2.1. Simulation of a Continuous Duckweed Cultivation System (Tray Experiment 1)
In a continuous system, the accumulation of ballast salts can occur [
19], which can result in toxic salt levels and lead to a culture crash. Therefore, experiments mimicking continuous systems for obtaining more accurate results and assessing their water consumption are needed. In this study, a continuous system with a continuous supply of NDNE and LF and a continuous discharge that meets the discharge standards in Flanders (Belgium) that works for 175 days was simulated. The medium composition was calculated for systems with different depths (0.4 m, 0.7 m and 1 m). After simulating the system, a tray experiment was carried out with the calculated compositions. The relative growth (RGR) and chlorophyll inhibitions results, obtained from this experiment, are shown in
Figure 2.
It was observed that the RGR inhibition of the continuous systems with a depth of 0.7 m or 0.4 m was significantly higher at the end of the growing season compared to the beginning of the season (p = 3.98 × 10−3 and 1.28 × 10−11, respectively). Regarding chlorophyll inhibition, it was shown that the reference medium had a significantly higher chlorophyll content compared to the other treatments (p < 8.99 × 10−7). When comparing the chlorophyll concentrations at the start of the growing season with those at the end of the growing season, only the system with a depth of 0.7 m showed a significant inhibition (p = 3.56 × 10−2). For the 0.4 m system, the duckweed obtained in the pre-cultivation exhibited a significant chlorophyll inhibition (p = 3.56 × 10−2) but seemed to recover during the cultivation step.
As expected, the RGR of duckweed grown in a deeper system was less inhibited after 175 days than when it was growing in a shallower system. Accordingly, the deeper the system, or the higher the buffering capacity for the accumulating salts that enter the system, the lower the inhibition after one growing season. Roughly, a similar trend was shown for chlorophyll inhibition. The observation that the reference medium had a higher chlorophyll content can be explained by the low N and P concentrations of the wastewater-based growing media. Additionally, the pH of the reference medium was closer to the optimal pH for duckweed growth (
Table A3) [
9].
It can be concluded that, when duckweed is grown in a system with a continuous supply of NDNE and LF and a continuous discharge that meets the legal standards in Flanders (Belgium), a buffering capacity of at least 1 m depth is recommended. This would ensure that the relative growth inhibition would not be significantly higher at the end of the growing season compared to the start of the cultivation. However, in practice, this would mean that the continuous cultivation of duckweed would consume a significant amount of water. A buffering capacity of at least 1 m depth means that, for the cultivation of one hectare for one growing season, 10,000 m³ water is needed. This is significantly higher compared to other crops such as maize, which has an irrigation water requirement of 900 to 1750 m³/ha/yr for optimal growth [
22]. In reality, the water consumption itself may be even higher than calculated in this simulation, as the experiment did not take into account the naturally occurring evaporation of water.
The high water consumption is a result of the toxicity that occurs due to the accumulation of nutrients because of the continuous addition of biological waste streams. According to our simulation, this toxicity is mainly determined by potassium, since this element exceeds the toxicity limit according to Landolt et al., in the shortest time (see
Table A3). However, if one considers the actual measured values before and after cultivation (
Table A1 and
Table A2), it can be observed that the toxicity limit of potassium (2000 mg/L) was not exceeded. None of the growing media, neither before nor after cultivation, exceeded the toxicity limit for any element according to Landolt and Frick et al. [
9,
23]. Nevertheless, some elements, such as K and Cl, lie further outside their optimum range when a shallower system is simulated, which may be the reason for increased stress and therefore growth and chlorophyll inhibition.
Next to the high K concentration, a possible driving factor determining toxicity may be an adverse balance of nutrients. For example, it is known that a ratio which favors Mg over Ca negatively affects
L. minor growth and its photosynthetic yield [
21]. Therefore, a Ca:Mg ratio of 1:1.6 or greater is recommended for
L. minor growth. In
Figure 3 it is shown that, during cultivation, the Mg/Ca ratio of the reference and start medium was significantly lower than the maximum ratio of 1.6 (
p = 8.33 × 10
−5 and 1.42 × 10
−7, respectively). For the least buffered medium (0.4 m) it was shown that the Mg/Ca ratio was significantly higher than this maximum ratio (
p = 9.16 × 10
−5).
