Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview
Abstract
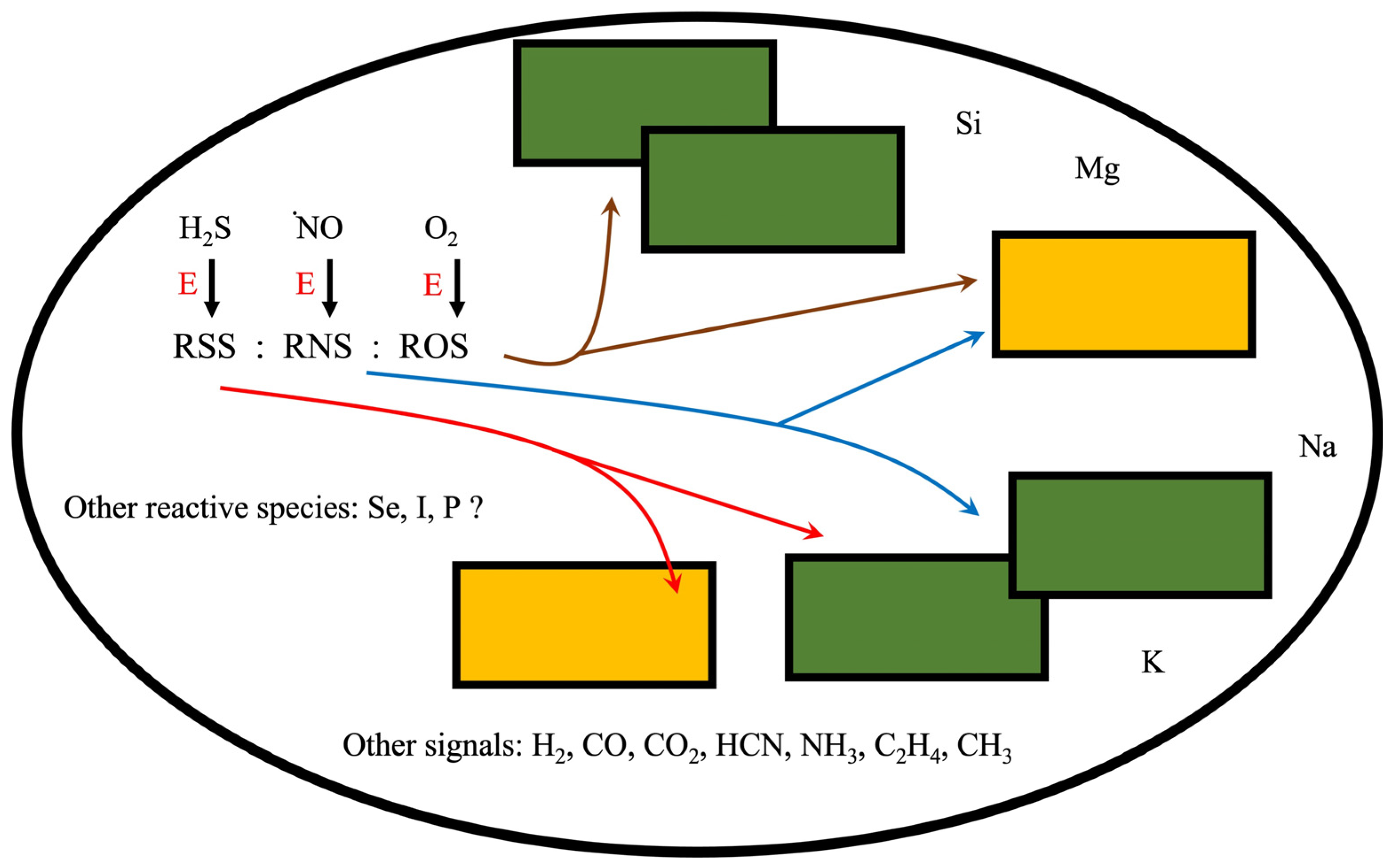
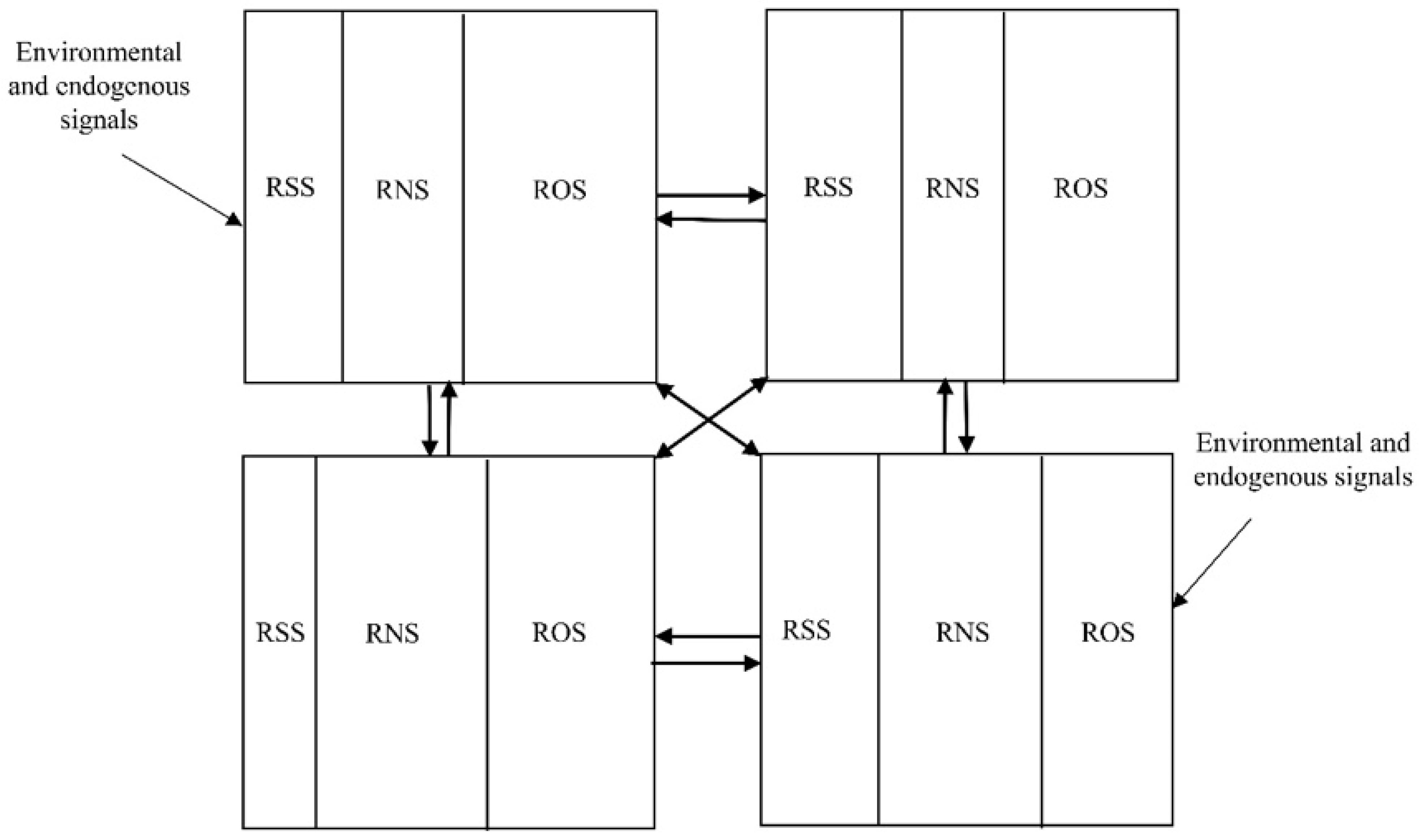
:1. RONSS Integration as a Metabolic Cluster
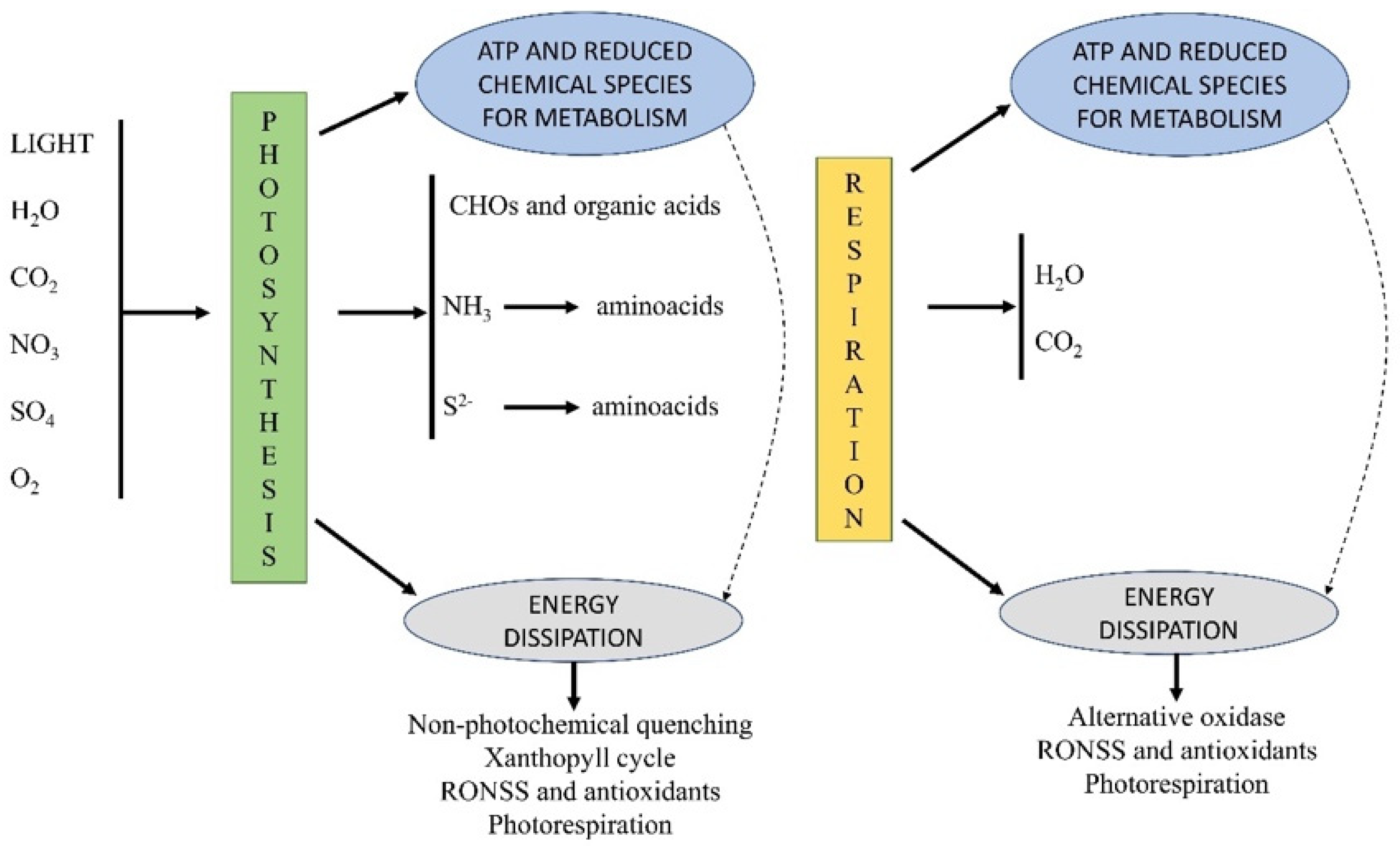
2. RONSS in Plant Metabolism
2.1. Reactive Oxygen Species
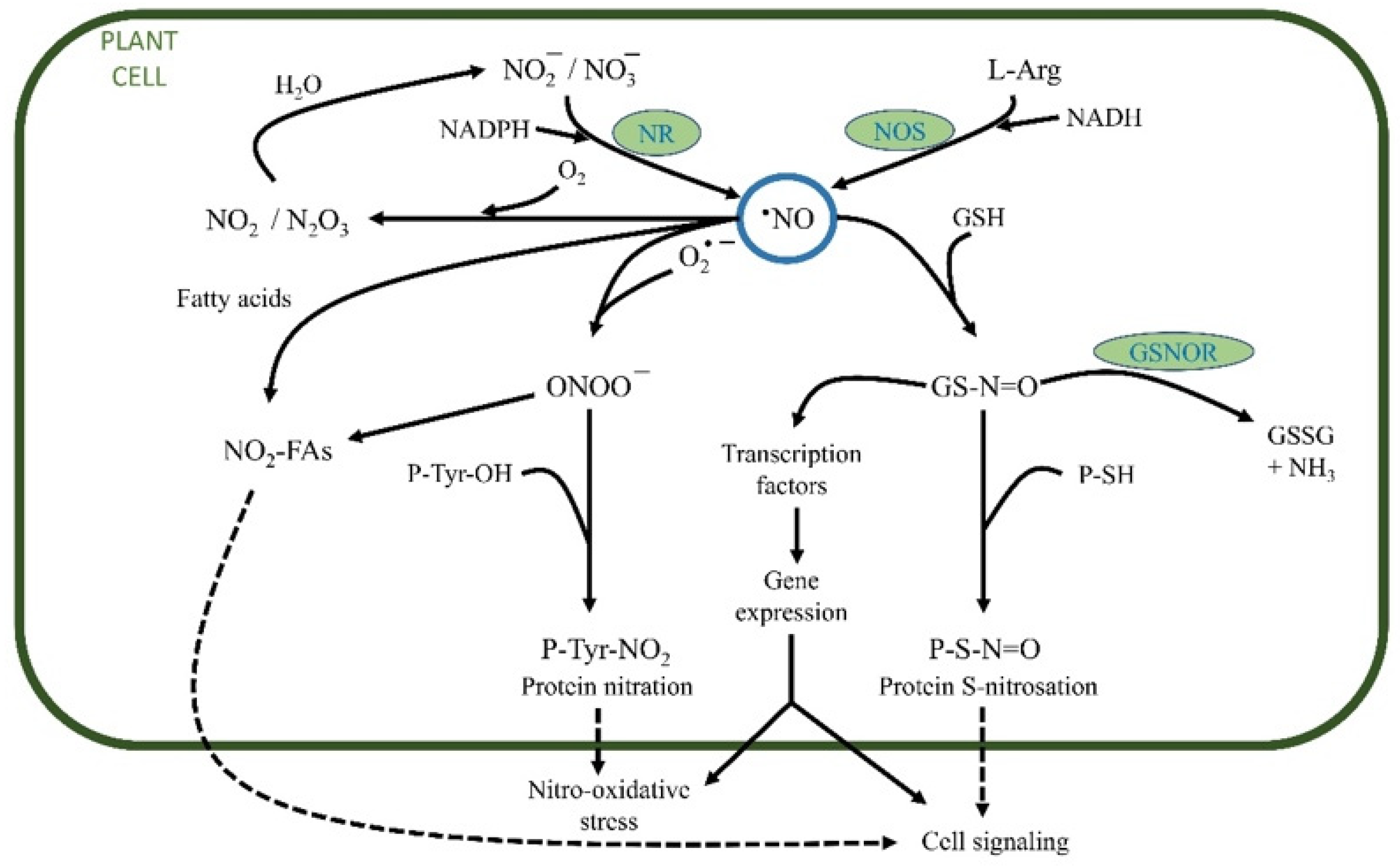
2.2. Reactive Nitrogen Species
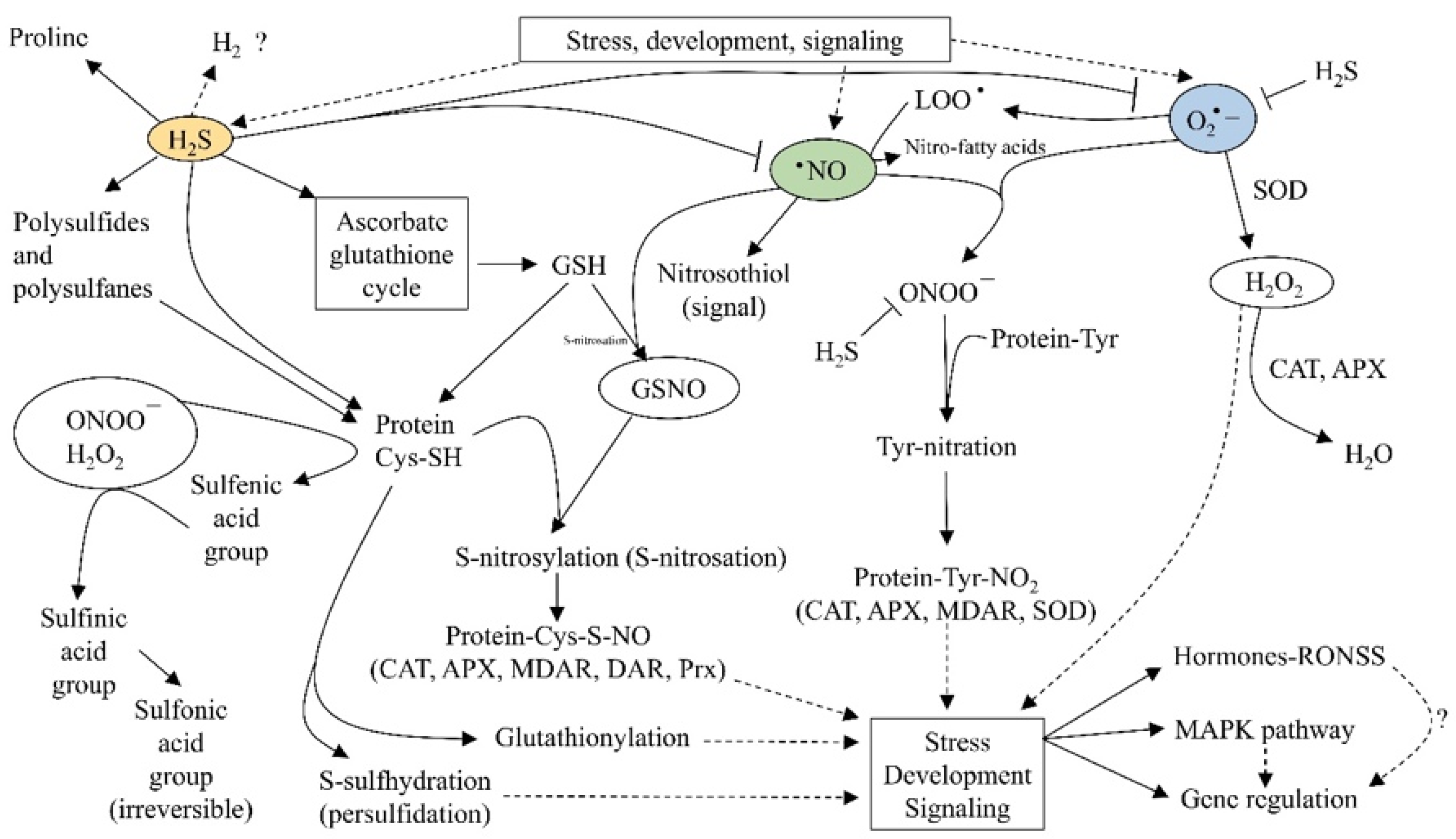
2.3. Reactive Sulfur Species
2.4. Reactive Oxygen, Nitrogen, Sulfur Species (RONSS)
3. RONSS as Biostimulants
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricard, J. Systems Biology and the Origins of Life? Part I. Are Biochemical Networks Possible Ancestors of Living Systems? Reproduction, Identity and Sensitivity to Signals of Biochemical Networks. Comptes Rendus Biol. 2010, 333, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Muchowska, K.B.; Varma, S.J.; Moran, J. Nonenzymatic Metabolic Reactions and Life’s Origins. Chem. Rev. 2020, 120, 7708–7744. [Google Scholar] [CrossRef] [PubMed]
- Becerra, A. The Semi-Enzymatic Origin of Metabolic Pathways: Inferring a Very Early Stage of the Evolution of Life. J. Mol. Evol. 2021, 89, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, H.J.; Deamer, D.W.; Smith, T. Biogenesis as an Evolutionary Process. J. Mol. Evol. 1991, 33, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. Non-Equilibrium Thermodynamic Foundations of the Origin of Life. Foundations 2022, 2, 308–337. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive Oxygen Species or Reactive Sulfur Species: Why We Should Consider the Latter. J. Exp. Biol. 2020, 223, jeb196352. [Google Scholar] [CrossRef]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [Green Version]
- Lotka, A.J. Contribution to the Energetics of Evolution. Proc. Natl. Acad. Sci. USA 1922, 8, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Corning, P.A. A Systems Theory of Biological Evolution. Biosystems 2022, 214, 104630. [Google Scholar] [CrossRef]
- Suki, B. The Major Transitions of Life from a Network Perspective. Front. Physiol. 2012, 3, 1–13. [Google Scholar] [CrossRef]
- De la Fuente, I.M. Elements of the Cellular Metabolic Structure. Front. Mol. Biosci. 2015, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, J.T. Hydrogen Sulfide and Environmental Stresses. Environ. Exp. Bot. 2019, 161, 50–56. [Google Scholar] [CrossRef]
- Hancock, J.T.; Whiteman, M. Hydrogen Sulfide Signaling: Interactions with Nitric Oxide and Reactive Oxygen Species. Ann. N. Y. Acad. Sci. 2016, 1365, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Heylighen, F.; Beigi, S.; Busseniers, E. The Role of Self-Maintaining Resilient Reaction Networks in the Origin and Evolution of Life. Biosystems 2022, 219, 104720. [Google Scholar] [CrossRef]
- De Bianchi, S.; Ballottari, M.; Dall’Osto, L.; Bassi, R. Regulation of Plant Light Harvesting by Thermal Dissipation of Excess Energy. Biochem. Soc. Trans. 2010, 38, 651–660. [Google Scholar] [CrossRef]
- Haupt-Herting, S.; Fock, H.P. Exchange of Oxygen and Its Role in Energy Dissipation during Drought Stress in Tomato Plants. Physiol. Plant 2000, 110, 489–495. [Google Scholar] [CrossRef]
- Baluška, F.; Reber, A.S.; Miller, W.B. Cellular Sentience as the Primary Source of Biological Order and Evolution. Biosystems 2022, 218, 104694. [Google Scholar] [CrossRef]
- Mahajan, A.S.; Oetjen, H.; Saiz-Lopez, A.; Lee, J.D.; McFiggans, G.B.; Plane, J.M.C. Reactive Iodine Species in a Semi-Polluted Environment. Geophys. Res. Lett. 2009, 36, L16803. [Google Scholar] [CrossRef]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for Reactive Reduced Phosphorus Species in the Early Archean Ocean. PNAS 2013, 110, 10089–10094. [Google Scholar] [CrossRef] [Green Version]
- Kharma, A.; Grman, M.; Misak, A.; Domínguez-Álvarez, E.; Nasim, M.J.; Ondrias, K.; Chovanec, M.; Jacob, C. Inorganic Polysulfides and Related Reactive Sulfur–Selenium Species from the Perspective of Chemistry. Molecules 2019, 24, 1359. [Google Scholar] [CrossRef]
- Alché, J.d.D. A Concise Appraisal of Lipid Oxidation and Lipoxidation in Higher Plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tola, A.J.; Jaballi, A.; Missihoun, T.D. Protein Carbonylation: Emerging Roles in Plant Redox Biology and Future Prospects. Plants 2021, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Straub, K.D. The Role of Hydrogen Sulfide in Evolution and the Evolution of Hydrogen Sulfide in Metabolism and Signaling. Physiology 2015, 31, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, H.; Cohen, M.F. Biological Consilience of Hydrogen Sulfide and Nitric Oxide in Plants: Gases of Primordial Earth Linking Plant, Microbial and Animal Physiologies. Nitric Oxide 2016, 55–56, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gumsley, A.P.; Chamberlain, K.R.; Bleeker, W.; Söderlund, U.; de Kock, M.O.; Larsson, E.R.; Bekker, A. Timing and Tempo of the Great Oxidation Event. PNAS 2017, 114, 1811–1816. [Google Scholar] [CrossRef] [Green Version]
- Santolini, J.; Wootton, S.A.; Jackson, A.A.; Feelisch, M. The Redox Architecture of Physiological Function. Curr. Opin. Physiol. 2019, 9, 34–47. [Google Scholar] [CrossRef]
- Allen, J.F.; Thake, B.; Martin, W.F. Nitrogenase Inhibition Limited Oxygenation of Earth’s Proterozoic Atmosphere. Trends Plant Sci. 2019, 24, 1022–1031. [Google Scholar] [CrossRef]
- Jacob, F. Evolution and Tinkering. Science 1977, 196, 1161–1166. [Google Scholar] [CrossRef] [Green Version]
- Macklem, P.T. Emergent Phenomena and the Secrets of Life. J. Appl. Physiol. 2008, 104, 1844–1846. [Google Scholar] [CrossRef]
