Evaluating Germplasm of Cultivated Oat Species from the VIR Collection under the Russian Northwest Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Description of Characters Valuable for Breeding
2.2. Description of Biochemical Indicators
2.3. Statistical Processing of the Results Obtained
3. Material and Methods
3.1. Research Materials
3.2. Assessment of Useful Agronomic Characters
3.3. Biochemical Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urubkov, S.A.; Khovanskaya, S.S.; Dremina, N.V.; Smirnov, S.O. Analysis of the chemical composition and nutritional value of grain raw materials for the production of baby food products. Food Ind. 2018, 8, 16–20. (In Russian) [Google Scholar]
- Lehtinen, P.; Kaukovirta-Norja, A. Oat lipids, enzymes, and quality. In Oats: Chemistry and Technology; Webster, F.H., Wood, P.J., Eds.; AAAC International Press: Toronto, ON, Canada, 2011; pp. 143–156. [Google Scholar] [CrossRef]
- Leonova, S.; Shelenga, T.; Hamberg, M.; Konarev, A.; Loskutov, I.; Carlsson, A. Analysis of oil composition in cultivars and wild species of oat (Avena sp.). Agric. Food Chem. 2008, 56, 7983–7991. [Google Scholar] [CrossRef] [PubMed]
- Loskutov, I.G. Oats: Functional properties and features of use. Bak. Confect. 2016, 3, 17. (In Russian) [Google Scholar]
- Bağcı, A.; Geçgel, Ü.; Özcan, M.M.; Dumlupınar, Z.; Uslu, N. Oil contents and fatty acid composition of oat (Avena sativa L.) seed and oils. J. Agroaliment. Process. Technol. 2019, 25, 181–186. [Google Scholar]
- Shelenga, T.V.; Kerv, Y.A.; Perchuk, I.N.; Solovyeva, E.A.; Khlestkina, E.K.; Loskutov, G.I.; Konarev, A.V. The potential of small grains crops in enhancing biofortification breeding strategies for human health benefit. Agronomy 2021, 11, 1420. [Google Scholar] [CrossRef]
- Vargach, Y.I.; Mertovishcheva, M.E.; Loskutov, I.G. Antioxidant activity of grain and oat films. Pomic. Small Fruits Cult. Russ. 2016, 47, 57–61. (In Russian) [Google Scholar]
- Özcan, M.M.; Bağcı, A.; Dursun, N.; Gezgin, S.; Hamurcu, M.; Dumlupınar, Z.; Uslu, N. Macro and micro element contents of several oat (Avena sativa L.) Genotype and Variety Grains. Iran. Chem. Chem. Eng. 2017, 36, 73–79. [Google Scholar]
- Bityutskii, N.P.; Yakkonen, K.L.; Loskutov, I.G. Content of iron, zinc and manganese in grains of Triticum aestivum, Secale cereale, Hordeum vulgare and Avena sativa cultivars registered in Russia. Genet. Resour. Crop Evol. 2017, 64, 1955–1961. [Google Scholar] [CrossRef]
- Abugalieva, A.I.; Loskutov, I.G.; Savin, T.V.; Chudinov, V.A. Evaluation of naked oat accessions from the VIR collection for their qualitative characteristics in Kazakhstan. Proc. Appl. Bot. Genet. Breed. 2021, 182, 9–21. (In Russian) [Google Scholar] [CrossRef]
- Abugalieva, A.I. The content of starch and amylose in the grain of oat varieties of Kazakhstan. Biotechnol. Theory Pract. 2011, 2, 25–31. (In Russian) [Google Scholar]
- Oat Grain Chemical Composition. Available online: http://agro-portal.su/oves/2607-himicheskiy-sostav-zerna-ovsa.html (accessed on 2 May 2022).