The shallower the system or the less buffering capacity the system has for accumulating salts, the higher the growth inhibition will be, and also, the more the nutrient uptake will decrease and will even become negative (
Table A7 and
Table A8). A negative nutrient uptake indicates leaching, which is a clear signal of plant stress. A negative removal was observed for the elements Ca, P and Mn. This is an issue, especially for phosphorous, as the system was created/simulated in such a way that the added P concentrations should equal the removed P concentration. These measured nutrient removals also do not match those published by Devlamynck et al. [
19], which were used to calculate the needed medium concentrations for the simulation. In addition, a constant removal was used to calculate all simulations for each nutrient, whereas we can see here that, for most nutrients, removal increases with increasing concentration. Even though more investigations are needed for continuous systems, this study indicates the importance of taking into account the salt accumulation in such setups.
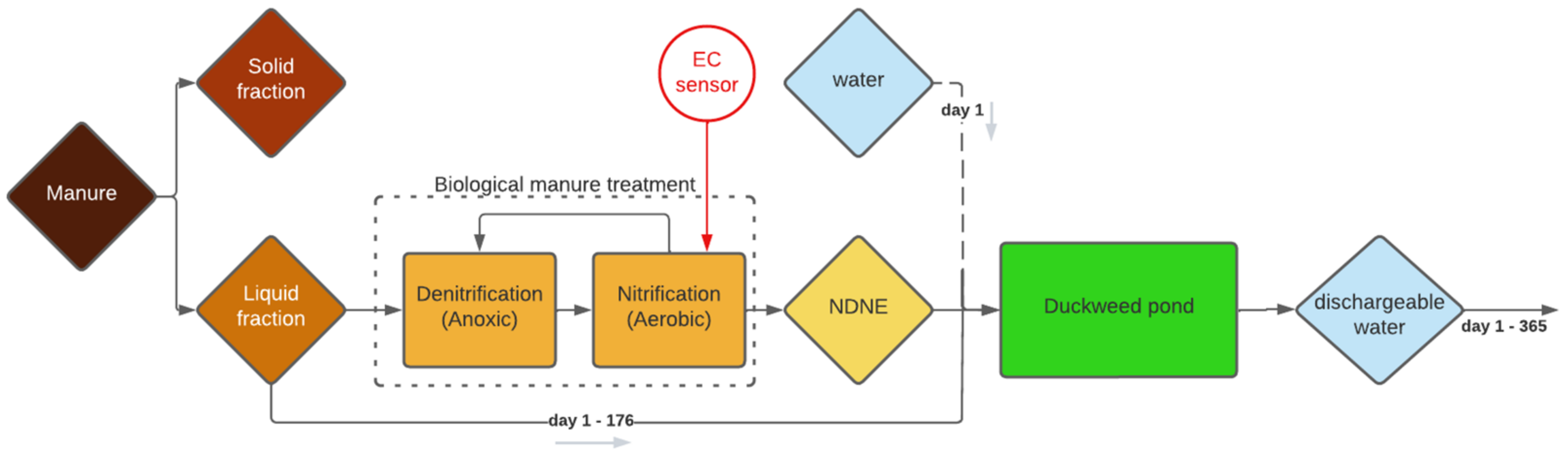
Besides the high water consumption, a big disadvantage of the simulated system is that the growth medium must be discharged after 175 days, at the end of the growing season. Discharging this quantity of growth medium involves some practical problems, as the medium is too salty to be discharged into nature as a single flush. A possible solution is to opt for 175 days of duckweed growth in one year and then to gradually discharge for the rest of the year until the system is empty on the 365th day, as illustrated in
Figure 1.
2.2. Duckweed Cultivation on NDNE (Tray Experiments 2 and 3)
2.2.1. NDNE as a Growing Medium
The simulation discussed in the previous section focuses on a system that treats NDNE and LF till a dischargeable effluent is obtained. However, it might be more interesting to treat a higher concentration of NDNE in a continuous system to achieve a higher biomass production and to reduce the water consumption needed for dilution. The purpose of the system is then changed from ‘treating a waste stream as a stand-alone system to a dischargeable effluent’ to ‘production of protein-rich biomass with recycled nutrients’. In order to still obtain a dischargeable effluent at the end, the system can be combined with a constructed wetland using reedbeds as a final purification step.
To assess the maximum accepted waste stream concentration to minimize the needed dilution, a second tray experiment was done. Nine different dilutions/treatments of NDNE were tested (
Table A2). It was investigated if the cultivation of duckweed on a biological medium (after dilution, addition of a salt solution or evaporation) resulted in better or worse growth compared to cultivation on a synthetic nutrient medium. In
Figure 4, the RGR inhibition and the chlorophyll inhibition of the duckweed grown on different media are shown.
