoxicity Activities. J. Braz. Chem. Soc. 2019, 30, 2404–2412. [Google Scholar] [CrossRef]
- Castro, K.N.d.C.; Chagas, A.C.d.S.; Costa-Júnior, L.M.; Canuto, K.M.; Brito, E.S.d.; Rodrigues, T.H.S.; de Andrade, I.M. Acaricidal Potential of Volatile Oils from Croton Species on Rhipicephalus Microplus. Rev. Bras. Farmacogn. 2019, 29, 811–815. [Google Scholar] [CrossRef]
- Carlos Dantas da Cruz, R.; da Silva Carvalho, K.; Justino Oliveira Costa, R.; Alexandre da Silva, P.; ucia da Cunha Silva, S.L.; Andrade Gualberto, S.; Buarque de Gusm, N.; Antonia de Souza, I. Phytochemical and Toxicological Evaluation of a Blend of Essential Oils of Croton Species on Aedes Aegypti and Mus Musculus. S. Afr. J. Bot. 2020, 132, 188–195. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Madeley, L.G.; Van Wyk, B.E. Volatiles from African Species of Croton (Euphorbiaceae), Including New Diterpenes in Essential Oil from Croton gratissimus. Heliyon 2019, 5, e02677. [Google Scholar] [CrossRef] [PubMed]
- Franssen, F.F.J.; Smeijsters, L.J.J.W.; Berger, I.; Medinilla Aldana, B.E. In Vivo and in Vitro Antiplasmodial Activities of Some Plants Traditionally Used in Guatemala against Malaria. Antimicrob. Agents Chemother. 1997, 41, 1500–1503. [Google Scholar] [CrossRef][Green Version]
- José Del Carmen, R.O.; Willam, H.M.J.; Del Carmen, G.M.A.; Nataly, J.G.; Stefany, C.O.S.; Anahi, C.A.; Domingo, P.T.J.; Leonardo, G.P.; De La Mora Miguel, P. Antinociceptive Effect of Aqueous Extracts from the Bark of Croton guatemalensis Lotsy in Mice. Res. Pharm. Sci. 2016, 11, 15–22. [Google Scholar]
- Andrade-Cetto, A.; Cruz, E.C.; Cabello-Hernández, C.A.; Cárdenas-Vázquez, R. Hypoglycemic Activity of Medicinal Plants Used among the Cakchiquels in Guatemala for the Treatment of Type 2 Diabetes. Evid.-Based Complement. Altern. Med. 2019, 2019, 2168603. [Google Scholar] [CrossRef]
- Henderson, M.S.; Murray, R.D.H.; McCrindle, R.; McMaster, D. Constituents of Solidago Species. Part III. The Constitution of Diterpenoids from Solidago juncea Ait. Can. J. Chem. 1973, 51, 1322–1331. [Google Scholar] [CrossRef]
- Lin, B.D.; Zhou, B.; Dong, L.; Wu, Y.; Yue, J.M. Formosins A–F: Diterpenoids with Anti-Microbial Activities from Excoecaria Formosana. Nat. Prod. Bioprospect. 2016, 6, 57–61. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, D.; Oliveros-Bastidas, A.; Alonso-Amelot, M.E.; Calcagno-Pissarelli, M.P. Diterpene Foliar Exudates of Blakiella Bartsiifolia and Phytotoxicity of Clerodanes. Nat. Prod. Commun. 2014, 9, 1407–1412. [Google Scholar] [CrossRef]
- Seto, R.; Nakamura, H.; Nanjo, F.; Hara, Y. Preparation of Epimers of Tea Catechins by Heat Treatment. Biosci. Biotechnol. Biochem. 1997, 61, 1434–1439. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane Diterpenes: Sources, Structures, and Biological Activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [PubMed]
- Vihakas, M. Flavonoids and Other Phenolic Compounds: Characterization and Interactions with Lepidopteran and Sawfly Larvae; Birth Defects Orig. Art. Ser; Turun yliopisto: Turku, Finland, 1975; Volume 11, ISBN 9789512959051. [Google Scholar]