- Brownlee, I.A. The physiological roles of dietary fiber. Food Hydrocoll. 2011, 25, 238–250. [Google Scholar] [CrossRef]
- Gematdinova, V.M.; Kanarsky, A.V.; Kanarskaya, Z.A.; Smetanskaya, I.I. The effect of alkaline and enzymatic processing of grain on the yield of beta-glucan. Voronezh State Univ. Eng. Technol. Bull. 2017, 79, 164–168. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Loskutov, I.G.; Polonskiy, V.I. Selection for the content of β-glucans in oat grain as a promising direction for obtaining healthy food products, raw materials and forage. Agric. Biol. 2017, 52, 646–657. (In Russian) [Google Scholar] [CrossRef]
- Shvachko, N.A.; Loskutov, I.G.; Semilet, T.V.; Popov, V.S.; Kovaleva, O.N.; Konarev, A.V. Bioactive components in oat and barley grain as a promising breeding trend for functional food production. Molecules 2021, 26, 2260. [Google Scholar] [CrossRef]
- Gilissen, L.J.W.J.; Meer, I.M.V.; Smulders, M.J.M. Why oats are safe and healthy for celiac disease patients. Med. Sci. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Loskutov, I.G. Oat (Avena L.). Distribution, Taxonomy, Evolution and Breeding Value; VIR: St. Petersburg, Russia, 2007; p. 336. (In Russian) [Google Scholar]
- Vavilov, N.I. Geographical Variability of Plants; Selected Works in 5 Volumes; Nauka: Moscow, Russia, 1965; Volume 5. (In Russian) [Google Scholar]
- Merezhko, A.F. A System for the Genetic study of Source Material for Plant Breeding; Methodical Instructions: Leningrad, Russia, 1984; p. 69. (In Russian) [Google Scholar]
- Horeva, V.I.; Shelenga, T.V.; Blinova, E.V.; Konarev, A.V.; Loskutov, I.G. Oats. Biochemical characteristics of samples. In Catalog of the World Collection VIR; VIR: St. Petersburg, Russia, 2018. (In Russian) [Google Scholar]
- Grib, S.I.; Kadyrova, M.A. The relationship of plant height with elements of productivity in barley during breeding for lodging resistance. In Proceedings of the Conference: Increasing the Resistance of Grain Crops to Lodging, Zhodino, Belarus, 15–17 May 1979; pp. 61–66. [Google Scholar]
- Pleshkov, B.P. Biochemistry of Agricultural Plants, 5th ed.; Agropromizdat: Moscow, Russia, 1987; p. 486. (In Russian) [Google Scholar]
- Loskutov, I.G.; Kovaleva, O.N.; Blinova, E.V. Guidelines for the Study and Conservation of the Global Collection of Barley and Oats; VIR: St. Petersburg, Russia, 2012; p. 63. (In Russian) [Google Scholar]
- Rodionova, N.F.; Soldatov, V.N.; Merezhko, V.E.; Yarosh, N.P. Cultural Flora. Oats; Kolos: Moscow, Russia, 1994; p. 367. (In Russian) [Google Scholar]
