Irrigation and Crop Load Management Lessen Rain-Induced Cherry Cracking
Abstract
:1. Introduction
2. Results
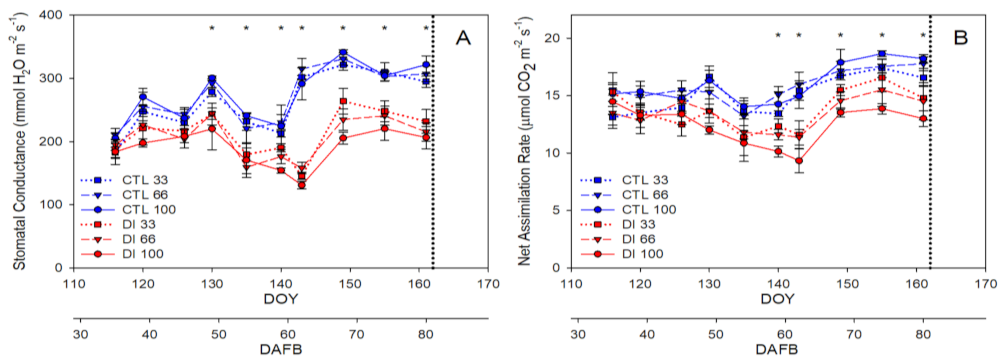
2.1. Soil-Plant-Atmosphere Water Status
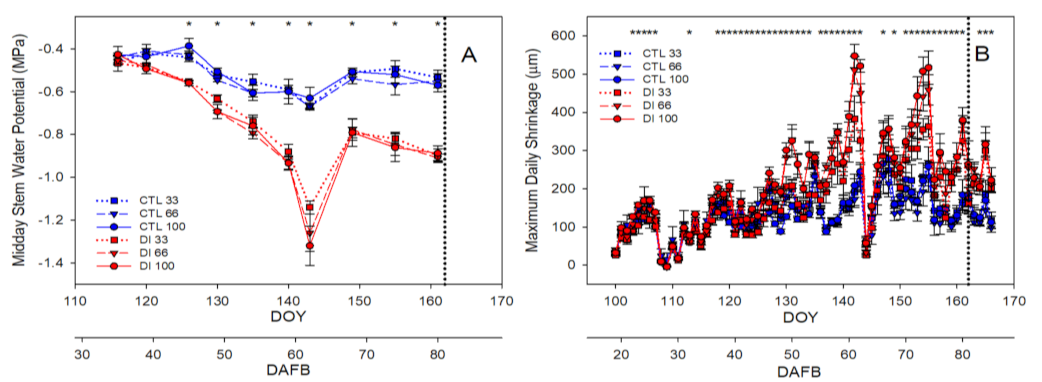
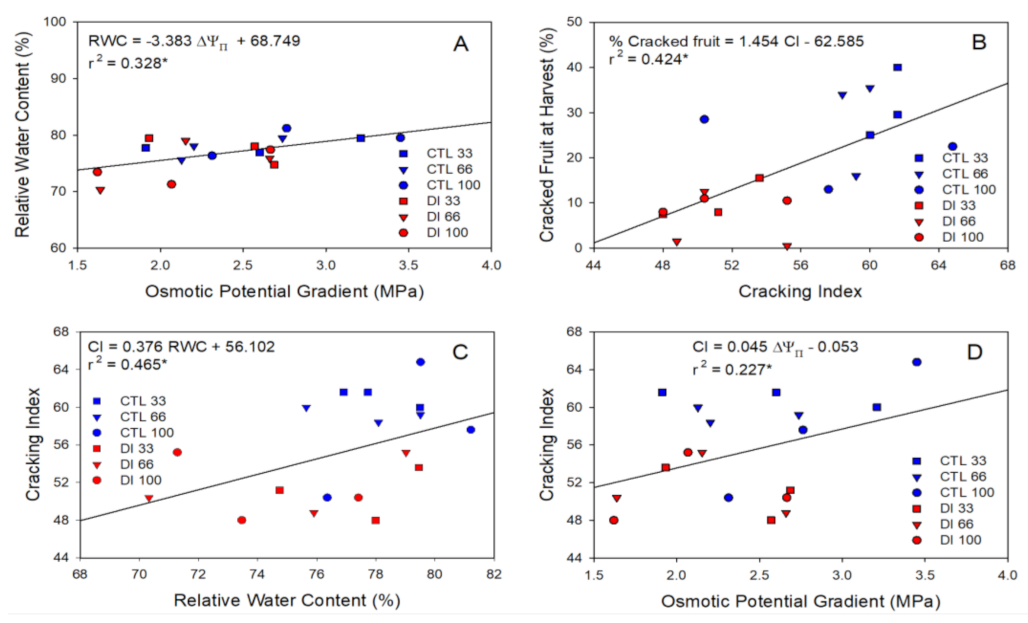
2.2. Fruit Water Relations
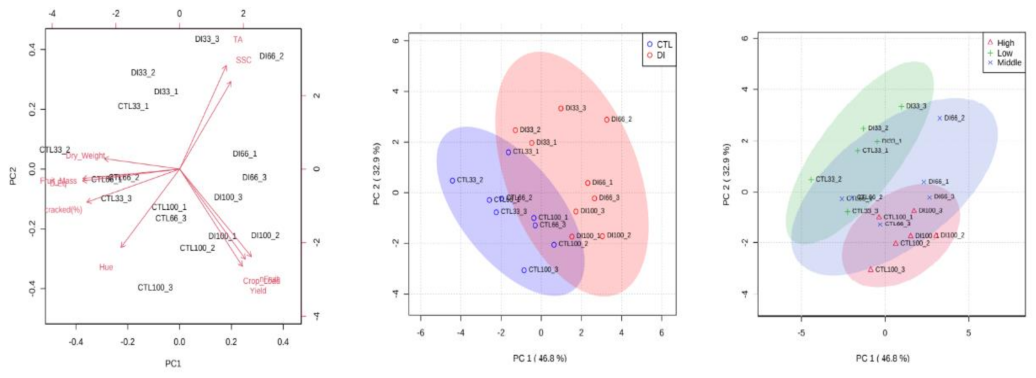
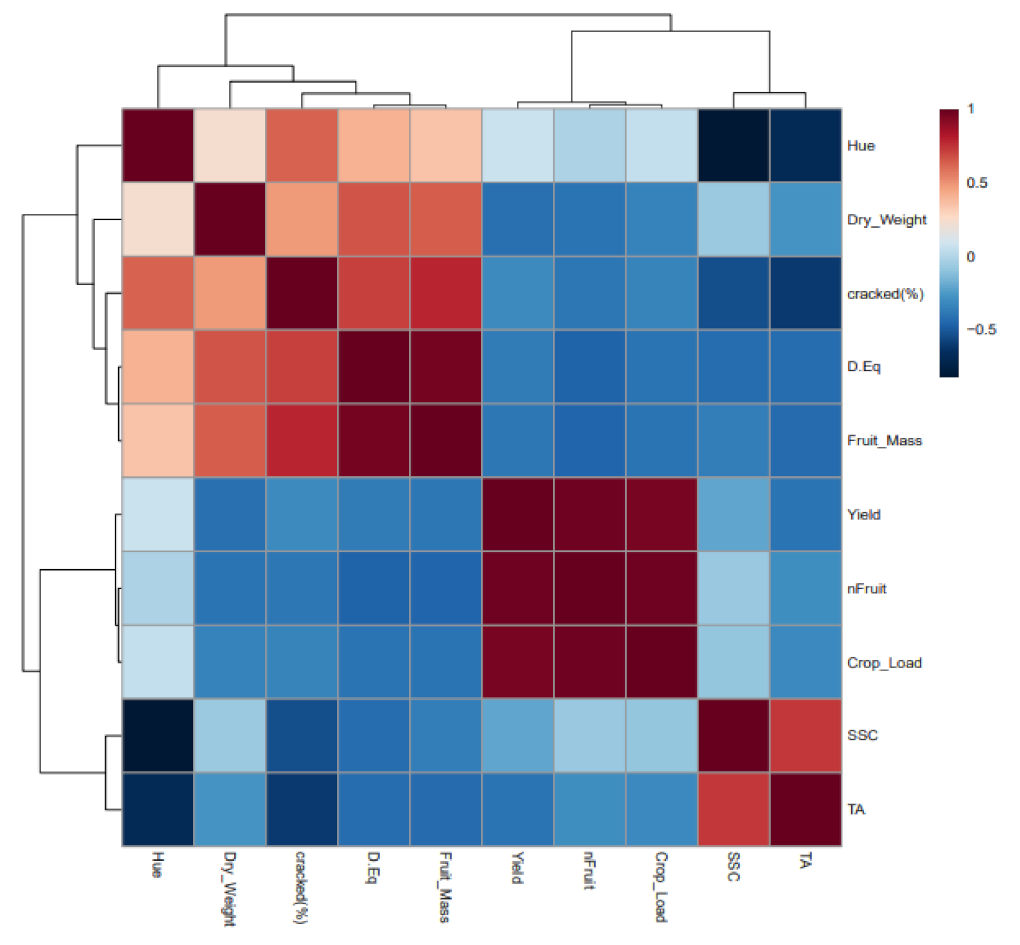
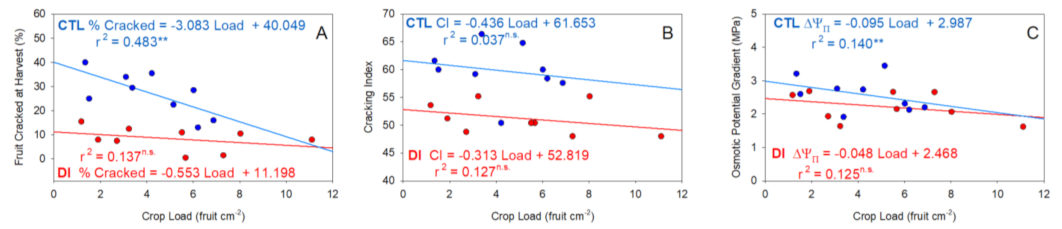
2.3. Fruit Yield and Quality
2.4. Cracking Susceptibility
3. Discussion
4. Materials and Methods
4.1. Experimental Site, Plant Material and Treatments
4.2. Soil, Tree, and Fruit Water Status Measurements
4.3. Fruit Yield and Quality
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Karamanoli, K.; Lazaridou, A.; Martens, S.; Molassiotis, A. Sweet Cherry Fruit Cracking: Follow-up Testing Methods and Cultivar-Metabolic Screening. Plant Methods 2020, 16, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangdal, E.; Hovland, K.L.; Børve, J.; Sekse, L.; Slimestad, R. Foliar Application of Calcium Reduces Postharvest Decay in Sweet Cherry Fruit by Various Mechanisms. Acta Hortic. 2008, 768, 143–148. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S.; Hinz, M.; Bukovac, M.J. Studies on Water Transport through the Sweet Cherry Fruit Surface: Characterizing Conductance of the Cuticular Membrane Using Pericarp Segments. Planta 2000, 212, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Measham, P.F.; Wilson, S.J.; Gracie, A.J.; Bound, S.A. Tree Water Relations: Flow and Fruit. Agric. Water Manag. 2014, 137, 59–67. [Google Scholar] [CrossRef]
- Peschel, S.; Beyer, M.; Knoche, M. Surface Characteristics of Sweet Cherry Fruit: Stomata-Number, Distribution, Functionality and Surface Wetting. Sci. Hortic. 2003, 97, 265–278. [Google Scholar] [CrossRef]
- Measham, P.; Bound, S.; Gracie, A.; Wilson, S. Incidence and Type of Cracking in Sweet Cherry (Prunus avium L.) Are Affected by Genotype and Season. Crop Pasture Sci. 2009, 60, 1002–1008. [Google Scholar] [CrossRef]