- Toyabe, S.; Sagawa, T.; Ueda, M.; Muneyuki, E.; Sano, M. Experimental Demonstration of Information-to-Energy Conversion and Validation of the Generalized Jarzynski Equality. Nat. Phys. 2010, 6, 988–992. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling Chemical Priming Machinery in Plants: The Role of Reactive Oxygen–Nitrogen–Sulfur Species in Abiotic Stress Tolerance Enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Carreras, A.; Valderrama, R.; Chaki, M.; Palma, J.M. Reactive Nitrogen Species and Nitrosative Stress in Plants. Plant Stress 2007, 1, 37–41. [Google Scholar]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive Oxygen Species, Antioxidants and Signaling in Plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Slusarenko, A.J. The Biology of Reactive Sulfur Species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–22. ISBN 978-3-319-20421-5. [Google Scholar]
- Giles, G.I.; Nasim, M.J.; Ali, W.; Jacob, C. The Reactive Sulfur Species Concept: 15 Years On. Antioxidants 2017, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Palma, J.M. Assessing Nitric Oxide (NO) in Higher Plants: An Outline. Nitrogen 2020, 1, 12–20. [Google Scholar] [CrossRef] [Green Version]
- González-Morales, S.; López-Sánchez, R.C.; Juárez-Maldonado, A.; Robledo-Olivo, A.; Benavides-Mendoza, A. A Transcriptomic and Proteomic View of Hydrogen Sulfide Signaling in Plant Abiotic Stress. In Hydrogen Sulfide and Plant Acclimation to Abiotic Stresses; Khan, M.N., Siddiqui, M.H., Alamri, S., Corpas, F.J., Eds.; Plant in Challenging Environments; Springer International Publishing: Cham, Switzerland, 2021; pp. 161–186. ISBN 978-3-030-73678-1. [Google Scholar]
- Khanna, K.; Sharma, N.; Kour, S.; Ali, M.; Ohri, P.; Bhardwaj, R. Hydrogen Sulfide: A Robust Combatant against Abiotic Stresses in Plants. Hydrogen 2021, 2, 319–342. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The Role of Reactive Sulfur Species in Oxidative Stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Del Río, L.A. ROS and RNS in Plant Physiology: An Overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkan, I. ROS and RNS: Key Signalling Molecules in Plants. J Exp Bot 2018, 69, 3313–3315. [Google Scholar] [CrossRef] [Green Version]
- Olson, K.R. Hydrogen Sulfide, Reactive Sulfur Species and Coping with Reactive Oxygen Species. Free Radic. Biol. Med. 2019, 140, 74–83. [Google Scholar] [CrossRef]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)-Releasing Synthetic Compounds as Novel Priming Agents against Drought Stress in Medicago Sativa Plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Elbasan, F.; Kucukoduk, M.; Turkan, I. Hydrogen Sulfide (H2S) and Nitric Oxide (NO) Alleviate Cobalt Toxicity in Wheat (Trriticum aestivum L.) by Modulating Photosynthesis, Chloroplastic Redox and Antioxidant Capacity. J. Hazard. Mater. 2020, 388, 122061. [Google Scholar] [CrossRef]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant Catalases as NO and H2S Targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Tomar, R.S.; Kataria, S.; Jajoo, A. Behind the Scene: Critical Role of Reactive Oxygen Species and Reactive Nitrogen Species in Salt Stress Tolerance. J. Agron. Crop. Sci. 2021, 207, 577–588. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Castroverde, C.D.M.; Kalaji, H.M.; Albaqami, M.; Aftab, T. Molecular Mechanisms of Nitric Oxide (NO) Signaling and Reactive Oxygen Species (ROS) Homeostasis during Abiotic Stresses in Plants. Int. J. Mol. Sci. 2021, 22, 9656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.J.; Chintagunta, A.D.; Reddy, Y.M.; Rajjou, L.; Garlapati, V.K.; Agarwal, D.K.; Prasad, S.R.; Simal-Gandara, J. Implications of Reactive Oxygen and Nitrogen Species in Seed Physiology for Sustainable Crop Productivity under Changing Climate Conditions. Curr. Plant Biol. 2021, 26, 100197. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, O. Interplay between Reactive Oxygen and Nitrogen Species in Living Organisms. Chem. Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Riaz, M.; Arif, M.S. Chemical Priming for Multiple Stress Tolerance. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer: Singapore, 2019; pp. 385–415. ISBN 9789811386251. [Google Scholar]
- Kaur, P.; Handa, N.; Verma, V.; Bakshi, P.; Kalia, R.; Sareen, S.; Nagpal, A.; Vig, A.P.; Mir, B.A.; Bhardwaj, R. Cross Talk Among Reactive Oxygen, Nitrogen and Sulfur During Abiotic Stress in Plants. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 857–871. ISBN 978-1-119-46867-7. [Google Scholar]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2021, 41, 119–142. [Google Scholar] [CrossRef]
- Mangal, V.; Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Sood, S.; Kumar, D.; Bharadwaj, V.; Singh, B.; Singh, R.K.; Aftab, T. Molecular Insights into the Role of Reactive Oxygen, Nitrogen and Sulphur Species in Conferring Salinity Stress Tolerance in Plants. J. Plant Growth Regul. 2022, 1–21. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019, 10, 1091. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Stress-Triggered Redox Signalling: What’s in PROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Hanke, G. ROS Production and Signalling in Chloroplasts: Cornerstones and Evolving Concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Mukherjee, S. Cysteine Modifications (OxPTM) and Protein Sulphenylation-Mediated Sulfenome Expression in Plants: Evolutionary Conserved Signaling Networks? Plant Signal. Behav. 2021, 16, 1831792. [Google Scholar] [CrossRef]
- Considine, M.J.; María Sandalio, L.; Helen Foyer, C. Unravelling How Plants Benefit from ROS and NO Reactions, While Resisting Oxidative Stress. Ann. Bot. 2015, 116, 469–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Considine, M.J.; Foyer, C.H. Oxygen and Reactive Oxygen Species-Dependent Regulation of Plant Growth and Development. Plant Physiol. 2021, 186, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Gow, A.J.; Stamler, J.S.; Glazebrook, J. Ancient Origins of Nitric Oxide Signaling in Biological Systems. Proc. Natl. Acad. Sci. USA 1999, 96, 14206–14207. [Google Scholar] [CrossRef] [Green Version]
