Root-Shoot Nutrient Dynamics of Huanglongbing-Affected Grapefruit Trees
Abstract
1. Introduction
2. Results
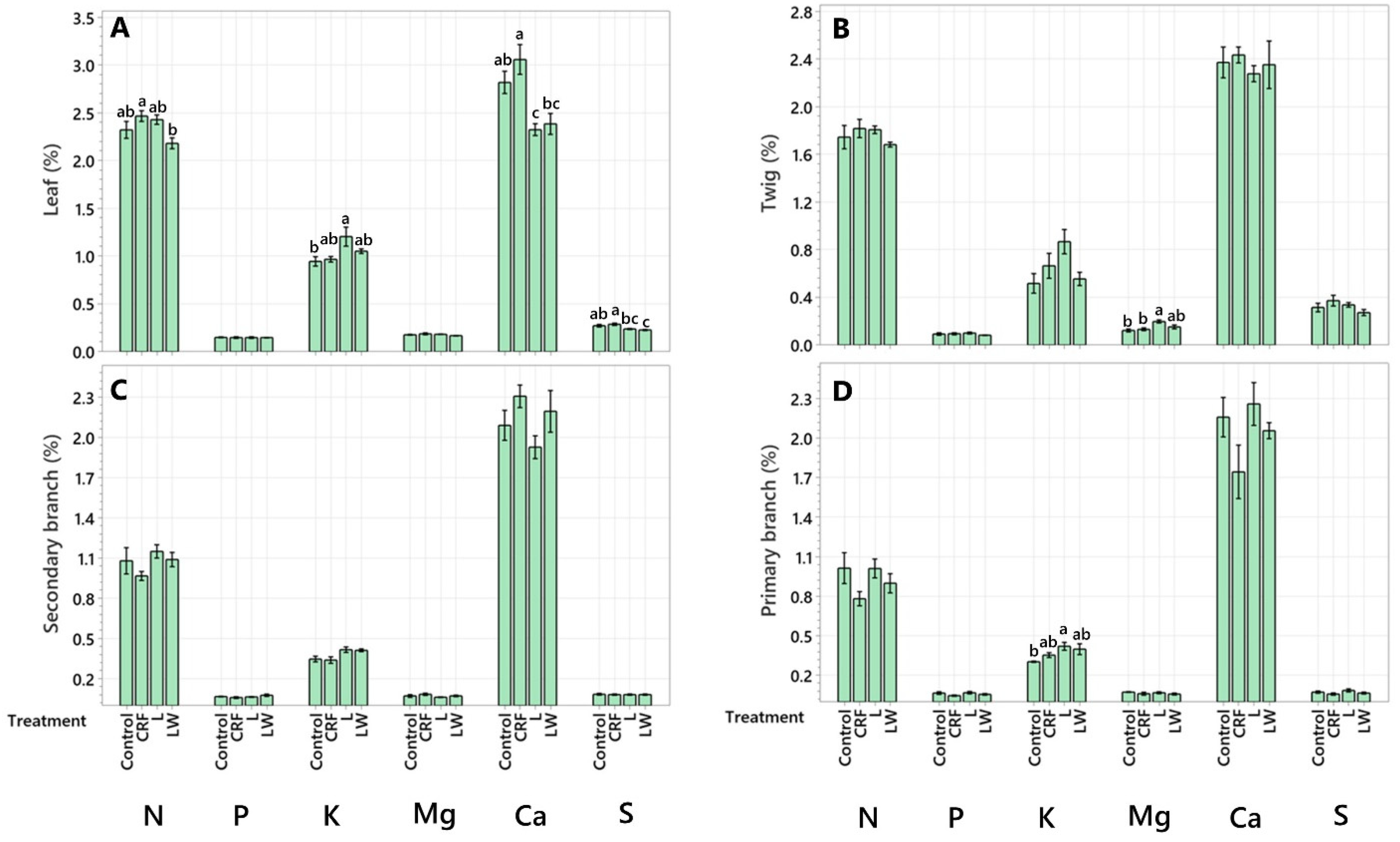
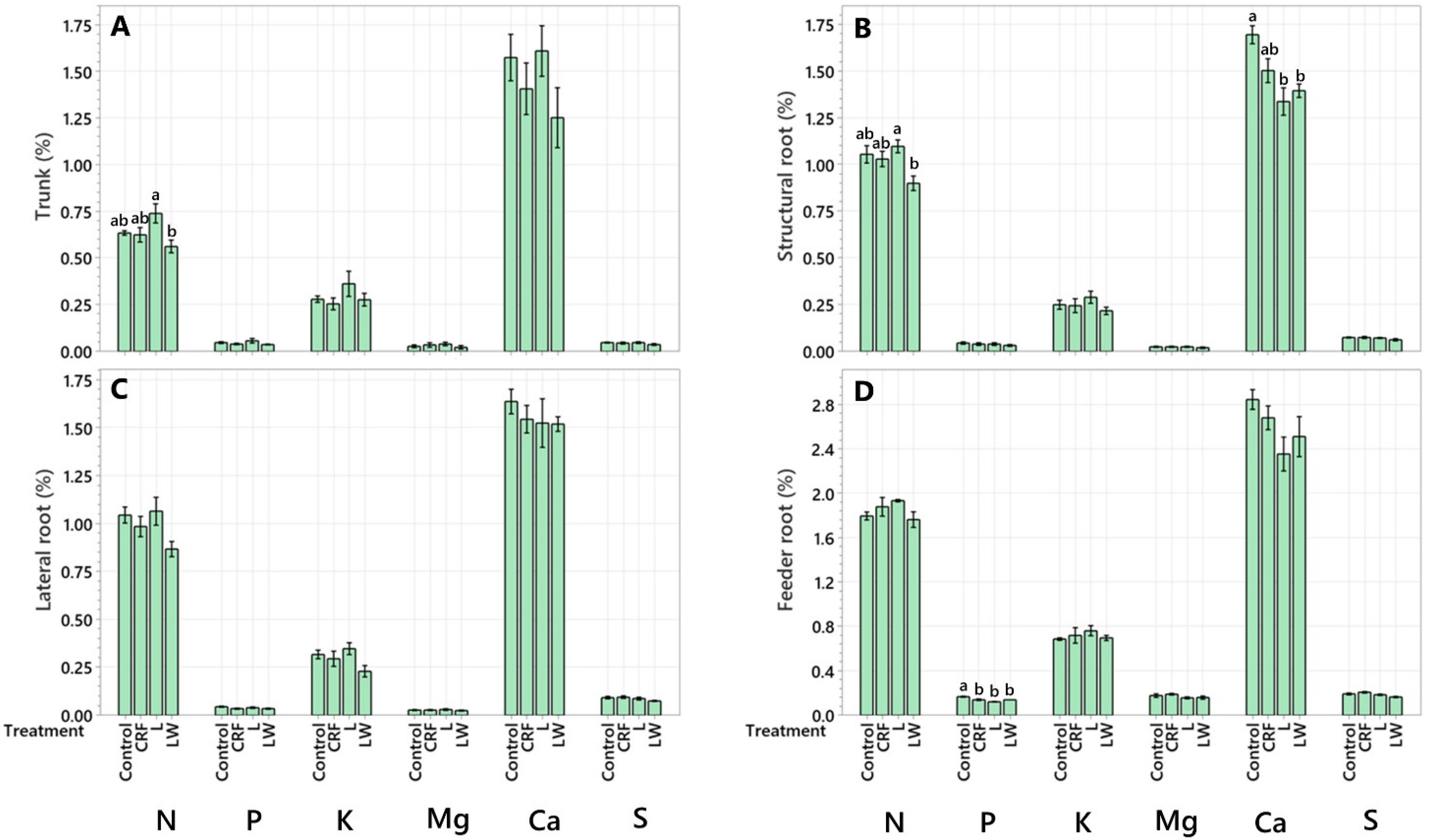
2.1. Macronutrient Concentration
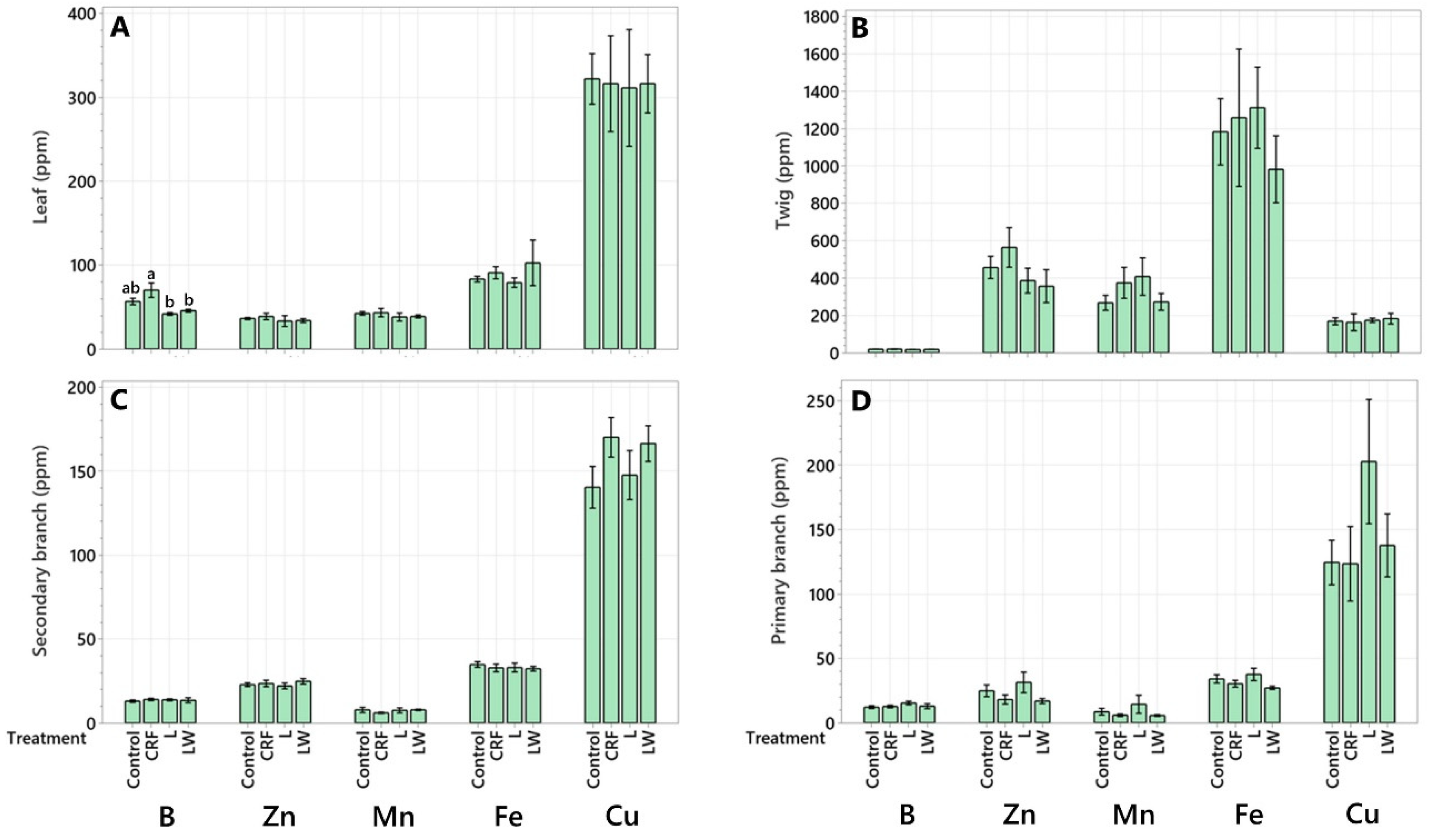
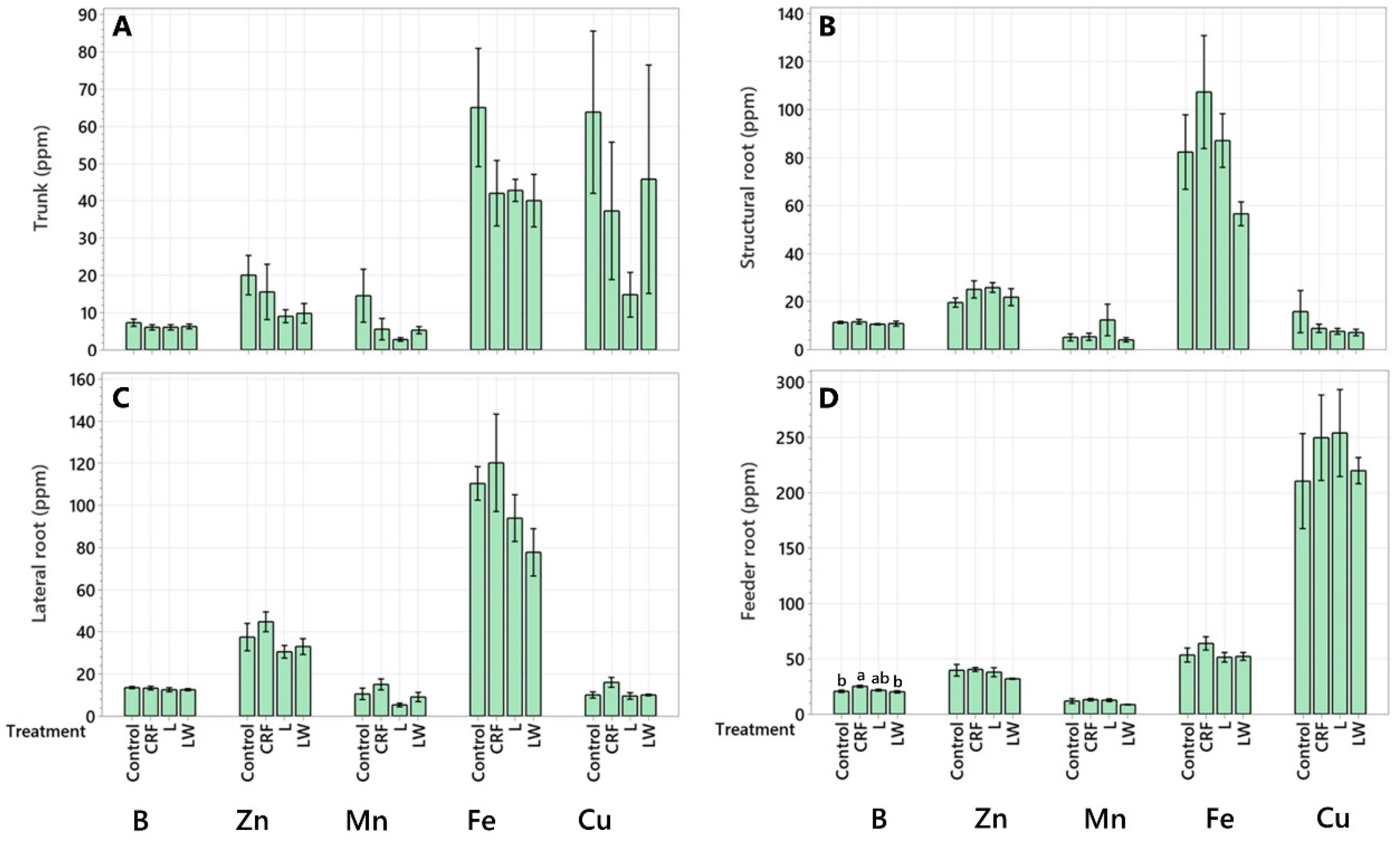
2.2. Micronutrient Concentration
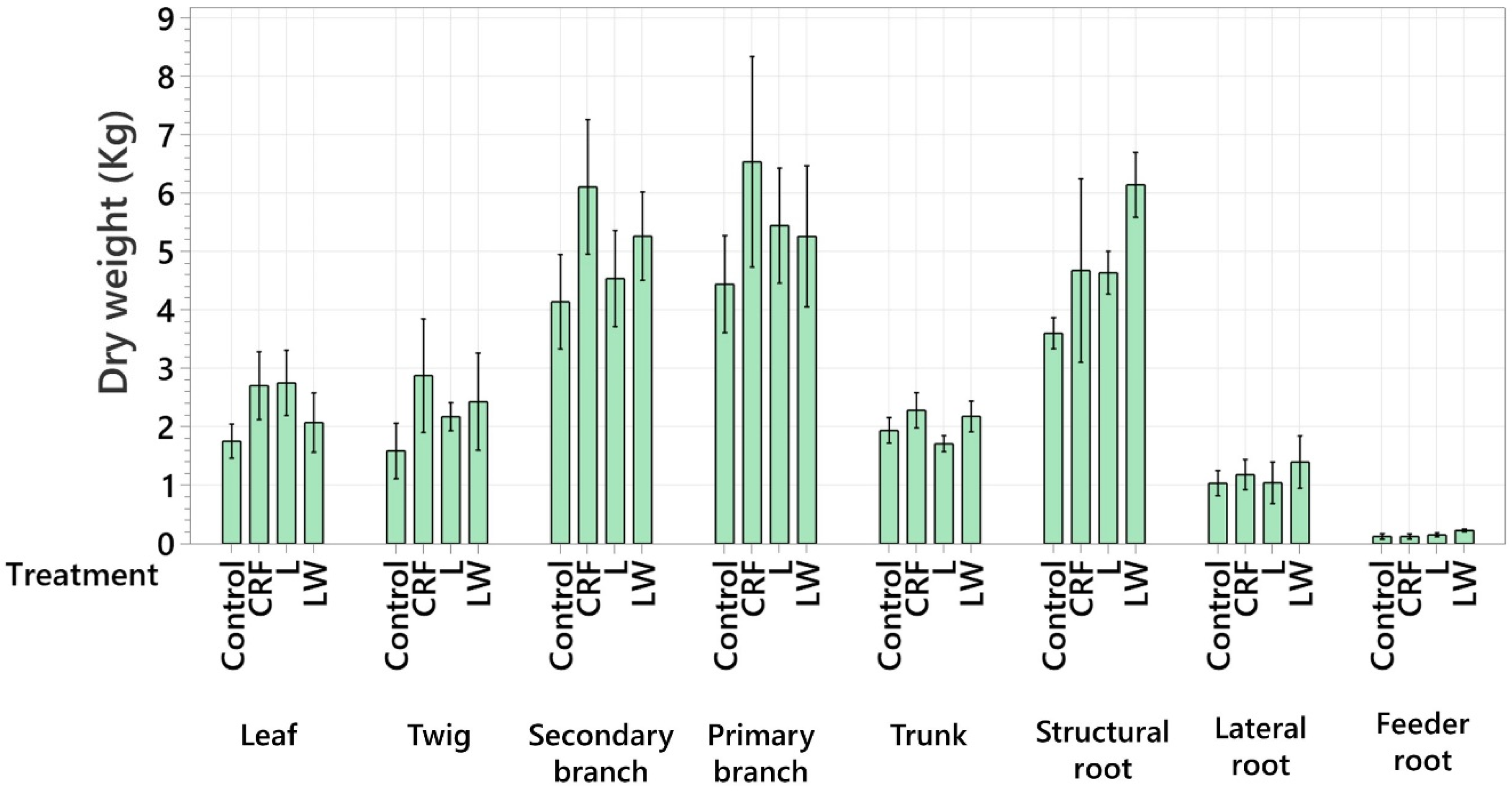
2.3. Total Tree Nutrient Content and Biomass
3. Discussion
4. Materials and Methods
4.1. Site Description
4.2. Tree Excavation and Dissection
4.3. Tree Biomass and Mineral Analysis
4.4. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obreza, T.A.; Tucker, D.P.H. Nutrient Management. In Florida Citrus: A Comprehensive Guide; Bird, C.J., Ed.; Cooperative Extension Service, University of Florida, University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2006; pp. 167–182. [Google Scholar]