- Quero-García, J.; Letourmy, P.; Campoy, J.A.; Branchereau, C.; Malchev, S.; Barreneche, T.; Dirlewanger, E. Multi-Year Analyses on Three Populations Reveal the First Stable QTLs for Tolerance to Rain-Induced Fruit Cracking in Sweet Cherry (Prunus avium L.). Hortic. Res. 2021, 8, 136. [Google Scholar] [CrossRef]
- Wermund, U.; Holland, A.; Reardon, S. Cracking Susceptibility of Sweet Cherries in the United Kingdom in Relation to Calcium Application and Cover Systems. Acta Hortic. 2005, 667, 475–482. [Google Scholar] [CrossRef]
- Measham, P.F.; Bound, S.A.; Gracie, A.J.; Wilson, S.J. Crop Load Manipulation and Fruit Cracking in Sweet Cherry (Prunus avium L.). Adv. Hortic. Sci. 2012, 26, 25–31. [Google Scholar] [CrossRef]
- Edin, M.; Lichou, J.; Saunier, R.; Garcin, A. Cerise, les Variétés et Leur Conduite; CTIFL, Ed.; CTIFL: Paris, France, 1997; ISBN 978-2-87911-084-4. [Google Scholar]
- Sekse, L. Fruit Cracking Mechanisms in Sweet Cherries (Prunus avium L.)—A Review. Acta Hortic. 1998, 468, 637–648. [Google Scholar] [CrossRef]
- Galindo, A.; Rodríguez, P.; Collado-González, J.; Cruz, Z.N.; Torrecillas, E.; Ondoño, S.; Corell, M.; Moriana, A.; Torrecillas, A. Rainfall Intensifies Fruit Peel Cracking in Water Stressed Pomegranate Trees. Agric. For. Meteorol. 2014, 194, 29–35. [Google Scholar] [CrossRef]
- Goodwin, I.; McClymont, L.; Green, S. The Effects of Water Deficits on Fruit Cracking and Sunburn Damage in ‘Cripps Pink’ Apple. Acta Hortic. 2022, 1335, 421–428. [Google Scholar] [CrossRef]
- Yan, G.; Youting, Y.; Ling, L.; Xin, Z.; Yuhui, B.; Huiqiong, M.; Lixinyi, W.; Bo, X. Study on the Effect Difference of Two Kinds of Control Techniques on Cracking Fruit of Qingcui Plum. IOP Conf. Ser. Earth Environ. Sci. 2021, 792, 012042. [Google Scholar] [CrossRef]