- Astier, J.; Mounier, A.; Santolini, J.; Jeandroz, S.; Wendehenne, D. The Evolution of Nitric Oxide Signalling Diverges between Animal and Green Lineages. J. Exp. Bot. 2019, 70, 4355–4364. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants– Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Staszek, P.; Gniazdowska, A. Peroxynitrite Induced Signaling Pathways in Plant Response to Non-Proteinogenic Amino Acids. Planta 2020, 252, 5. [Google Scholar] [CrossRef]
- Murad, F.; Barber, R. A Hypothesis about Cellular Signaling with Nitric Oxide in the Earliest Life Forms in Evolution. Free Radic. Biol. Med. 2009, 47, 1325–1327. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. NO Source in Higher Plants: Present and Future of an Unresolved Question. Trends Plant Sci. 2022, 27, 116–119. [Google Scholar] [CrossRef]
- Gupta, K.J.; Stoimenova, M.; Kaiser, W.M. In Higher Plants, Only Root Mitochondria, but Not Leaf Mitochondria Reduce Nitrite to NO, in Vitro and in Situ. J. Exp. Bot. 2005, 56, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Protein Nitration: A Connecting Bridge between Nitric Oxide (NO) and Plant Stress. Plant Stress 2021, 2, 100026. [Google Scholar] [CrossRef]
- Di Fino, L.; Arruebarrena Di Palma, A.; Perk, E.A.; García-Mata, C.; Schopfer, F.J.; Laxalt, A.M. Nitro-Fatty Acids: Electrophilic Signaling Molecules in Plant Physiology. Planta 2021, 254, 120. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From Elemental Sulfur to Hydrogen Sulfide in Agricultural Soils and Plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lancaster, J.R. Chemical Foundations of Hydrogen Sulfide Biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Arif, M.S.; Yasmeen, T.; Abbas, Z.; Ali, S.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; Abdel-Daim, M.M. Role of Exogenous and Endogenous Hydrogen Sulfide (H2S) on Functional Traits of Plants Under Heavy Metal Stresses: A Recent Perspective. Front. Plant Sci. 2021, 11, 545453. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Pluth, M.D. Reactive Sulfur Species (RSS): Persulfides, Polysulfides, Potential, and Problems. Curr. Opin. Chem. Biol. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Koike, S.; Ogasawara, Y. Sulfur Atom in Its Bound State Is a Unique Element Involved in Physiological Functions in Mammals. Molecules 2016, 21, 1753. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.R.; Farmer, P.J. Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S. Molecules 2019, 24, 3090. [Google Scholar] [CrossRef] [Green Version]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Cao, X.; Wang, C.; Luo, X.; Yue, L.; White, J.C.; Elmer, W.; Dhankher, O.P.; Wang, Z.; Xing, B. Elemental Sulfur Nanoparticles Enhance Disease Resistance in Tomatoes. ACS Nano 2021, 15, 11817–11827. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, Q.; Guo, Z.; Fu, J.; Sun, Y.; Gu, C.; Xing, B.; Dhankher, O.P. Sulfur Nanoparticles Improved Plant Growth and Reduced Mercury Toxicity via Mitigating the Oxidative Stress in Brassica napus L. J. Clean. Prod. 2021, 318, 128589. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Rhim, J.-W. New Insight into Sulfur Nanoparticles: Synthesis and Applications. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2329–2356. [Google Scholar] [CrossRef]
- Grman, M.; Nasim, M.J.; Leontiev, R.; Misak, A.; Jakusova, V.; Ondrias, K.; Jacob, C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants 2017, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Lum, H.K.; Butt, Y.K.C.; Lo, S.C.L. Hydrogen Peroxide Induces a Rapid Production of Nitric Oxide in Mung Bean (Phaseolus aureus). Nitric Oxide 2002, 6, 205–213. [Google Scholar] [CrossRef]
- Li, Z.G.; Luo, L.J.; Sun, Y.F. Signal Crosstalk between Nitric Oxide and Hydrogen Sulfide May Be Involved in Hydrogen Peroxide-Induced Thermotolerance in Maize Seedlings. Russ. J. Plant Physiol. 2015, 62, 507–514. [Google Scholar] [CrossRef]
- Wu, D.; Chu, H.; Jia, L.; Chen, K.; Zhao, L. A Feedback Inhibition between Nitric Oxide and Hydrogen Peroxide in the Heat Shock Pathway in Arabidopsis Seedlings. Plant Growth Regul. 2015, 75, 503–509. [Google Scholar] [CrossRef]
- Jian, W.; Zhang, D.; Zhu, F.; Wang, S.; Pu, X.; Deng, X.-G.; Luo, S.-S.; Lin, H. Alternative Oxidase Pathway Is Involved in the Exogenous SNP-Elevated Tolerance of Medicago Truncatula to Salt Stress. J. Plant Physiol. 2016, 193, 79–87. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Q.; Zhang, Y.; Yang, N.; Wu, G.; Li, Q.; Wang, W. Alleviation of Osmotic Stress by H2S Is Related to Regulated PLDα1 and Suppressed ROS in Arabidopsis Thaliana. J. Plant Res. 2020, 133, 393–407. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen Sulfide Alleviates Cadmium-Induced Cell Death through Restraining ROS Accumulation in Roots of Brassica rapa L. Ssp. Pekinensis. Oxidative Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen Sulfide Alleviates Chromium Stress on Cauliflower by Restricting Its Uptake and Enhancing Antioxidative System. Physiol. Plant 2020, 168, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Lisjak, M.; Srivastava, N.; Teklic, T.; Civale, L.; Lewandowski, K.; Wilson, I.; Wood, M.E.; Whiteman, M.; Hancock, J.T. A Novel Hydrogen Sulfide Donor Causes Stomatal Opening and Reduces Nitric Oxide Accumulation. Plant Physiol. Biochem. 2010, 48, 931–935. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Mollica, D.C.F.; Vicente, M.H.; Peres, L.E.P.; Modolo, L.V. NO, Hydrogen Sulfide Does Not Come First during Tomato Response to High Salinity. Nitric Oxide 2018, 76, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.-H.; Wu, F.-H.; He, E.-M.; Liu, X.; Shangguan, Z.-P.; Zheng, H.