- Srivastava, A.K.; Singh, S. Diagnosis of Nutrient Constraints in Citrus Orchards of Humid Tropical India. J. Plant Nutr. 2006, 29, 1061–1076. [Google Scholar] [CrossRef]
- Alva, A.K.; Mattos, D.; Paramasivam, S.; Patil, B.; Dou, H.; Sajwan, K.S. Potassium Management for Optimizing Citrus Production and Quality. Int. J. Fruit Sci. 2006, 6, 3–43. [Google Scholar] [CrossRef]
- Mattos-Jr, D.; Kadyampakeni, D.M.; da Silva, J.R.; Vashisth, T.; Boaretto, R.M. Reciprocal effects of huanglongbing infection and nutritional status of citrus trees: A review. Trop. Plant Pathol. 2020, 45, 586–596. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long-term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungau, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Chinyukwi, T. Are macronutrients and micronutrients therapeutic for restoring performance of trees affected by citrus greening? A discussion of current practices and future research opportunities. J. Plant Nutr. 2021, 44, 2949–2969. [Google Scholar] [CrossRef]
- Hendricks, G.S.; Shukla, S. Water and Nitrogen Management Effects on Water and Nitrogen Fluxes in Florida Flatwoods. J. Environ. Qual. 2011, 40, 1844–1856. [Google Scholar] [CrossRef]
- Obreza, T.A.; Collins, M.E. Common Soils Used for Citrus Production in Florida. 2002. Available online: https://ufdcimages.uflib.ufl.edu/IR/00/00/31/34/00001/SS40300.pdf (accessed on 18 October 2022).
- Obreza, T.A.; Schumann, A. Keeping Water and Nutrients in the Florida Citrus Tree Root Zone. HortTechnology 2010, 20, 67–73. [Google Scholar] [CrossRef]
- Huang, J.-H.; Cai, Z.-J.; Wen, S.-X.; Guo, P.; Ye, X.; Lin, G.-Z.; Chen, L.-S. Effects of boron toxicity on root and leaf anatomy in two Citrus species differing in boron tolerance. Trees 2014, 28, 1653–1666. [Google Scholar] [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Achor, D.; Albrigo, G. Anatomical distribution of abnormally high levels of starch in HLB-affected Valencia orange trees. Physiol. Mol. Plant Pathol. 2009, 74, 76–83. [Google Scholar] [CrossRef]
- Gottwald, T.R. Current Epidemiological Understanding of Citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2013, 63, 290–298. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Irey, M. Balancing Resources for Management of Root Health in HLB Affected Groves. 2012. Available online: https://citrusagents.ifas.ufl.edu/media/crecifasufledu/citrus-agents/growers-institutes/2012/2012-Graham.pdf (accessed on 18 October 2022).