Surprisingly, the different dilutions and the undiluted NDNE showed no RGR inhibition compared to the reference medium. Even for the treatments where the EC was artificially increased there was only a significantly higher RGR inhibition after evaporation until an EC of 11.8 mS/cm was obtained (p = 2.38 × 10—9). However, for chlorophyll inhibition, a different trend was shown. There was a significantly higher chlorophyll inhibition for the undiluted NDNE and for most of the treatments where the EC was increased, indicating plant stress. For the diluted NDNE to an EC of 4, 6, or 8 mS/cm, the chlorophyll inhibitions were significantly lower than the reference (p = 1.48 × 10−6, 1.77 × 10−12 and 1.28 × 10−13, respectively). The RGR and chlorophyll inhibitions of these dilutions were even below 0%.
From the results shown in
Figure 4, we can conclude that duckweed grew better on the diluted NDNE than on the reference medium. This is surprising, as the N medium is described in the literature as one of the best nutrient media used to support the fast growth of duckweed [
24]. A possible explanation lies in the presence of organic components in the used waste streams. Organic matter contains humic substances that can be divided into three classes: fulvic acids (FA), humic acid (HA), and humin [
25]. Humic substances might increase the uptake of both macro and micronutrients, such as N, P, K, Fe and Zn [
26]. Additionally, they might also reduce the plant uptake of certain toxic metal ions, like Cd [
27]. Thus, one might reason that the application of humic substances could improve plant response to salinity. However, in the literature, opinions are very much divided on this hypothesis. Liu et al., studied the influence of HA on the salt tolerance of hydroponically grown creeping bentgrass. They found out that, in general, the application of HA did not improve the salinity tolerance of the plant [
28]. However, different findings were observed for other crops. Ghulam et al., studied the influence of HA on salt tolerance and nutrient uptake in wheat. They found out that a combined application of K and HA was promising for increasing wheat salt tolerance and nutrient uptake [
29]. As both studies show very different results and worked with other crops than duckweed, it is difficult to make any concrete conclusions other than a possible indication that duckweed might behave like wheat and have higher salt tolerance in the presence of humic substances. Another explanation lies in the form in which nitrogen is present in the growth medium. Ammonium is the preferred nitrogen source of duckweed [
30] and NDNE has more nitrogen present in the form of ammonium compared to the reference medium (
Table A1), in which all the nitrogen is present in the form of nitrate.
In this study, it was shown that the duckweed grew better on the diluted NDNE media than on the reference medium. However, it should be considered that only data on growth and chlorophyll concentration was studied here. Osmotic stress can cause a reduction in protein concentration. Therefore, an important next step to this study should be biomass quality assessment of the duckweed grown on these different media.
2.2.2. Salt Tolerance of Duckweed
From the previous tray experiment, it seemed that duckweed is less salt-sensitive when grown on a biological medium compared to when grown on a synthetic medium. In order to better understand the salt tolerance of duckweed, an additional tray experiment was done. In this third experiment, a dose-response curve of
Lemna minor was determined after adding different concentrations of NaCl to the synthetic reference medium. This approach is similar to how toxicity tests are usually done in literature [
31]. However, this may not be the best way to determine salt tolerance as it is also important to take into account the ratio of certain elements. This was already demonstrated for the Mg/Ca ratio for example. Therefore, the results of this tray experiment were compared with another experiment where the same amount of Na
+ was added to a synthetic medium by adding a mixture of NaCl, Na
2SO
4 and K
2SO
4. This mixture had an SO
42−/Cl
− ratio of 0.25, similar to the ratio measured in NDNE in our previous research.
In
Figure 5 both the RGR- and the chlorophyll inhibition are plotted as a function of the EC and the concentration of sodium in the mixture. It is shown that the EC is lower when Na
+ is added as NaCl than when the same concentration of Na
+ is added as a combination of NaCl, Na
2SO
4 and K
2SO
4. This is because SO
42− has twice as many charges as Cl
− and will therefore have a greater influence on the conductivity. Next, it was shown that the RGR inhibition was significantly higher at a certain EC when only NaCl was added compared to when a combination of NaCl, Na
2SO
4 and K
2SO
4 was added. A significant higher growth inhibition was observed after adding 46.6 mM Cl
− to the medium (up to EC = 6.51–6.65 mS/cm), compared to the reference medium (0 mM NaCl added—EC = 1.5 mS/cm) (
p = 2 × 10
−3 for pre-cultivation,
p < 1.08 × 10
−9 for cultivation).