-L. Hydrogen Sulfide Enhances Salt Tolerance through Nitric Oxide-Mediated Maintenance of Ion Homeostasis in Barley Seedling Roots. Sci. Rep. 2015, 5, 12516. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen Sulfide: A Novel Component in Arabidopsis Peroxisomes Which Triggers Catalase Inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-W.; Liu, F.-J.; Zhai, J.; Li, F.-D.; Bi, H.-G.; Ai, X.-Z. Auxin Acts as a Downstream Signaling Molecule Involved in Hydrogen Sulfide-Induced Chilling Tolerance in Cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative Roles of Nitric Oxide and Hydrogen Sulfide in Melatonin-Induced Tolerance of Pepper (Capsicum annuum L.) Plants to Iron Deficiency and Salt Stress Alone or in Combination. Physiol. Plant 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic Acid-Induced Nitric Oxide Enhances Arsenic Toxicity Tolerance in Maize Plants by Upregulating the Ascorbate-Glutathione Cycle and Glyoxalase System. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Kaya, C.; Sarıoğlu, A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Gibberellic Acid-Induced Generation of Hydrogen Sulfide Alleviates Boron Toxicity in Tomato (Solanum lycopersicum L.) Plants. Plant Physiol. Biochem. 2020, 153, 53–63. [Google Scholar] [CrossRef]
- Kolbert, Z.; Szőllősi, R.; Feigl, G.; Kónya, Z.; Rónavári, A. Nitric Oxide Signalling in Plant Nanobiology: Current Status and Perspectives. J. Exp. Bot. 2021, 72, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Wang, S.; Zou, J.; Ding, W.; Shen, W. Magnesium Hydride-Mediated Sustainable Hydrogen Supply Prolongs the Vase Life of Cut Carnation Flowers via Hydrogen Sulfide. Front. Plant Sci. 2020, 11, 595376. [Google Scholar] [CrossRef] [PubMed]
- Kou, N.; Xiang, Z.; Cui, W.; Li, L.; Shen, W. Hydrogen Sulfide Acts Downstream of Methane to Induce Cucumber Adventitious Root Development. J. Plant Physiol. 2018, 228, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Corpas, F.J. Crosstalk among Hydrogen Sulfide (H2S), Nitric Oxide (NO) and Carbon Monoxide (CO) in Root-System Development and Its Rhizosphere Interactions: A Gaseous Interactome. Plant Physiol. Biochem. 2020, 155, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, J.; Cheng, D.; Wang, R.; Mei, Y.; Hu, H.; Shen, W.; Zhang, Y. Nitric Oxide Contributes to Methane-Induced Osmotic Stress Tolerance in Mung Bean. BMC Plant Biol. 2018, 18, 207. [Google Scholar] [CrossRef] [Green Version]
- Pyrzynska, K.; Sentkowska, A. Selenium in Plant Foods: Speciation Analysis, Bioavailability, and Factors Affecting Composition. Crit. Rev. Food Sci. Nutr. 2021, 61, 1340–1352. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Warzea, L.; Jiang, Y.; Hawkesfors, M. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Springer: Berlin/Heidelberg, Germany, 2017; Volume 114, p. 231. ISBN 978-3-319-56248-3. [Google Scholar]
- Ghuge, S.A.; Kadam, U.S.; Hong, J.C. Selenoprotein: Potential Player in Redox Regulation in Chlamydomonas Reinhardtii. Antioxidants 2022, 11, 1630. [Google Scholar] [CrossRef]
- García Márquez, V.; Morelos Moreno, Á.; Benavides Mendoza, A.; Medrano Macías, J. Ionic Selenium and Nanoselenium as Biofortifiers and Stimulators of Plant Metabolism. Agronomy 2020, 10, 1399. [Google Scholar] [CrossRef]
- González-Morales, S.; Pérez-Labrada, F.; García-Enciso, E.L.; Leija-Martínez, P.; Medrano-Macías, J.; Dávila-Rangel, I.E.; Juárez-Maldonado, A.; Rivas-Martínez, E.N.; Benasvides-Mendoza, A. Selenium and Sulfur to Produce Allium Functional Crops. Molecules 2017, 22, 558. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Han, G.; Ye, S.; Luo, Y.; Zhou, X. Effects of Selenium on Serotonin Synthesis and the Glutathione Redox Cycle in Plum Leaves. J. Soil Sci. Plant Nutr. 2020, 20, 2212–2221. [Google Scholar] [CrossRef]
- Kopriva, S.; Suter, M.; von Ballmoos, P.; Hesse, H.; Krähenbühl, U.; Rennenberg, H.; Brunold, C. Interaction of Sulfate Assimilation with Carbon and Nitrogen Metabolism in Lemna Minor. Plant Physiol. 2002, 130, 1406–1413. [Google Scholar] [CrossRef] [Green Version]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium Biofortification in Fragaria × Ananassa: Implications on Strawberry Fruits Quality, Content of Bioactive Health Beneficial Compounds and Metabolomic Profile. Front. Plant Sci. 2017, 8, 1887. [Google Scholar] [CrossRef] [Green Version]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; dos Reis, A.R. Selenate and Selenite Affect Photosynthetic Pigments and ROS Scavenging through Distinct Mechanisms in Cowpea (Vigna unguiculata (L.) Walp) Plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Yeasmin, M.; Lamb, D.; Choppala, G.; Rahman, M.M. Selenium Accumulation and Speciation in Chickpea (Cicer arietinum) Impacted by S in Soils: Potential for Biofortification. ACS Agric. Sci. Technol. 2022, 2, 135–143. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Narvaéz-Ortiz, W.A. Selenium and Nano-Selenium as a New Frontier of Plant Biostimulant. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Sustainable Plant Nutrition in a Changing World; Springer International Publishing: Cham, Switzerland, 2022; pp. 41–54. ISBN 978-3-031-07063-1. [Google Scholar]
- Solórzano, E.; Corpas, F.J.; González-Gordo, S.; Palma, J.M. Reactive Oxygen Species (ROS) Metabolism and Nitric Oxide (NO) Content in Roots and Shoots of Rice (Oryza sativa L.) Plants under Arsenic-Induced Stress. Agronomy 2020, 10, 1014. [Google Scholar] [CrossRef]
- Tanou, G.; Job, C.; Belghazi, M.; Molassiotis, A.; Diamantidis, G.; Job, D. Proteomic Signatures Uncover Hydrogen Peroxide and Nitric Oxide Cross-Talk Signaling Network in Citrus Plants. J. Proteome Res. 2010, 9, 5994–6006. [Google Scholar] [CrossRef]