- Lang, G.A.; Sage, L.; Wilkinson, T. Ten Years of Studies on Systems to Modify Sweet Cherry Production Environments: Retractable Roofs, High Tunnels, and Rain-Shelters. Acta Hortic. 2016, 1130, 83–90. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. Influence of High Tunnel Microclimate on Fruit Quality and Calcium Concentration in ‘Santina’ Sweet Cherries in a Mediterranean Climate. Agronomy 2021, 11, 1186. [Google Scholar] [CrossRef]
- Balbontín, C.; Ayala, H.; Bastías, M.R.; Tapia, G.; Ellena, M.; Torres, C.; Yuri, J.A.; Quero-García, J.; Ríos, J.C.; Silva, H. Cracking in Sweet Cherries: A Comprehensive Review from a Physiological, Molecular, and Genomic Perspective. Chil. J. Agric. Res. 2013, 73, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, S.; Qu, Z.; Xiu, T.; Hu, Y.; Chen, C.; Lv, N. Reducing Cherry Rain-Cracking: Development and Characterization of Cold-Water Fish Gelatin Films Reinforced by Dual Rod-Spherical Nanoscale Structures Formed under Magnetic Fields. Food Hydrocoll. 2022, 128, 107579. [Google Scholar] [CrossRef]
- Heacox, L. Curb Cherry Cracking. Fruit Grow. 2001, 121, 16. [Google Scholar]
- Blanco, V.; Domingo, R.; Pérez-Pastor, A.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and Plant Water Indicators for Deficit Irrigation Management of Field-Grown Sweet Cherry Trees. Agric. Water Manag. 2018, 208, 83–94. [Google Scholar] [CrossRef]
- Conejero, W.; Ortuño, M.F.; Mellisho, C.D.; Torrecillas, A. Influence of Crop Load on Maximum Daily Trunk Shrinkage Reference Equations for Irrigation Scheduling of Early Maturing Peach Trees. Agric. Water Manag. 2010, 97, 333–338. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Crop Load Affects Maximum Daily Trunk Shrinkage of Plum Trees. Tree Physiol. 2007, 27, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.; Martínez-Hernández, G.B.; Artés-Hernández, F.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Water Relations and Quality Changes throughout Fruit Development and Shelf Life of Sweet Cherry Grown under Regulated Deficit Irrigation. Agric. Water Manag. 2019, 217, 243–254. [Google Scholar] [CrossRef]