- Wolfender, J.L. HPLC in Natural Product Analysis: The Detection Issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.T.; Goldblatt, L.A. Correlation of Ultraviolet and Infrared Spectra of Terpene Hydrocarbons. Anal. Chem. 1954, 26, 1726–1737. [Google Scholar] [CrossRef]
- De Heluani, C.S.; Catalán, C.A.N.; Hernández, L.R.; Burgueño-Tapia, E.; Joseph-Nathan, P. 13C NMR Assignments and Conformational Evaluation of Diterpenes from Croton sarcopetalus Muell. Magn. Reson. Chem. 1998, 36, 947–950. [Google Scholar] [CrossRef]
- Aguilar-Guadarrama, A.B.; Rios, M.Y. Three New Sesquiterpenes from Croton arboreous. J. Nat. Prod. 2004, 67, 914–917. [Google Scholar] [CrossRef]
- Capasso, A.; Piacente, S.; De Tommasi, N.; Ragucci, M.; Pizza, C. Constituents of Croton menthodorus and Their Effects on Electrically Induced Contractions of the Guinea-Pig Isolated Ileum. Phyther. Res. 2000, 14, 156–159. [Google Scholar] [CrossRef]
- Zou, G.A.; Su, Z.H.; Zhang, H.W.; Wang, Y.; Yang, J.S.; Zou, Z.M. Flavonoids from the Stems of Croton caudatus Geisel. Var. Tomentosus Hook. Molecules 2010, 15, 1097–1102. [Google Scholar] [CrossRef]
- Dos Santos, K.P.; Motta, L.B.; Santos, D.Y.A.C.; Salatino, M.L.F.; Salatino, A.; Ferreira, M.J.P.; Lago, J.H.G.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Furlan, C.M. Antiproliferative Activity of Flavonoids from Croton sphaerogynus Baill. (Euphorbiaceae). Biomed. Res. Int. 2015, 2015, 212809. [Google Scholar] [CrossRef]
- Aponte-Buitrago, R.; Mayorga-Wandurraga, H.; Moreno-Murillo, B. Flavonols and Sesquiterpenoids from Outer Bark and Leaves of Croton Polycarpus Benth. (Euphorbiaceae). Blacpma 2017, 16, 471–485. [Google Scholar]
- Li, S.; Han, Q.; Qiao, C.; Song, J.; Xu, H. Chinese Medicine Chemical Markers for the Quality Control of Herbal Medicines: An Overview. Chin. Med. 2008, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.S.; Qin, C.S.; Tan, S.; Xuan, S.; Ying, P.J.; Le, H.Y.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid.-Based Complement. Altern. Med. 2021, 2021, 2057333. [Google Scholar] [CrossRef]
- Escandón-Rivera, S.M.; Mata, R.; Andrade-Cetto, A. Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules 2020, 25, 4145. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Evans, F.J.; Roberts, M.F.; Phillipson, J.D.; Zenk, M.H.; Gleba, Y.Y. Polyphenolic Compounds from Croton lechleri. Phytochemistry 1991, 30, 2033–2040. [Google Scholar] [CrossRef]
- Cordeiro, K.W.; Felipe, J.L.; Malange, K.F.; Do Prado, P.R.; De Oliveira Figueiredo, P.; Garcez, F.R.; De Cássia Freitas, K.; Garcez, W.S.; Toffoli-Kadri, M.C. Anti-Inflammatory and Antinociceptive Activities of Croton urucurana Baillon Bark. J. Ethnopharmacol. 2016, 183, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in Physiological Functions and Mechanisms of (-)-Epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of Catechin, Epicatechin, and Rutin: Optimization of a Novel Complete Antidiabetic Formulation Using a Mixture Design Approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef]