- Tanou, G.; Filippou, P.; Belghazi, M.; Job, D.; Diamantidis, G.; Fotopoulos, V.; Molassiotis, A. Oxidative and Nitrosative-Based Signaling and Associated Post-Translational Modifications Orchestrate the Acclimation of Citrus Plants to Salinity Stress. Plant J. 2012, 72, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Jain, M.; Tripathi, D.K.; Singh, V.P. Involvement of Nitrate Reductase-Dependent Nitric Oxide Production in Magnetopriming-Induced Salt Tolerance in Soybean. Physiol. Plant 2020, 168, 422–436. [Google Scholar] [CrossRef]
- Kaur, H.; Bhatla, S.C. Melatonin and Nitric Oxide Modulate Glutathione Content and Glutathione Reductase Activity in Sunflower Seedling Cotyledons Accompanying Salt Stress. Nitric Oxide 2016, 59, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Al-Huqail, A.A.; Alqahtani, M.A.; Ahmad, P. Silicon Is Dependent on Hydrogen Sulphide to Improve Boron Toxicity Tolerance in Pepper Plants by Regulating the AsA-GSH Cycle and Glyoxalase System. Chemosphere 2020, 257, 127241. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Shen, W. Roles of Hydrogen Sulfide and Nitric Oxide in the Alleviation of Cadmium-Induced Oxidative Damage in Alfalfa Seedling Roots. Biometals 2012, 25, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Zangene-Madar, F.; Enteshari, S. Role of Two-Sided Crosstalk between NO and H2S on Improvement of Mineral Homeostasis and Antioxidative Defense in Sesamum Indicum under Lead Stress. Ecotoxicol. Environ. Saf. 2017, 139, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of Nitric Oxide and Hydrogen Sulfide in Regulating Oxidative Defence System in Wheat Plants Grown under Cadmium Stress. Physiol. Plant 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chan, Z. Nitric Oxide-Activated Hydrogen Sulfide Is Essential for Cadmium Stress Response in Bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zheng, P.; Li, S.; Li, K.; Xu, H. Nitrate Reductase-Dependent NO Production Is Involved in H2S-Induced Nitrate Stress Tolerance in Tomato via Activation of Antioxidant Enzymes. Sci. Hortic. 2018, 229, 207–214. [Google Scholar] [CrossRef]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between Hydrogen Peroxide and Sodium Nitroprusside Following Chemical Priming of Ocimum basilicum L. against Salt Stress. Physiol. Plant 2020, 168, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of Hydrogen Peroxide and Nitric Oxide on Both Salt and Heat Stress Tolerance in Rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhary, M.A.M.; Rehman, A. Foliage-Applied Sodium Nitroprusside and Hydrogen Peroxide Improves Resistance against Terminal Drought in Bread Wheat. J. Agron. Crop. Sci. 2017, 203, 473–482. [Google Scholar] [CrossRef]
- Christou, A.; Manganaris, G.A.; Fotopoulos, V. Systemic Mitigation of Salt Stress by Hydrogen Peroxide and Sodium Nitroprusside in Strawberry Plants via Transcriptional Regulation of Enzymatic and Non-Enzymatic Antioxidants. Environ. Exp. Bot. 2014, 107, 46–54. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Cui, W.; Xu, S.; Shen, W.; Wang, R. Hydrogen Sulfide Enhances Alfalfa (Medicago sativa) Tolerance against Salinity during Seed Germination by Nitric Oxide Pathway. Plant Soil 2012, 351, 107–119. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Belghazi, M.; Filippou, P.; Fotopoulos, V.; Grigorios, D.; Molassiotis, A. Roles of Sodium Hydrosulfide and Sodium Nitroprusside as Priming Molecules during Drought Acclimation in Citrus Plants. Plant Mol. Biol. 2015, 89, 433–450. [Google Scholar] [CrossRef]
- Tanou, G.; Job, C.; Rajjou, L.; Arc, E.; Belghazi, M.; Diamantidis, G.; Molassiotis, A.; Job, D. Proteomics Reveals the Overlapping Roles of Hydrogen Peroxide and Nitric Oxide in the Acclimation of Citrus Plants to Salinity. Plant J. 2009, 60, 795–804. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing Oxidative Stress through the Lens of Oxidative Signalling Rather than Damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freschi, L. Nitric Oxide and Phytohormone Interactions: Current Status and Perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Garcia-Mata, C.; He, L.-F. Interaction between Hydrogen Sulfide and Hormones in Plant Physiological Responses. Plant Growth Regul 2019, 87, 175–186. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Kashfi, K.; Fotopoulos, V. P77: Exploring the Potential of NOSH-Aspirin as a Plant Priming Agent against Abiotic Stress Factors. Nitric Oxide 2014, 39, S39. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S.; et al. Humic Substances: Determining Potential Molecular Regulatory Processes in Plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [Green Version]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic Acid and Nitric Oxide Signaling in Plant Heat Stress. Physiol. Plant 2020, 168, 241–255. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculík, M.; Corpas, F.J. Silicon Crosstalk with Reactive Oxygen Species, Phytohormones and Other Signaling Molecules. J. Hazard. Mater. 2020, 408, 124820. [Google Scholar] [CrossRef] [PubMed]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of Biostimulation of Plants under Abiotic Stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Dai, H.; Sun, Y. Hydrogen Sulfide Protects Wheat Seedlings against Copper Stress by Regulating the Ascorbate and Glutathione Metabolism in Leaves. Aust. J. Crop. Sci. 2012, 6, 248–254. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.-Y.; Hu, K.-D.; He, Y.-D.; Wang, S.-H.; Luo, J.-P. Hydrogen Sulfide Promotes Wheat Seed Germination and Alleviates Oxidative Damage against Copper Stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, Z.H.; Naeem, M.; Abbas, Z.K.; Ansari, M.W. Chapter 8—Nitric Oxide and Hydrogen Sulfide Interactions in Plants under Adverse Environmental Conditions. In Emerging Plant Growth Regulators in Agriculture; Aftab, T., Naeem, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 215–244. ISBN 978-0-323-91005-7. [Google Scholar]