- Gelly, M.; Marsal, J.; Mata, M.; Arbonès, A.; Rufat, J.; Girona, J. Fruit Water Potential: A Comparison Study to Other Water Potential Parameters. Acta Hortic. 2004, 646, 35–40. [Google Scholar] [CrossRef]
- McFadyen, L.M.; Hutton, R.J.; Barlow, E.W.R. Effects of Crop Load on Fruit Water Relations and Fruit Growth in Peach. J. Hortic. Sci. 1996, 71, 469–480. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Behboudian, M.H.; Dixon, J.; Neal, S.M.; Caspari, H.W. Improvement of Fruit Quality and Storage Potential of ‘Braeburn’ Apple through Deficit Irrigation. J. Hortic. Sci. Biotechnol. 2000, 75, 615–621. [Google Scholar] [CrossRef]
- Kelner, J.-J.; Regnard, J.-L.; Lauri, P.-É. Crop Load and Rootstock Effects on Maturation Rate and Harvest Quality of cv. Braeburn Apples. Fruits 2000, 55, 73–81. [Google Scholar]
- Moing, A.; Renaud, C.; Christmann, H.; Fouilhaux, L.; Tauzin, Y.; Zanetto, A.; Gaudillère, M.; Laigret, F.; Claverie, J. Is There a Relation between Changes in Osmolarity of Cherry Fruit Flesh or Skin and Fruit Cracking Susceptibility? J. Am. Soc. Hortic. Sci. 2004, 129, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.; Riedel, D.; Neuwald, D.A.; Knoche, M. Water Influx through the Wetted Surface of a Sweet Cherry Fruit: Evidence for an Associated Solute Efflux. Plants 2020, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Matthews, M.A.; Shackel, K.A. Growth and Water Transport in Fleshy Fruit. In Vascular Transport in Plants; Elsevier: Amsterdam, The Netherlands, 2005; pp. 181–197. ISBN 978-0-12-088457-5. [Google Scholar]
- Blaya-Ros, P.J.; Blanco, V.; Torres-Sánchez, R.; Domingo, R. Drought-Adaptive Mechanisms of Young Sweet Cherry Trees in Response to Withholding and Resuming Irrigation Cycles. Agronomy 2021, 11, 1812. [Google Scholar] [CrossRef]