- Eshetie, A.M.; Berhanu, A.W.; Yeshambel, M.C. Evaluation of biomass yield and nutritional quality of oats–vetch mixtures at different harvesting stage under residual moisture in Fogera District, Ethiopia. Agric. Food Secur. 2018, 7, 88. [Google Scholar] [CrossRef]
- Gebremedhn, B.W.; Alemu, A. Evaluation of different oat varieties for fodder yield and yield related traits in Debre Berhan area, Central Highlands of Ethiopia. Livest. Res. Rural Develop. 2015, 27, 9. [Google Scholar]
- Premkumar, R.; Nirmalakumari; Anandakumar, C.R. Studies on genetic variability and character association among yield and yield attributing traits in oats (Avena sativa L.). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 4075–4083. [Google Scholar] [CrossRef]
- Seryakova, L.P. Meteorological conditions and plants. In Textbook on Agrometeorology; LGI: Leningrad, Russia, 1971; p. 158. (In Russian) [Google Scholar]
- Tamm, I. Genetic and environmental variation of grain yield of oat varieties. Agron. Res. 2003, 1, 93–97. [Google Scholar]
- Polonskiy, V.I.; Surin, N.A.; Gerasimov, S.A.; Lipshin, A.G.; Sumina, A.V.; Zyute, S.A. The study of oat varieties (Avena sativa L.) of various geographical origin for grain quality and productivity. Vavilov J. Genet. Breed. 2019, 23, 683–690. (In Russian) [Google Scholar] [CrossRef]
- Yarosh, N.P.; Nisova, G.K.; Rodionova, N.A. Variability of biochemical characteristics of sowing oat varieties depending on natural and climatic growing zones. VIR Bull. 1988, 180, 3–9. (In Russian) [Google Scholar]
- Redaelli, R.; Sgrulletta, D.; De Stefanis, E. Genetic variability for chemical components in sixty European oat (Avena sativa L.) cultivars. Cereal Res. Commun. 2003, 31, 185–192. [Google Scholar] [CrossRef]
- Sykut-Domańska, E.; Rzedzicki, Z.; Nita, Z. Chemical composition variability of naked and husked oat grain (Avena sativa L.). Cereal Res. Commun. 2013, 41, 327–337. [Google Scholar] [CrossRef]
- Heneen, W.K.; Banas, A.; Leonova, S.; Carlsson, A.S.; Marttila, S.; Debski, H.; Stymne, S. The distribution of oil in the oat grain. Plant Signal. Behav. 2009, 4, 55–56. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.W. Nutrient composition and nutritional quality of oats and comparisons with other cereals. In Oats: Chemistry and Technology; Webster, F.H., Wood, P.J., Eds.; AAAC International Press: Toronto, ON, Canada, 2011; pp. 95–107. [Google Scholar] [CrossRef]
- Kozlova, G.Y.; Akimova, O.V. Comparative assessment of the bare-grained and filmy oats varieties on main parameters of corn quality. Agric. Biol. 2009, 44, 87–89. (In Russian) [Google Scholar]