- Pandey, N.; Verma, L. Nitric Oxide Alleviates Iron Toxicity by Reducing Oxidative Damage and Growth Inhibition in Wheat (Triticum aestivum L.) Seedlings. Int. J. Plant Environ. 2019, 5, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous Nitric Oxide (NO) Ameliorates Salinity-Induced Oxidative Stress in Tomato (Solanum Lycopersicum) Plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, W.; Niu, Y.; Jin, C.; Chai, R.; Tang, C.; Zhang, Y. Nitric Oxide Enhances Development of Lateral Roots in Tomato (Solanum lycopersicum L.) under Elevated Carbon Dioxide. Planta 2013, 237, 137–144. [Google Scholar] [CrossRef]
- Ghorbani, A.; Pishkar, L.; Roodbari, N.; Pehlivan, N.; Wu, C. Nitric Oxide Could Allay Arsenic Phytotoxicity in Tomato (Solanum lycopersicum L.) by Modulating Photosynthetic Pigments, Phytochelatin Metabolism, Molecular Redox Status and Arsenic Sequestration. Plant Physiol. Biochem. 2021, 167, 337–348. [Google Scholar] [CrossRef]
- Beligni, M.V.; Lamattina, L. Nitric Oxide Protects against Cellular Damage Produced by Methylviologen Herbicides in Potato Plants. Nitric Oxide 1999, 3, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Noritake, T.; Kawakita, K.; Doke, N. Nitric Oxide Induces Phytoalexin Accumulation in Potato Tuber Tissues. Plant Cell Physiol. 1996, 37, 113–116. [Google Scholar] [CrossRef]
- París, R.; Lamattina, L.; Casalongué, C.A. Nitric Oxide Promotes the Wound-Healing Response of Potato Leaflets. Plant Physiol. Biochem. 2007, 45, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Skrobacz, K.; Bobrecka-Jamro, D.; Balawejder, M. Response of Potato (Solanum tuberosum L.) Plants to Spraying by Hydrogen Peroxide. Sustainability 2020, 12, 2469. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eum, H.-L.; Lee, S.-K. The Responses of Yukbo Strawberry (Fragaria Ananassa Duch.) Fruit to Nitric Oxide. Food Sci. Biotechnol. 2007, 16, 123–126. [Google Scholar]
- Wills, R.B.H.; Ku, V.V.V.; Leshem, Y.Y. Fumigation with Nitric Oxide to Extend the Postharvest Life of Strawberries. Postharvest Biol. Technol. 2000, 18, 75–79. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M. Influence of Exogenously Applied Nitric Oxide on Strawberry (Fragaria × Ananassa) Plants Grown under Iron Deficiency and/or Saline Stress. Physiol. Plant 2019, 165, 247–263. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.; Ashraf, M. The Mechanism of Hydrogen Sulfide Mitigation of Iron Deficiency-Induced Chlorosis in Strawberry (Fragaria × Ananassa) Plants. Protoplasma 2019, 256, 371–382. [Google Scholar] [CrossRef]
- Manafi, H.; Baninasab, B.; Gholami, M.; Talebi, M. Nitric Oxide Induced Thermotolerance in Strawberry Plants by Activation of Antioxidant Systems and Transcriptional Regulation of Heat Shock Proteins. J. Hortic. Sci. Biotechnol. 2021, 96, 783–796. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Pan, Y.; Ren, B.; Chen, Q.; Li, X.; Xiong, X.; Tao, J.; Cheng, Q.; Ma, M. Beneficial Behavior of Nitric Oxide in Copper-Treated Medicinal Plants. J. Hazard. Mater. 2016, 314, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Naeem, M.; Khan, M.M.M.A.; Uddin, M. Vincristine and Vinblastine Anticancer Catharanthus Alkaloids: Pharmacological Applications and Strategies for Yield Improvement. In Catharanthus roseus: Current Research and Future Prospects; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 277–307. ISBN 978-3-319-51620-2. [Google Scholar]
- Gupta, M.M.; Singh, D.V.; Tripathi, A.K.; Pandey, R.; Verma, R.K.; Singh, S.; Shasany, A.K.; Khanuja, S.P.S. Simultaneous Determination of Vincristine, Vinblastine, Catharanthine, and Vindoline in Leaves of Catharanthus Roseus by High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2005, 43, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Towler, M.; Hassanali, A.; Lutgen, P.; Engeu, P.O. Dried-Leaf Artemisia Annua: A Practical Malaria Therapeutic for Developing Countries? World J. Pharm. 2014, 3, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Nomani, L.; Zehra, A.; Choudhary, S.; Wani, K.I.; Naeem, M.; Siddiqui, M.H.; Khan, M.M.A.; Aftab, T. Exogenous Hydrogen Sulphide Alleviates Copper Stress Impacts in Artemisia annua L.: Growth, Antioxidant Metabolism, Glandular Trichome Development and Artemisinin Biosynthesis. Plant Biol. 2022, 24, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.-S.; Kim, W. Anti-Inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharm. 2015, 19, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Adamska, I.; Biernacka, P. Bioactive Substances in Safflower Flowers and Their Applicability in Medicine and Health-Promoting Foods. Int. J. Food Sci. 2021, 2021, e6657639. [Google Scholar] [CrossRef]
- Amir, S.B.; Rasheed, R.; Ashraf, M.A.; Hussain, I.; Iqbal, M. Hydrogen Sulfide Mediates Defense Response in Safflower by Regulating Secondary Metabolism, Oxidative Defense, and Elemental Uptake under Drought. Physiol. Plant 2021, 172, 795–808. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, O.K.; Hegazy, A.E. Molecular Role of Nitric Oxide in Secondary Products Production in Ginkgo biloba Cell Suspension Culture. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.-P.; Du, X.-H.; Shi, R.-J. Exogenous nitric oxide accelerates soluble sugar, proline and secondary metabolite synthesis in Ginkgo biloba under drought stress. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2007, 33, 499–506. [Google Scholar]
- Post-White, J.; Ladas, E.J.; Kelly, K.M. Advances in the Use of Milk Thistle (Silybum marianum). Integr. Cancer 2007, 6, 104–109. [Google Scholar] [CrossRef]
- Zangani, E.; Zehtab-Salmasi, S.; Andalibi, B.; Zamani, A.-A. Protective Effects of Nitric Oxide on Photosynthetic Stability and Performance of Silybum Marianum under Water Deficit Conditions. Agron. J. 2018, 110, 555–564. [Google Scholar] [CrossRef]