Remarkably, when adding a combination of Cl− and SO42−, a significantly higher tolerance was observed in terms of growth inhibition in function of the EC, as total growth inhibition was only obtained after the addition of 37.3 mM Cl− and 9.32 mM SO42− to the medium (up to EC = 8.18–8.43 mS/cm) (p = 4.88 × 10−7 for cultivation). Presumably, total inhibition could have occurred even at lower concentrations than 46.6 mM NaCl. Therefore, it may be assumed that, in a synthetic medium, the duckweed was less inhibited when an equal amount of Cl−/SO42− was added than when only Cl− was added.
The same trends are shown in
Figure 5C,D for chlorophyll inhibition. However, it is possible to see significant differences at lower EC values or after the addition of lower Na
+ concentrations. A significantly higher chlorophyll inhibition was observed for pre-cultivation after adding 18 mM Cl
− to the medium (up to EC = 3.5 mS/cm), compared to the reference medium (0 mM NaCl added—EC = 1.5 mS/cm) (
p = 3.24 × 10
−4 for pre-cult). Remarkably, when adding a combination of Cl
− and SO
42− ions, a significantly higher tolerance was observed for the pre-cultivation, in terms of both growth inhibition in function of the EC and the Na
+ concentration. However, total chlorophyll inhibition was only obtained for the pre-cultivation after the addition of 25.6 mM Cl
− and 6.4 mM SO
42− to the medium (up to EC = 6.25 mS/cm) (
p = 2.19 × 10
−3). For the cultivation step, there was, for both experiments, a significant chlorophyll inhibition after the addition of 32 mM Na
+ (up to EC = 4.96–5.05 for Cl
− addition; EC = 6.25–6.44 for Cl
− and SO
42− addition) (
p = 1.02 × 10
−5 for Cl
− addition;
p = 5.63 × 10
−5 for Cl
− and SO
42− addition). The chlorophyll content of a plant can be used as a measure to assess oxidative damage in salt treatments. Oxidative stress is usual before a decrease in growth or die-off. In this sense, it is expected that chlorophyll inhibition already occurs at lower concentrations and EC values.
To conclude, in this experiment, duckweed was less inhibited when an equal amount of Cl−/SO42− was added than when only Cl− was added. This proves that the composition of the anions plays a role in the salt tolerance of duckweed. As a result, the observation from the previous experiment that duckweed is less salt-sensitive when grown on a biological medium may be partly explained by the composition of the anions in this medium.
2.2.3. Variation in NDNE
From the second tray test, it could be concluded that the best medium for duckweed consists of a mixture of 75% NDNE and 25% demineralized water (EC = 8), both in terms of growth and chlorophyll concentration. However, the composition of the NDNE is not constant over time. In order to demonstrate that variation, the EC of NDNE was monitored in situ in a treatment facility. Over the same period, also the precipitation was monitored to calculate the dilution of the treatment system over its retention period (36 days).
Figure 6 shows that there is variation in the EC of NDNE over time. In fact, for 49% of the time, the measured EC was higher than 10.9 mS/cm, which is the maximal EC for duckweed survival according to Landolt et al. [
9].
It was investigated whether the precipitation influenced the variation of the EC, as the rainwater that enters the system may dilute the stream and thus lower the EC, causing a higher variation. In this way, a lower EC could be expected in periods with high precipitation. To visualise this impact in the graph, a corrected EC was calculated by assuming that the rainwater entering the NDNE has an EC of 0. This leads to an underestimation but clearly shows that rainwater has no to little effect on the variation of the EC on NDNE.
More likely, the variable concentration of anions and cations in the NDNE is mainly caused by variations in the inlet stream and other process parameters. In this case, the monitored pig manure processing treatment has an inlet stream that consists of a combination of manure from fattening pigs and sows. The manure of sows is worth more for fertilisation and is preferred in times of high demand. Thus, around 10/05, the manure of sows was all diverted for fertilizing the lands. Hence, the manure from fattening pigs was fed to the treatment. The latter is thicker and has a higher conductivity. As a result, we can observe increasing conductivity from then on.
To conclude, the variation of the source material determines to a large extent the variation in the final growth medium. This variation can be reduced by the installation of buffering or storage lagoons, which would allow for a more stable effluent composition and an easier formulation of an adequate medium for duckweed growth on NDNE.