- da Silva, J.R.; De Alvarenga, F.V.; Boaretto, R.M.; Lopes, J.R.S.; Quaggio, J.A.; Coletta-Filho, H.; Mattos, D. Following the effects of micronutrient supply in HLB-infected trees: Plant responses and ‘Candidatus Liberibacter asiaticus’ acquisition by the Asian citrus psyllid. Trop. Plant Pathol. 2020, 45, 597–610. [Google Scholar] [CrossRef]
- Shahzad, F.; Chun, C.; Schumann, A.; Vashisth, T. Nutrient Uptake in Huanglongbing-affected Sweet Orange: Transcriptomic and Physiological Analysis. J. Am. Soc. Hortic. Sci. 2020, 145, 349–362. [Google Scholar] [CrossRef]
- Hallman, L.M.; Kadyampakeni, D.M.; Ferrarezi, R.S.; Wright, A.L.; Ritenour, M.A.; Johnson, E.G.; Rossi, L. Impact of Ground Applied Micronutrients on Root Growth and Fruit Yield of Severely Huanglongbing-Affected Grapefruit Trees. Horticulturae 2022, 8, 763. [Google Scholar] [CrossRef]
- Esteves, E.; Kadyampakeni, D.M.; Zambon, F.; Ferrarezi, R.S.; Maltais-Landry, G. Magnesium fertilization has a greater impact on soil and leaf nutrient concentrations than nitrogen or calcium fertilization in Florida orange production. Nutr. Cycl. Agroecosyst. 2022, 122, 73–87. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Jani, A.D.; James, H.T.; Gil, C.; Ritenour, M.A.; Wright, A.L. Sweet Orange Orchard Architecture Design, Fertilizer, and Irrigation Management Strategies under Huanglongbing-endemic Conditions in the Indian River Citrus District. HortScience 2020, 55, 2028–2036. [Google Scholar] [CrossRef]
- Phuyal, D.; Nogueira, T.A.R.; Jani, A.D.; Kadyampakeni, D.M.; Morgan, K.T.; Ferrarezi, R.S. ‘Ray Ruby’ Grapefruit Affected by Huanglongbing II. Planting Density, Soil, and Foliar Nutrient Management. HortScience 2020, 55, 1420–1432. [Google Scholar] [CrossRef]
- Alva, A.K.; Fares, A.; Dou, H. Managing Citrus Trees to Optimize Dry Mass and Nutrient Partitioning. J. Plant Nutr. 2003, 26, 1541–1559. [Google Scholar] [CrossRef]
- Zekri, M.; Schumann, A.W.; Vashisth, T.; Kadyampakeni, D.M.; Morgan, K.T.; Boman, B.; Obreza, T. 2019–2020 Florida Citrus Production Guide: Fertilizer Application Methods; University of Florida George A Smathers Libraries: Gainesville, FL, USA, 2019. [Google Scholar]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- Dou, H.; Alva, A.K. Nitrogen uptake and growth of two citrus rootstock seedlings in a sandy soil receiving different controlled-release fertilizer sources. Biol. Fertil. Soils 1998, 26, 169–172. [Google Scholar] [CrossRef]
- Schumann, A. Optimizing Citrus Fertigation. 2014. Available online: https://citrusagents.ifas.ufl.edu/media/crecifasufledu/citrus-agents/growers-institutes/2014/Schumann--Citrus-Institute-2014V2.pdf (accessed on 18 October 2022).
- El Bey, N.; Aounallah, M.K.; Chammam, M.R.; Bettaieb, T. Effect of controlled-release fertilizers (CRF) on vegetative growth, nutritional status, fruit yield and quality of ‘Maltaise Ballerin’ orange trees. Plant Physiol. Rep. 2021, 26, 699–708. [Google Scholar] [CrossRef]
- Gao, Y.; Song, X.; Liu, K.; Li, T.; Zheng, W.; Wang, Y.; Liu, Z.; Zhang, M.; Chen, Q.; Li, Z.; et al. Mixture of controlled-release and conventional urea fertilizer application changed soil aggregate stability, humic acid molecular composition, and maize nitrogen uptake. Sci. Total Environ. 2021, 789, 147778. [Google Scholar] [CrossRef] [PubMed]
- Bettaga, N.; Ben Mimoun, M. Effects of controlled-release fertilizer on fruit yield and quality of clementine citrus trees. Acta Hortic. 2010, 868, 429–432. [Google Scholar] [CrossRef]
- Vashisth, T.; Grosser, J. Comparison of Controlled Release Fertilizer (CRF) for Newly Planted Sweet Orange Trees under Huanglongbing Prevalent Conditions. J. Hortic. 2018, 5, 244. [Google Scholar] [CrossRef]
- Singerman, A. Cost of Producing Processed Oranges in Southwest Florida in 2017/18; Citrus Research and Education Center, University of Florida, IFAS: Lake Alfred, FL, USA, 2019. [Google Scholar]
- Willis, L.E.; Davies, F.S.; Graetz, D.A. Fertigation and Growth of Young ‘Hamlin’ Orange Trees in Florida. HortScience 1991, 26, 106–109. [Google Scholar] [CrossRef]
- Syvertsen, J.; Jifon, J. Frequent fertigation does not affect citrus tree growth, fruit yield, nitrogen uptake, and leaching losses. Annu. Meet. Fla. State Hortic. Soc. 2001, 114, 88–93. [Google Scholar]
- Obreza, T.A.; Sartain, J.B. Improving Nitrogen and Phosphorus Fertilizer Use Efficiency for Florida’s Horticultural Crops. HortTechnology 2010, 20, 23–33. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2020, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Humble, G.D.; Hsiao, T.C. Light-dependent Influx and Efflux of Potassium of Guard Cells during Stomatal Opening and Closing. Plant Physiol. 1970, 46, 483–487. [Google Scholar] [CrossRef]
- Chen, M.; Mishra, S.; Heckathorn, S.A.; Frantz, J.M.; Krause, C. Proteomic analysis of Arabidopsis thaliana leaves in response to acute boron deficiency and toxicity reveals effects on photosynthesis, carbohydrate metabolism, and protein synthesis. J. Plant Physiol. 2014, 171, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alcántara, B.; Quiñones, A.; Primo-Millo, E.; Legaz, F. Nitrogen remobilization response to current supply in young citrus trees. Plant Soil 2011, 342, 433–443. [Google Scholar] [CrossRef]
- Feigenbaum, S.; Bielorai, H.; Erner, Y.; Dasberg, S. The fate of 15N labeled nitrogen applied to mature citrus trees. Plant Soil 1987, 97, 179–187. [Google Scholar] [CrossRef]
- USDA. Supplement to the Soil Survey of Osceola County Area, Florida; United States Department of Agriculture: Washington, DC, USA, 2011; p. 35.
- Morgan, K.T.; Kadyampakeni, D.M.; Zekri, M.; Schumann, A.W.; Vashisth, T.; Obreza, T.A. 2020–2021 Florida Citrus Production Guide: Nutrition Management for Citrus Trees. 2020. Available online: https://www.researchgate.net/publication/344061579_2020-2021_Florida_Citrus_Production_Guide_Nutrition_Management_for_Citrus_Trees (accessed on 18 October 2022).