- Zangani, E.; Zehtab-Salmasi, S.; Andalibi, B.; Zamani, A.A.; Hashemi, M. Exogenous Nitric Oxide Improved Production and Content of Flavonolignans in Milk Thistle Seeds under Water Deficit System. Acta Physiol. Plant 2021, 43, 87. [Google Scholar] [CrossRef]
- Saller, R.; Meier, R.; Brignoli, R. The Use of Silymarin in the Treatment of Liver Diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018, 9, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, S.; Kim, Y.-J.; Devi, B.S.R.; Oh, J.Y.; Kim, S.-Y.; Kwon, W.-S.; Yang, D.-C. Sodium Nitroprusside Enhances the Elicitation Power of Methyl Jasmonate for Ginsenoside Production in Panax Ginseng Roots. Res. Chem. Intermed. 2016, 42, 2937–2951. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Huqail, A.A. Sodium Nitroprusside Application Regulates Antioxidant Capacity, Improves Phytopharmaceutical Production and Essential Oil Yield of Marjoram Herb under Drought. Ind. Crops Prod. 2020, 158, 113034. [Google Scholar] [CrossRef]
- Nasrin, F.; Fatemeh, N.; Ramezan, R. Comparison the Effects of Nitric Oxide and Spermidin Pretreatment on Alleviation of Salt Stress in Chamomile Plant (Matricaria recutita L.). J. Stress Physiol. Biochem. 2012, 8, 214–223. [Google Scholar]
- Murkowski, A. Heat Stress and Spermidine: Effect on Chlorophyll Fluorescence in Tomato Plants. Biol. Plant 2001, 44, 53–57. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Amjad Kamal, M.; Paul Kamdem, J.; Zaman, B.; da Rocha, J. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological Efficacy of Medicinal Plant Extracts in Preventing Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, e7904349. [Google Scholar] [CrossRef] [PubMed]
- Vranješ, M.; Štajner, D.; Vranješ, D.; Blagojevic, B.; Pavlović, K.; Milanov, D.; Popović, B.M. Medicinal Plants Extracts Impact on Oxidative Stress in Mice Brain Under the Physiological Conditions: The Effects of Corn Silk, Parsley, and Bearberry. Acta Chim. Slov. 2021, 68, 896–903. [Google Scholar] [CrossRef]
- Gu, R.; Wang, Y.; Wu, S.; Wang, Y.; Li, P.; Xu, L.; Zhou, Y.; Chen, Z.; Kennelly, E.J.; Long, C. Three New Compounds with Nitric Oxide Inhibitory Activity from Tirpitzia Sinensis, an Ethnomedicinal Plant from Southwest China. BMC Chem. 2019, 13, 47. [Google Scholar] [CrossRef]
- Singh, A.L.; Singh, S.; Kurella, A.; Verma, A.; Mahatama, M.K.; Venkatesh, I. Chapter 12—Plant Bio-Stimulants, Their Functions and Use in Enhancing Stress Tolerance in Oilseeds. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 239–259. ISBN 978-0-323-85579-2. [Google Scholar]
- keya Tudu, C.; Dey, A.; Pandey, D.K.; Panwar, J.S.; Nandy, S. Chapter 8—Role of Plant Derived Extracts as Biostimulants in Sustainable Agriculture: A Detailed Study on Research Advances, Bottlenecks and Future Prospects. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 159–179. ISBN 978-0-323-85579-2. [Google Scholar]
- Karthiga, D.; Chozhavendhan, S.; Gandhiraj, V.; Aniskumar, M. The Effects of Moringa Oleifera Leaf Extract as an Organic Bio-Stimulant for the Growth of Various Plants: Review. Biocatal. Agric. Biotechnol. 2022, 43, 102446. [Google Scholar] [CrossRef]
- Yuniati, N.; Kusumiyati, K.; Mubarok, S.; Nurhadi, B. The Role of Moringa Leaf Extract as a Plant Biostimulant in Improving the Quality of Agricultural Products. Plants 2022, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa Plant-Derived Biostimulant Stimulate Short-Term Growth of Salt Stressed Zea mays L. Plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021, 16. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.R.; Pelegrino, M.T.; Seabra, A.B. Nitric Oxide-Releasing Chitosan Nanoparticles Alleviate the Effects of Salt Stress in Maize Plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-Nitrosoglutathione into Chitosan Nanoparticles Improves Drought Tolerance of Sugarcane Plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Nahar, K.; Mohsin, S.M.; Parvin, K.; Fujita, M. Interaction of Sulfur with Phytohormones and Signaling Molecules in Conferring Abiotic Stress Tolerance to Plants. Plant Signal. Behav. 2018, 13, e1477905. [Google Scholar] [CrossRef]
- Mikkelsen, R.; Norton, R. Soil and Fertilizer Sulfur. Better Crops Plant Food 2013, 97, 7–9. [Google Scholar]
- Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Treatment of Nitric Oxide Supplemented with Nitrogen and Sulfur Regulates Photosynthetic Performance and Stomatal Behavior in Mustard under Salt Stress. Physiol. Plant 2020, 168, 490–510. [Google Scholar] [CrossRef] [PubMed]

| Oxidation State | Representative Compound and Formula |
|---|---|
| +2 | OF2 |
| +1 | O2F2 |
| 0 | O2 |
| −1/2 | All superoxides, O2−, O2−, HO2 |
| −1 | All the peroxides, H2O2, HO2−, HO |
| −2 | All the oxides, H2O, CO2 |
| Oxidation State | Representative Compound and Formula |
|---|---|
| +5 | HNO3, NO3−, ONOO− (peroxynitrite) |
| +4 | ·NO2 (nitrogen dioxide), N2O4 |
| +3 | HNO2, NO2−, S-nitrosothiols (RS-N=O), NO+ |
| +2 | ·NO (nitric oxide) |
| +1 | N2O (nitrous oxide), NO− |
| 0 | N2 |
| −1 | NH2OH (hydroxylamine) |
| −2 | N2H4 (hydrazine) |
| −3 | NH3, NH4+ |
| Oxidation State | Representative Compound and Formula |
|---|---|
| +6 | Sulfate, SO42− |
| +6 and −2 | Thiosulfate, S2O32− |
| +5 and −2 | Polythionates (-O3S-Sn-SO3-)2− Dithionate, S2O62− Trithionate, S3O62− Tetrathionate, S4O62− |
| +4 | Sulfur dioxide, SO2 Sulfite, SO32− Disulfite, S2O52− Sulfonic acid (RSO3H) from ROS-mediated protein sulfonylation. Sulfone, OS(S) the oxidation product of sulfoxides. |
| +3 | Dithionite, S2O42− Disulfide-S-dioxide (thiosulfonate) RS(O2)SR |
| +2 | Carbonyl Sulfide (COS), OCS. Sulfinic acid (RSO2H) from ROS-mediated protein sulfinylation. |
| +1 | Disulfide-S-monoxide (thiosulfinate) RS(O)SR |