- Aderogba, M.A.; Ndhlala, A.R.; Van Staden, J. Acetylcholinesterase Inhibitors from Croton Sylvaticus Ethyl Acetate Leaf Extract and Their Mutagenic Effects. Nat. Prod. Commun. 2013, 8, 795–798. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Klllç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- ICH. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmon. Tripart. Guidel. 2005, 1–13. [Google Scholar] [CrossRef]
- Salgado-Garciglia, R.; Hernández-García, A.; Montiel-Montoya, J.; Valdez-Morales, M.; López-Valdez, L.G.; Herrera-Cabrera, B.E.; Zaragoza-Martínez, F.; Lucho Constantino, G.G.; Barrales-cureño, H.J. Flavonoids Quantification in Acer Negundo L., Extracts by Hplc Analysis. Agro Product. 2021, II, 1–8. [Google Scholar] [CrossRef]
- Saraf, A.; Sankhala, S. Simultaneous Determination of Rutin and Quercetin in Different Parts of Tecomella Undulata (Seem): An Endangered Medicinal Plant. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 434–439. [Google Scholar]
- Fan, M.; Chen, G.; Sun, B.; Wu, J.; Li, N.; Sarker, S.D.; Nahar, L.; Guo, M. Screening for Natural Inhibitors of Human Topoisomerases from Medicinal Plants with Bio-Affinity Ultrafiltration and LC–MS. Phytochem. Rev. 2020, 19, 1231–1261, ISBN 0123456789. [Google Scholar] [CrossRef]
- Rosas-Ramírez, D.; Pereda-Miranda, R.; Escandón-Rivera, S.; Arreguín-Espinosa, R. Identification of α-Glucosidase Inhibitors from Ipomoea Alba by Affinity-Directed Fractionation-Mass Spectrometry. Rev. Bras. Farmacogn. 2020, 30, 336–345. [Google Scholar] [CrossRef]
- Kimura, A.; Lee, J.H.; Lee, I.S.; Lee, H.S.; Park, K.H.; Chiba, S.; Kim, D. Two Potent Competitive Inhibitors Discriminating α-Glucosidase Family I from Family II. Carbohydr. Res. 2004, 339, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Qin, X.; Cao, X.; Wang, L.; Bai, F.; Bai, G.; Shen, Y. Structural Insight into Substrate Specificity of Human Intestinal Maltase-Glucoamylase. Protein Cell 2011, 2, 827–836. [Google Scholar] [CrossRef]
- Rosas-Ramírez, D.; Escandón-Rivera, S.; Pereda-Miranda, R. Morning Glory Resin Glycosides as α-Glucosidase Inhibitors: In Vitro and in Silico Analysis. Phytochemistry 2018, 148, 39–47. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]







| 2 a | 3 a | |||
|---|---|---|---|---|
| Position | δH | δC | δH | δC |
| 1 | α 1.85 b, m β 1.93 b, m | 19.8 | α 1.84 b, m β 1.93 b, m | 20.1 |
| 2 | α 1.99 b, m β 2.07 b, m | 27.1 | α 2.02 b, m β 2.10 b, m | 27.2 |
| 3 | 5.30, dd (2.87, 1.39) | 123.6 | 5.31, m | 123.1 |
| 4 | - | 141.5 | - | 143.0 |
| 5 | - | 42.9 | - | 44.7 |
| 6 | 4.73 dd (11.34, 5.00) | 78.2 | 3.59, dd (11.35, 4.97) | 76.1 |
| 7 | α 2.20 b, m β 1.76 b, m | 33.0 | α 2.20 b, dd (13.71, 2.26) β 1.70 b, m | 37.4 |
| 8 | 1.76 b, m | 34.3 | 1.71 ddd (14.06, 6.21, 3.58) | 34.7 |
| 9 | - | 49.3 | - | 49.4 |
| 10 | 1.66, d (11.50) | 47.1 | 1.58 dd (11.63, 1.30) | 47.2 |