- Isaac, R.A.; Johnson, W.C. Elemental Analysis of Plant Tissue by Plasma Emission Spectroscopy: Collaborative Study. J. AOAC Int. 1985, 68, 499–505. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2022. [Google Scholar]

| N | ||||
| g tree−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 40.64 ± 7.14 abc | 67.51 ± 14.9 a | 66.42 ± 13.31 a | 45.35 ± 10.88 abc |
| Twig | 26.81 ± 7.11 abcd | 50.75 ± 15.27 abc | 39.4 ± 4.89 ab | 40.89 ± 13.86 abc |
| Secondary Branch | 46.67 ± 13.03 ab | 59.59 ± 12.84 ab | 51.57 ± 8.57 a | 56.72 ± 7.17 a |
| Primary Branch | 47.43 ± 12.17 a | 48.55 ± 9.36 abc | 54.07 ± 8.53 a | 48.38 ± 14.41 ab |
| Trunk | 12.34 ± 1.51 bcd | 14.34 ± 2.16 bc | 12.67 ± 1.33 bc | 12.40 ± 1.99 bc |
| Structural Root | 38.31 ± 4.22 abc | 46.38 ± 15.56 abc | 50.68 ± 3.46 a | 55.69 ± 6.78 a |
| Lateral Root | 10.68 ± 2.22 cd | 11.30 ± 2.22 bc | 10.50 ± 2.87 bc | 12.40 ± 4.37 bc |
| Feeder Root | 2.15 ± 0.84 d | 2.19 ± 0.76 c | 2.88 ± 0.65 c | 3.9 ± 0.24 c |
| Total | 225.07 ± 1.81 | 300.64 ± 5.55 | 288.22 ± 5.17 | 275.77 ± 4.60 |
| P | ||||
| g tree−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 2.6 ± 0.40 ab | 4.00 ± 0.87 a | 4.11 ± 0.97 a | 3.0 ± 0.76 a |
| Twig | 1.34 ± 0.31 abc | 2.78 ± 0.91 ab | 2.2 ± 0.41 ab | 1.98 ± 0.65 abc |
| Secondary Branch | 2.83 ± 0.68 ab | 3.87 1.18 a | 2.95 ± 0.58 ab | 3.91 ± 0.31 a |
| Primary Branch | 3.14 ± 0.77 a | 2.96 ± 0.62 ab | 4.00 ± 1.09 a | 2.94 ± 0.72 ab |
| Trunk | 0.93 ± 0.16 bc | 0.91 ± 0.15 ab | 0.98± 0.24 b | 0.81± 0.11 bc |
| Structural Root | 1.64 ± 0.28 abc | 1.90 ± 0.82 ab | 1.88 ± 0.44 ab | 2.02 ± 0.40 abc |
| Lateral Root | 0.46 ± 0.09 c | 0.42 ± 0.10 b | 0.45 ± 0.20 b | 0.47 ± 0.13 c |
| Feeder Root | 0.2 ± 0.08 c | 0.16 ± 0.05 b | 0.18 ± 0.04 b | 0.31 ± 0.03 c |
| Total | 13.19 ± 0.23 | 17.03 ± 1.50 | 16.78 ± 1.45 | 15.53 ± 3.38 |
| K | ||||
| g tree−1 | ||||
| Tree component | Control | CRF | L | |
| Leaf | 16.33 ± 2.55 a | 26.19 ± 5.58 a | 33.91 ± 8.82 a | |
| Twig | 7.59 ± 2.23 bcd | 19.17 ± 6.31 abc | 19.4 ± 4.48 abc | |
| Secondary Branch | 13.88 ± 1.90 ab | 21.04 ± 4.92 ab | 18.96 ± 3.55 ab | |
| Primary Branch | 13.64 ± 2.67 abc | 22.52 ± 5.22 ab | 23.63 ± 5.69 abc | |
| Trunk | 5.52 ± 0.95 cd | 5.83 ± 1.06 bc | 6.18 ± 1.16 bc | |
| Structural Root | 9.05 ± 1.16 abcd | 10.04 ± 3.64 abc | 13.36 ± 1.63 bc | |
| Lateral Root | 3.29 ± 0.66 d | 3.33 ± 0.70 bc | 3.59 ± 1.22 bc | |
| Feeder Root | 0.83 ± 0.32 d | 0.78 ± 0.25 c | 1.17 ± 0.29 c | |
| Total | 70.17 ± 0.58 | 108.96 ± 3.93 | 120.25 ± 4.01 | |
| Mg | ||||
| g tree−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 3.09 ± 0.50 a | 5.17 ± 1.22 a | 4.98 ± 0.97 a | 3.43 ± 0.78 a |
| Twig | 1.89 ± 0.59 abc | 3.5 ± 0.91 abc | 4.33 ± 0.68 a | 3.59 ± 1.03 a |
| Secondary Branch | 2.97 ± 0.71 ab | 5.19 ± 1.07 a | 2.79 ± 0.44 a | 3.93 ± 0.87 a |
| Primary Branch | 3.33 ± 0.60 a | 3.66 ± 0.51 ab | 3.65 ± 0.47 a | 3.02 ± 0.67 ab |
| Trunk | 0.57 ± 0.22 c | 0.82 ± 0.30 bc | 0.67 ± 0.16 bc | 0.49 ± 0.19 b |
| Primary Root | 0.90 ± 0.12 bc | 1.10 ± 0.41 bc | 1.19 ± 0.22 bc | 1.24 ± 0.32 ab |
| Secondary Root | 0.27 ± 0.04 c | 0.31 ± 0.06 c | 0.31 ± 0.11 c | 0.31 ± 0.07 b |
| Feeder Root | 0.22 ± 0.09 c | 0.22 ± 0.07 c | 0.23 ± 0.05 c | 0.35 ± 0.03 b |
| Total | 13.268 ± 0.33 | 20.01 ± 1.96 | 18.17 ± 1.90 | 16.40 ± 1.67 |
| Ca | ||||
| g tree−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 49.71 ± 9.34 abc | 83.65 ± 18.45 abc | 63.39 ± 12.60 bc | 47.77 ± 9.53 bcd |