- Grimm, E.; Knoche, M. Sweet Cherry Skin Has a Less Negative Osmotic Potential than the Flesh. J. Am. Soc. Hortic. Sci. 2015, 140, 472–479. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet Cherry Fruit Cracking Mechanisms and Prevention Strategies: A Review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Measham, P.F.; Gracie, A.J.; Wilson, S.J.; Bound, S.A. An Alternative View of Rain-Induced Cracking of Sweet Cherries (Prunus avium L.). Acta Hortic. 2014, 1020, 217–222. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Tindjau, R.; Wong, D.C.J.; Matzat, T.; Haslam, T.; Song, C.; Gambetta, G.A.; Kunst, L.; Castellarin, S.D. Drought Stress Modulates Cuticular Wax Composition of the Grape Berry. J. Exp. Bot. 2020, 71, 3126–3141. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Hu, X.; Zhang, Y.-L.; Liu, Y.; Wang, G.-L.; You, C.-X.; Li, Y.-Y.; Hao, Y.-J. An Apple Long-Chain Acyl-CoA Synthetase 2 Gene Enhances Plant Resistance to Abiotic Stress by Regulating the Accumulation of Cuticular Wax. Tree Physiol. 2020, 40, 1450–1465. [Google Scholar] [CrossRef]
- Quero-García, J.; Schuster, M.; López-Ortega, G.; Charlot, G. Sweet Cherries Varieties and Improvement. In Cherries: Botany, Production and Uses; Quero-García, J., Iezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Boston, MA, USA, 2017; ISBN 978-1-78064-837-8. [Google Scholar]
- Neilsen, G.H.; Neilsen, D.; Kappel, F.; Forge, T. Interaction of Irrigation and Soil Management on Sweet Cherry Productivity and Fruit Quality at Different Crop Loads That Simulate Those Occurring by Environmental Extremes. HortScience 2014, 49, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Bound, S.A.; Close, D.C.; Quentin, A.G.; Measham, P.F.; Whiting, M.D. Crop Load and Time of Thinning Interact to Affect Fruit Quality in Sweet Cherry. J. Agric. Sci. 2013, 5, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Marsal, J.; Lopez, G.; del Campo, J.; Mata, M.; Arbones, A.; Girona, J. Postharvest Regulated Deficit Irrigation in ‘Summit’ Sweet Cherry: Fruit Yield and Quality in the Following Season. Irrig. Sci. 2010, 28, 181–189. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. FAO Irrigation and drainage paper. In Crop Evapotranspiration-Guidelines for Computing Crop. Water Requirements; Food and Agriculture Organization: Rome, Italy, 1998; ISBN 9253042192. [Google Scholar]
- Marsal, J. Sweet Cherry. In Crop Yield Response Water; FAO Irrigation and Drainage; FAO: Rome, Italy, 2012; pp. 449–457. [Google Scholar]
- Fereres, E.; Martinich, D.A.; Aldrich, T.M.; Castel, J.; Holzapfel, E.; Schulbach, H. Drip Irrigation Saves Money in Young Almond Orchards. Calif. Agric. 1982, 36, 12–13. [Google Scholar]
- McCutchan, H.; Shackel, K.A. Stem-Water Potential as a Sensitive Indicator of Water Stress in Prune Trees (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Lechaudel, M. Croissance et Qualité Organoleptique de La Mangue (Mangifera indica): Analyse Expérimentale et Modélisation de L’effet de La Disponibilité Hydrique et Carbonée. Ph.D. Dissertation, Institut National Agronomique Paris-Grignon, Paris, France, 2004. [Google Scholar]
- Milad, R.E.; Shackel, K.A. Water Relations of Fruit End Cracking in French Prune (Prunus domestica L. cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 824–828. [Google Scholar] [CrossRef]
- Christensen, J.V. Cracking in Cherries: III. Determination of Cracking Susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]


| Yield (kg Tree−1) | n Fruit(n Fruit Tree−1) | FE (n Fruit cm−2 Trunk) | Double Fruit (%) | Cracked Fruit (%) | Cracking Index | WP (kg m−3) | |
|---|---|---|---|---|---|---|---|
| CTL33 | 8.13 b | 599 b | 2.35 b | 0.17 | 31.50 | 63.47 | 2.67 b |
| CTL66 | 18.19 a | 1401 ab | 5.34 ab | 0.17 | 28.50 | 59.73 | 6.07 a |
| CTL100 | 25.31 a | 1944 a | 7.04 a | 0.50 | 21.33 | 60.00 | 8.44 a |
| ANOVA | 0.005 | 0.006 | 0.025 | 0.492 | 0.412 | 0.153 | 0.004 |
| DI33 | 8.91 b | 680 b | 2.40 b | 1.00 | 10.33 | 53.87 | 4.75 b |