| 11 | α 1.85 b, m β 2.26 b, m | 33.9 | α 1.93, m β 2.26 b, m | 34.0 |
| 12 | 2.33 b,c, m | 17.8 | 2.33 b,c, m | 17.8 |
| 13 | - | 124.4 | - | 124.5 |
| 14 | 6.26, dd (1.83, 0.90) | 110.9 | 6.26, dd (1.83, 0.95) | 110.9 |
| 15 | 7.35, t (1.69) | 143.1 | 7.35, t (1.69) | 143.0 |
| 16 | 7.23, dd (1.61, 0.88) | 138.7 | 7.23, dd (1.62, 0.91) | 138.7 |
| 17 | 1.14, d (6.69) | 16.1 | 1.15, d (6.71) | 16.3 |
| 18 | 1.59, br s | 21.2 | 1.84, br s | 22.6 |
| 19 | 1.09, s | 13.9 | 0.98, s | 12.8 |
| 20 | - | 182.3 | - | 181.9 |
| -OAc | 2.04 | 22.0 | - | - |
| 170.8 | ||||
| Rt | Linear Range (μg/mL) | Calibration Equation | R2 a | LOD (μg/mL) | LOQ (μg/mL) | Precision | Recovery (%mean) | |
|---|---|---|---|---|---|---|---|---|
| Intraday (%RSD) | Interday (%RSD) | |||||||
| 14.52 | 20–250 | Y = 9.29484284x + 17.083753 | 0.9996 | 0.19 | 0.57 | 0.79 | 0.22 | 100.74 |
| Batch | %EWE a | Content in mg/g b |
|---|---|---|
| 09–2013 | 20.5 | 0.6067 ± 0.0025 |
| 06–2015 | 19.3 | 0.5585 ± 0.0042 |
| 10–2019 | 18.7 | 0.6440 ± 0.0068 |
| Compound | Formula a | ESI-MS [M–H]– b | ESI-MS [M + H]+ b | MAL12 | MGAM | ||
|---|---|---|---|---|---|---|---|
| Theoretical Ki | Hydrogen Bond | Theoretical Ki | Hydrogen Bond | ||||
| 1 | C20H28O3 (316) | 315.2467 | - | 7.12 μM | His279, Arg312 | 3.02 μM | Gln1372, Arg1377 |
| 2 | C22H30O5 (374) | - | - | 17.1 μM | His279, Arg312 | 6.31 μM | Gln1372, Arg1377 |
| 3 | C20H28O4 (332) | 331.1823 | 333.2444 | 13 μM | Arg312 | 5.09 μM | Gln1372, Arg1377 |
| 4 | C20H26O4 (330) | 329.1670 | - | 6.73 μM | His279, Arg312 | 1.95 μM | Gln1372, Arg1377 |
| 5 | C20H28O4 (332) | 331.1823 | 333.2444 | 4.14 μM | Ser156, His279, Arg312 | 2.4 μM | Try1251, Gln1372, Arg1377 |
| Acarbose c | C25H43NO18 (645) | - | 646 | 51.4 nM | His279, Gln322, Glu304, Arg312 | 35.7 nM | Tyr1251, Gln1372, Arg1377, Gln1561, Gly1588 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escandón-Rivera, S.M.; Andrade-Cetto, A.; Rosas-Ramírez, D.G.; Arreguín-Espinosa, R. Phytochemical Screening and Isolation of New Ent-Clerodane Diterpenoids from Croton guatemalensis Lotsy. Plants 2022, 11, 3159. https://doi.org/10.3390/plants11223159
Escandón-Rivera SM, Andrade-Cetto A, Rosas-Ramírez DG, Arreguín-Espinosa R. Phytochemical Screening and Isolation of New Ent-Clerodane Diterpenoids from Croton guatemalensis Lotsy. Plants. 2022; 11(22):3159. https://doi.org/10.3390/plants11223159
Chicago/Turabian StyleEscandón-Rivera, Sonia Marlen, Adolfo Andrade-Cetto, Daniel Genaro Rosas-Ramírez, and Roberto Arreguín-Espinosa. 2022. "Phytochemical Screening and Isolation of New Ent-Clerodane Diterpenoids from Croton guatemalensis Lotsy" Plants 11, no. 22: 3159. https://doi.org/10.3390/plants11223159
APA StyleEscandón-Rivera, S. M., Andrade-Cetto, A., Rosas-Ramírez, D. G., & Arreguín-Espinosa, R. (2022). Phytochemical Screening and Isolation of New Ent-Clerodane Diterpenoids from Croton guatemalensis Lotsy. Plants, 11(22), 3159. https://doi.org/10.3390/plants11223159