| Twig | 36.42 ± 9.76 bc | 71.09 ± 24.22 abcd | 49.9 ± 7.06 bcd | 54.23 ± 15.39 abcd |
| Secondary Branch | 85.05 ± 14.59 a | 138.67 ± 23.22 a | 87.72 ± 17.63 ab | 118.75 ± 25.01 a |
| Primary Branch | 97.40 ± 19.79 a | 103.83 ± 13.01 ab | 122.57 ± 22.45 a | 106.77 ± 23.41 ab |
| Trunk | 30.46 ± 3.73 bc | 32.32 ± 5.41 bcd | 27.63 ± 3.29 cd | 27.69 ± 5.44 cd |
| Structural Root | 60.93 ± 4.34 ab | 72.91 ± 24.80 abcd | 62.56 ± 7.43 bc | 85.98 ± 9.17 abc |
| Lateral Root | 16.99 ± 3.68 bc | 18.38 ± 4.14 cd | 15.64 ± 5.04 cd | 21.44 ± 7.05 cd |
| Feeder Root | 3.54 ± 1.46 c | 3.15 ± 1.06 d | 3.34 ± 0.65 d | 5.59 ± 0.61 d |
| Total | 380.53 ± 1.24 | 524.05 ± 8.08 | 432.79 ± 7.60 | 468.24 ± 7.57 |
| S | ||||
| g tree−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 4.78 ± 0.87 a | 7.96 ± 1.90 ab | 6.59 ± 1.39 ab | 4.64 ± 1.01 ab |
| Twig | 4.92 ± 1.52 a | 9.69 ± 2.31 a | 7.42 ± 1.19 a | 6.19 ± 1.72 a |
| Secondary Branch | 3.63 ± 0.89 ab | 5.05 ± 1.09 abc | 3.67 ± 0.57 bc | 4.39 ± 0.76 abc |
| Primary Branch | 3.49 ± 0.87 ab | 3.54 ± 0.44 bc | 4.73 ± 1.02 ab | 3.55 ± 1.05 abc |
| Trunk | 0.93 ± 0.13 b | 1.03 ± 0.17 c | 0.81 ± 0.09 c | 0.82 ± 0.15 bc |
| Structural Root | 2.71 ± 0.27 ab | 3.43 ± 1.19 bc | 3.37 ± 0.34 bc | 3.87 ± 0.63 abc |
| Lateral Root | 0.92 ± 0.16 b | 1.07 ± 0.20 c | 0.88 ± 0.27 c | 1.03 ± 0.30 abc |
| Feeder Root | 0.22 ± 0.07 b | 0.24 ± 0.08 c | 0.27 ± 0.06 c | 0.36 ± 0.04 c |
| Total | 21.63 ± 0.53 | 32.04 ± 2.50 | 27.78 ± 2.12 | 24.89 ± 1.63 |
| B | ||||
| mg kg−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 99.77 ± 20.85 a | 199.19 ± 58.39 a | 115.65 ± 24.16 a | 92.58 ± 20.11 a |
| Twig | 29.16 ± 9.14 bc | 52.38 ± 17.25 b | 35.68 ± 3.57 bcd | 41.02 ± 12.78 abc |
| Secondary Branch | 52.70 ± 8.74 b | 85.73 ± 18.14 b | 61.16 ± 9.01 bc | 74.04 ± 17.64 ab |
| Primary Branch | 55.58 ± 13.56 ab | 77.05 ± 14.13 b | 81.80 ± 14.40 ab | 70.92 ± 26.73 abc |
| Trunk | 14.00 ± 2.14 bc | 13.77 ± 2.40 b | 10.28 ± 1.43 d | 13.69 ± 2.10 bc |
| Structural Root | 40.42 ± 3.32 bc | 56.37 ± 20.04 b | 48.99 ± 5.16 bcd | 66.23 ± 9.86 abc |
| Lateral Root | 13.67 ± 2.58 bc | 15.54 ± 3.43 b | 12.51 ± 3.65 cd | 17.44 ± 5.42 bc |
| Feeder Root | 2.35 ± 0.82 c | 3.09 ± 1.15 b | 3.22 ± 0.73 d | 4.43 ± 0.35 c |
| Total | 307.70 ± 1.77 | 503.17 ± 9.18 | 369.32 ± 6.85 | 380.40 ± 5.79 |
| Zn | ||||
| mg kg−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 62.45 ± 8.47 b | 109.01 ± 29.73 b | 93.43 ± 23.72 b | 71.35 ± 20.47 b |
| Twig | 756.91 ± 254.33 a | 1467.75 ± 403.15 a | 815.33 ± 128.33 a | 870.69 ± 342.09 a |
| Secondary Branch | 92.43 ± 15.89 b | 142.7 6± 30 31 b | 96.53 ± 13.51 b | 127.33 12.56 b |
| Primary Branch | 113.91 ± 27.63 b | 102.58 ± 11.14 b | 169.62 ± 54.63 b | 86.82 ± 24.17 b |
| Trunk | 37.67 ± 9.94 b | 36.44 ± 17.89 b | 15.70 ± 3.66 b | 21.72 ± 6.13 b |
| Structural Root | 70.97 ± 9.83 b | 127.51 ± 45.64 b | 119.87 ± 14.28 b | 131.17 ± 21.74 b |
| Lateral Root | 34.87 ±c4.05 b | 49.10 ± 7.81 b | 28.78 ± 6.13 b | 43.17 ± 10.13 b |
| Feeder Root | 4.46 ± 1.39 b | 5.05 ± 1.93 b | 5.39 ± 1.13 b | 7.08 ± 0.65 b |
| Total | 1173.31 ± 7.36 | 2040.23 ± 31.68 | 1344.69 ± 21.45 | 1359.36 ± 22.08 |
| Mn | ||||
| mg kg−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 73.07 ± 11.10 b | 121.10 ± 34.10 b | 104.64 ± 22.53 b | 80.04 ± 19.03 b |
| Twig | 453.87 ± 157.22 a | 931.26 ± 265.62 a | 843.84 ± 157.15 a | 686.03 ± 246.98 a |