| 0 | S0 (sulfane sulfur), elemental sulfur, mainly S8 (cycloocta-S). Sulfoxide (R-S(-O)-R such as the dimethyl sulfoxide (DMSO). Oxidized derivatives of sulfide and sulfenic acid (RSOH) from ROS-mediated protein sulfenylation. Near the six electrons, valence S0 never exists by itself. Sulfane sulfur (S0, S-S, or S2) is labile. There are a variety of compounds such as S8, thiosulfate, polysulfanes, and polysulfides, that contain S0 |
| −1 | Disulfide (RSSR) is a persulfide −S-S− found in the linkages between two cysteine residues in proteins. RSSH denotes persulfides (also called hydrosulfides or hydropersulfides) obtained by the action of H2S on cysteine residues (R-SH). Thioethers and thiols can be oxidized to disulfides. Persulfides such as CysSSH, GSSH, and protein-SSH act as signaling compounds in organisms. Major products of the decomposition of persulfides are polysulfanes Disulfide-S-monoxide (thiosulfinate) RS(O)SR Disulfide-S-dioxide (thiosulfonate) RS(O2)SR Thiyl-radical HS· or RS· |
| −2 | Sulfide, S2− and organic polysulfides, S22−, S32−, S52− Disulfides (R-S-S-R) Carbon disulfide (CS2) FeS2 NaHS and Na2S are sources of S2− and of its conjugated acids SH− and H2S. Organic and inorganic polysulfides (with Sn > 2) contain S0 atoms, which allows a diversity of oxidation states. |
| −2 | Hydrogen sulfide (H2S), disulfane or hydrogen persulfide (H2S2), H2S3, other inorganic polysulfides (H2Sx) x ≥ 1, and polysulfanes (RSSnH, RSSnSR, n > 2). Polysulfanes contain S0 atoms, which allows a diversity of oxidation states. |
| −2 | Thioethers (C-S-C) such as dimethyl sulfide (DMS), CH3-S-CH3 and dimethyl disulfide (DMDS), CH3-S-S-CH3. |
| −2 | Thiols (R-SH) such as glutathione (GSH) and methyl mercaptan, CH3-SH. Thiols are derived from the sulfhydryl group -SH of cysteine that enables multiple oxidation states (−2 to +6). Thiolates are anionic derivatives of thiols in which a metal or other cation replaces H. |
| Impact on the Plant | Reactive Species | Plant Species | Reference |
|---|---|---|---|
| Decreased absorption and/or toxicity of heavy metals | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO | Medicago sativa Sesamum indicum Triticum aestivum Triticum aestivum | [129] [130] [131] [51] |
| Increase in the concentration of essential elements | H2S, ·NO H2S, ·NO | Triticum aestivum Sesamum indicum | [131] [130] |
| Increase in Relative Growth Rate (RGR) and/or biomass | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2O2, ·NO H2O2, ·NO H2O2, ·NO | Cynodon dactylon Medicago sativa Sesamum indicum Solanum lycopersicum Triticum aestivum Triticum aestivum Ocimum basilicum Oriza sativa Triticum aestivum | [129] [132] [130] [133] [51] [131] [134] [135] [136] |
| Improved crop yield and/or quality | H2O2, ·NO | Ocimum basilicum | [134] |
| Increase in Relative Water Content (RWC) | H2S, ·NO H2O2, ·NO | Triticum aestivum Fragaria × ananassa | [51] [137] |
| Increment in stomatal conductance (gs) | H2S, ·NO H2S, ·NO | Medicago sativa Triticum aestivum | [50] [51] |
| Increase in the quantum efficiency of PSII (Fv/Fm) | H2S, ·NO H2S, ·NO H2O2, ·NO H2O2, ·NO | Medicago sativa Triticum aestivum Citrus aurantium Fragaria × ananassa | [50] [51] [125] [137] |
| Increase in CO2 assimilation (A) | H2O2, ·NO | Citrus aurantium | [125] |
| Increment in the concentration of photosynthetic pigments | H2S, ·NO H2S, ·NO H2O2, ·NO H2O2, ·NO H2O2, ·NO | Sesamum indicum Triticum aestivum Citrus aurantium Fragaria × ananassa Ocimum basilicum | [130] [131] [125] [137] [134] |
| Increased activity of antioxidant enzymes (e.g., SOD and CAT) and the ascorbate–glutathione (AsA–GSH) cycle | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2O2, ·NO | Cynodon dactylon Medicago sativa Medicago sativa Medicago sativa Solanum lycopersicum Triticum aestivum Triticum aestivum Ocimum basilicum | [132] [138] [129] [50] [133] [131] [51] [134] |
| Proteome reprogramming through reversible or irreversible posttranslational modifications (PTM) and changes in gene expression | H2S, ·NO H2S, ·NO H2O2, ·NO | Citrus aurantium Citrus aurantium Citrus aurantium | [139] [140] [124] |
| Mitigation of the relative electrolyte leakage under stress | H2S, ·NO H2O2, ·NO H2O2, ·NO H2O2, ·NO | Cynodon dactylon Citrus aurantium Citrus aurantium Fragaria × ananassa | [132] [124] [125] [137] |
| Mitigation of lipid peroxidation under stress | H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2S, ·NO H2O2, ·NO | Cynodon dactylon Medicago sativa Medicago sativa Solanum lycopersicum Triticum aestivum Fragaria × ananassa | [132] [50] [129] [133] [131] [137] |
| Increased accumulation of proline and other osmolytes | H2S, ·NO H2O2, ·NO | Medicago sativa Triticum aestivum | [50] [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano-Macías, J.; Flores-Gallegos, A.C.; Nava-Reyna, E.; Morales, I.; Tortella, G.; Solís-Gaona, S.; Benavides-Mendoza, A. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants 2022, 11, 3203. https://doi.org/10.3390/plants11233203
Medrano-Macías J, Flores-Gallegos AC, Nava-Reyna E, Morales I, Tortella G, Solís-Gaona S, Benavides-Mendoza A. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants. 2022; 11(23):3203. https://doi.org/10.3390/plants11233203
Chicago/Turabian StyleMedrano-Macías, Julia, Adriana Carolina Flores-Gallegos, Erika Nava-Reyna, Isidro Morales, Gonzalo Tortella, Susana Solís-Gaona, and Adalberto Benavides-Mendoza. 2022. "Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview" Plants 11, no. 23: 3203. https://doi.org/10.3390/plants11233203
APA StyleMedrano-Macías, J., Flores-Gallegos, A. C., Nava-Reyna, E., Morales, I., Tortella, G., Solís-Gaona, S., & Benavides-Mendoza, A. (2022). Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants, 11(23), 3203. https://doi.org/10.3390/plants11233203