| DI66 | 20.06 ab | 1694 a | 6.09 ab | 0.83 | 4.83 | 50.67 | 10.70 ab |
| DI100 | 27.52 a | 2351 a | 9.28 a | 1.33 | 9.83 | 49.07 | 14.68 a |
| ANOVA | 0.007 | 0.004 | 0.019 | 0.911 | 0.354 | 0.092 | 0.007 |
| CTL | 17.21 | 1304 | 4.91 | 0.28 | 27.11 | 61.07 | 5.73 |
| DI | 18.83 | 1598 | 5.92 | 1.06 | 8.33 | 51.20 | 10.05 |
| ANOVA | 0.695 | 0.412 | 0.550 | 0.094 | 0.001 | 0.001 | 0.033 |
| Equatorial Diameter (mm) | Fresh Mass (g) | Dry Mass (g) | Relative Water Content (%) | Skin Color (hue°) | Soluble Solids Concentration (%) | Titratable Acidity (%) | |
|---|---|---|---|---|---|---|---|
| CTL33 | 31.73 a | 13.40 | 3.14 | 77.59 | 17.05 | 21.57 | 0.99 |
| CTL66 | 31.48 a | 13.01 | 2.94 | 78.43 | 21.98 | 20.50 | 0.94 |
| CTL100 | 30.48 b | 13.06 | 2.75 | 78.79 | 19.78 | 18.93 | 0.91 |
| ANOVA | 0.035 | 0.239 | 0.297 | 0.760 | 0.226 | 0.253 | 0.140 |
| DI33 | 31.05 a | 13.14 a | 3.17 | 76.21 | 10.33 b | 24.83 | 1.19 a |
| DI66 | 29.32 b | 11.82 b | 2.75 | 76.26 | 14.17 ab | 22.97 | 1.08 ab |
| DI100 | 29.15 b | 11.70 b | 3.03 | 74.05 | 16.60 a | 22.30 | 0.99 b |
| ANOVA | 0.014 | 0.016 | 0.482 | 0.706 | 0.047 | 0.089 | 0.045 |
| CTL | 31.23 | 13.17 | 2.94 | 78.27 | 19.60 | 20.33 | 0.94 |
| DI | 29.84 | 12.21 | 2.98 | 75.51 | 13.70 | 23.37 | 1.09 |
| ANOVA | 0.004 | 0.004 | 0.825 | 0.043 | 0.002 | 0.002 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Irrigation and Crop Load Management Lessen Rain-Induced Cherry Cracking. Plants 2022, 11, 3249. https://doi.org/10.3390/plants11233249
Blanco V, Blaya-Ros PJ, Torres-Sánchez R, Domingo R. Irrigation and Crop Load Management Lessen Rain-Induced Cherry Cracking. Plants. 2022; 11(23):3249. https://doi.org/10.3390/plants11233249
Chicago/Turabian StyleBlanco, Victor, Pedro J. Blaya-Ros, Roque Torres-Sánchez, and Rafael Domingo. 2022. "Irrigation and Crop Load Management Lessen Rain-Induced Cherry Cracking" Plants 11, no. 23: 3249. https://doi.org/10.3390/plants11233249