| Secondary Branch | 32.95 ± 11.28 b | 35.23 ± 4.32 b | 32.16 ± 7.24 b | 39.76 ± 3.60 b |
| Primary Branch | 38.26 ± 10.90 b | 32.56 ± 1.24 b | 79.98 ± 44.31 b | 29.90 ± 9.83 b |
| Trunk | 27.62 ± 13.46 b | 12.91 ± 6.97 b | 4.82 ± 1.13 b | 11.43 ± 2.36 b |
| Structural Root | 18.95 ± 5.85 b | 23.25 ± 11.78 b | 61.36 ± 36.51 b | 23.47 ± 4.25 b |
| Lateral Root * | 9.60 ± 2.30 b | 16.26 ± 3.50 b | 4.67 ± 0.69 b | 9.98 ± 1.15 b |
| Feeder Root | 1.26 ± 0.33 b | 1.67 ± 0.66 b | 1.75 ± 0.32 b | 1.89 ± 0.17 b |
| Total | 655.63 ± 6.34 | 1174.27 ± 29.55 | 1133.25 ± 26.47 | 882.53 ± 24.53 |
| Fe | ||||
| mg kg−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 144.04 ± 21.07 b | 251.31 ± 59.80 b | 215.68 ± 40.79 b | 218.14 ± 73.26 b |
| Twig | 2060 ± 863.63 a | 3305.2 ± 1182.37 a | 2827.4 ± 507.11 a | 2380.39 ± 818.77 a |
| Secondary Branch | 140.61 ± 22.13 b | 197.23 ± 33.46 b | 149.03 ± 28.58 b | 170.09 ± 25.40 b |
| Primary Branch | 151.01 ± 28.07 b | 204.10 ± 67.91 b | 197.80 ± 35.09 b | 137.59 ± 25.97 b |
| Trunk | 122.70 ± 29.65 b | 98.34 ± 24.10 b | 74.21 ± 10.74 b | 87.74 ±17.99 b |
| Structural Root | 304.55 ± 77.35 b | 473.65 ± 202.92 b | 403.70 ± 57.21 b | 342.15 ± 31.18 b |
| Lateral Root | 115.10 ± 24.12 b | 123.89 ± 14.00 b | 88.32 ± 18.88 b | 104.99 ± 29.36 b |
| Feeder Root | 6.22 ± 2.07 b | 7.34 ± 2.68 b | 7.31 ± 1.53 b | 11.49 ± 0.86 b |
| Total | 3044.27 ± 16.42 | 4661.09 ± 47.00 | 3963.51 ± 43.75 | 3452.63 ± 39.62 |
| Cu | ||||
| mg kg−1 | ||||
| Tree component | Control | CRF | L | LW |
| Leaf | 551.08 ± 77.48 a | 908.36 ± 310.29 a | 880.74 ± 249.09 a | 660.71 ± 194.10 ab |
| Twig | 260.7 ± 69.64 ab | 425.37 ± 149.26 abc | 377.82 ± 52.99 ab | 377.4 ± 94.32 ab |
| Secondary Branch | 574.28 ± 109.89 a | 1000.56 ± 127.06 a | 653.89 ± 114.90 ab | 859.96 ± 102.49 a |
| Primary Branch | 570.21 ± 131.41 a | 663.72 ± 81.37 ab | 1113.84 ± 346.38 a | 790.24 ± 325.53 a |
| Trunk | 123.32 ± 41.76 b | 86.529 ± 44.81 bc | 25.97 ± 11.06 b | 98.32 ± 62.54 b |
| Structural Root | 58.39 ± 32.95 b | 42.88 ± 16.66 bc | 35.77 ± 8.15 b | 40.88 ± 3.62 b |
| Lateral Root | 9.49 ± 1.34 b | 17.98 ± 4.28 c | 9.83 ± 3.34 b | 13.64 ± 3.90 b |
| Feeder Root | 22.64 ± 6.00 b | 33.97 ± 14.97 c | 35.89 ± 8.29 b | 49.85 ± 7.69 b |
| Total | 2170.15 ± 198.15 | 3179.4 ± 1649.66 | 3133.80 ± 1735.76 | 2891.04 ± 1520.87 |
| Treatment | Total (DW) |
|---|---|
| kg | |
| Control | 18.61 ± 1.81 |
| CRF | 26.47 ± 4.88 |
| L | 18.45 ± 2.74 |
| LW | 20.98 ± 3.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallman, L.M.; Kadyampakeni, D.M.; Fox, J.-P.; Wright, A.L.; Rossi, L. Root-Shoot Nutrient Dynamics of Huanglongbing-Affected Grapefruit Trees. Plants 2022, 11, 3226. https://doi.org/10.3390/plants11233226
Hallman LM, Kadyampakeni DM, Fox J-P, Wright AL, Rossi L. Root-Shoot Nutrient Dynamics of Huanglongbing-Affected Grapefruit Trees. Plants. 2022; 11(23):3226. https://doi.org/10.3390/plants11233226
Chicago/Turabian StyleHallman, Lukas M., Davie M. Kadyampakeni, John-Paul Fox, Alan L. Wright, and Lorenzo Rossi. 2022. "Root-Shoot Nutrient Dynamics of Huanglongbing-Affected Grapefruit Trees" Plants 11, no. 23: 3226. https://doi.org/10.3390/plants11233226
APA StyleHallman, L. M., Kadyampakeni, D. M., Fox, J.-P., Wright, A. L., & Rossi, L. (2022). Root-Shoot Nutrient Dynamics of Huanglongbing-Affected Grapefruit Trees. Plants, 11(23), 3226. https://doi.org/10.3390/plants11233226