- Vargach, Y.I.; Horeva, V.I.; Loskutov, I.G. The content of protein, lipids and starch in grains of naked and hulled forms of oat. Pomic. Small Fruits Cult. Russ. 2017, 51, 67–71. (In Russian) [Google Scholar]
- Yusova, O.A.; Vasyukevich, S.V. Evaluation of oat collection accessions in terms of productivity and biochemical indices under the conditions of the southern forest-steppe of West Siberia. Bull. Altai State Agrar. Univ. 2014, 7, 33–37. (In Russian) [Google Scholar]
- Broeck, H.C.; Londono, D.M.; Timmer, R.; Smulders, M.J.M.; Gilissen, L.J.W.J.; Meer, I.M. Profiling of nutritional and health-related compounds in oat varieties. Foods 2016, 5, 2. [Google Scholar] [CrossRef]
- Khoury, D.E.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta-glucan: Health benefits in obesity and metabolic syndrome. Hindawi Publ. Corp. J. Nutr. Metab. 2012, 28. [Google Scholar] [CrossRef]
- Hallfrisch, J.; Behall, K.M. Physiological responses of men and women to barley and oat extracts (Nu-trimX). I. Breath hydrogen, methane, and gastrointestinal symptoms. Cereal Chem. 2003, 80, 76–79. [Google Scholar] [CrossRef]
- Gerasimov, S.A.; Polonskiy, V.I.; Sumina, A.V.; Surin, N.A.; Lipshin, A.G.; Zyute, S.A. The influence of the genotype and growing conditions of oats on the content of biologically active components in the grain. Chem. Plant Raw Mater. 2020, 2, 65–71. (In Russian) [Google Scholar] [CrossRef]
- Ermakov, A.I.; Yarosh, N.P. Chemical composition of cotton seeds depending on growing conditions. Bull. Agric. Sci. 1958, 4, 41–45. (In Russian) [Google Scholar]
- Ermakov, A.I.; Arasimovich, V.V. Methods of Biochemical Research of Plants; Agropromizdat: Leningrad, Russia, 1987; p. 430. (In Russian) [Google Scholar]
- Popov, V.S.; Perchuk, I.N.; Khoreva, V.I. Gravimetric method for the quantitative determination of soluble β-glucans in oat grain. Biotechnol. Plant Breed. 2021, 4, 5–12. (In Russian) [Google Scholar] [CrossRef]
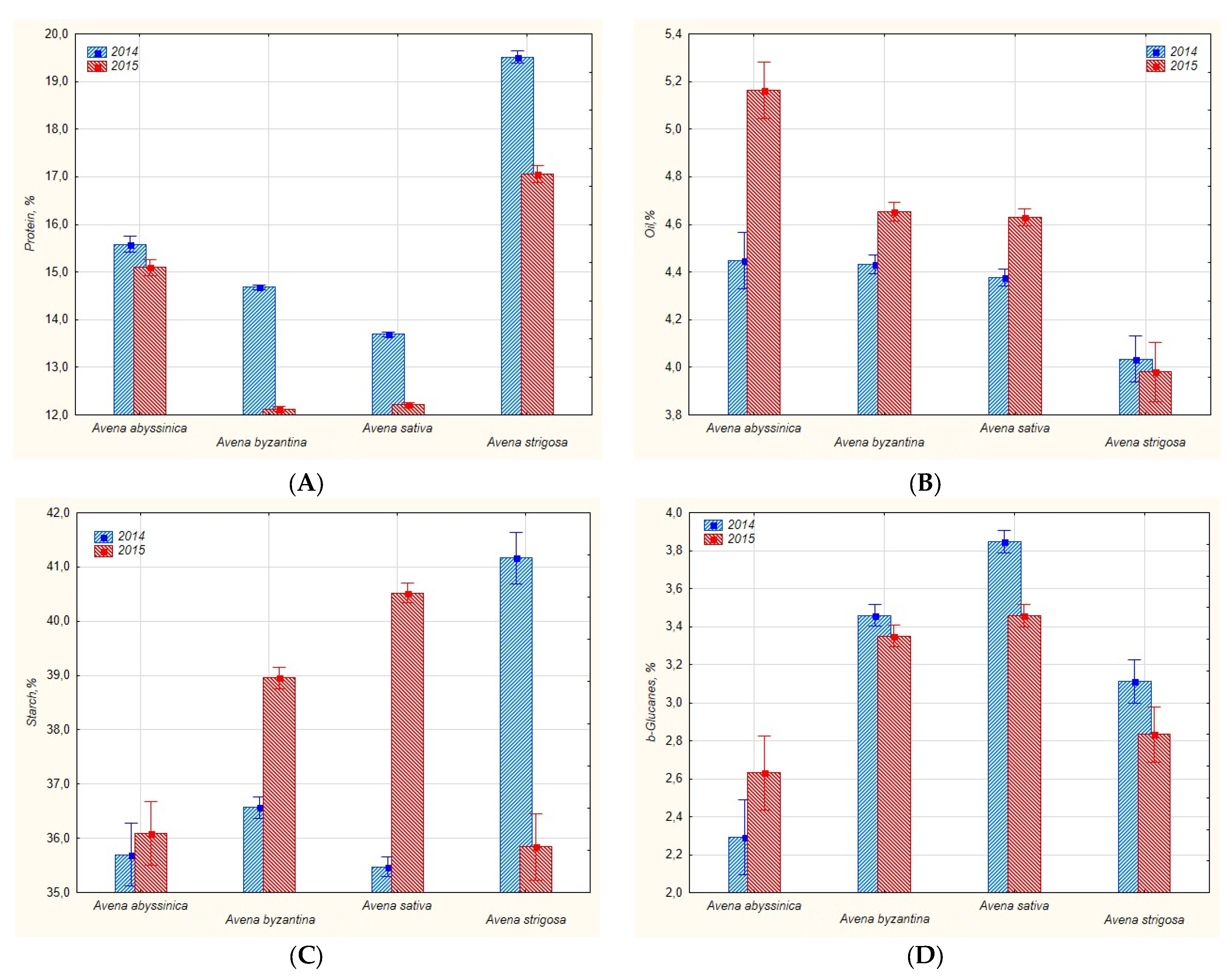


| Avena spp. | Years of Study | Number of Entries | Protein | Oil | Starch | β-Glucans |
|---|---|---|---|---|---|---|
| A. sativa L. | 2014 | 23 | 13.65 ± 1.79 | 4.40 ± 0.69 | 35.58 ± 3.17 | 3.78 ± 0.83 |
| 2015 | 23 | 12.20 ± 1.04 | 4.64 ± 0.70 | 40.45 ± 3.10 | 3.41 ± 0.56 | |
| Mean for 2 years | 12.92 | 4.52 | 38.02 | 3.60 | ||
| A. abyssinica Hochst. | 2014 | 3 | 15.58 ± 1.22 | 4.45 ± 0.02 | 35.70 ± 1.84 | 2.29 ± 0.06 |
| 2015 | 3 | 15.03 ± 0.17 | 5.24 ± 0.18 | 35.26 ± 3.47 | 2.63 ± 0.11 | |
| Mean for 2 years | 15.31 | 4.84 | 35.48 | 2.46 | ||
| A. strigosa Schreb. | 2014 | 3 | 19.51 ± 0.98 | 4.03 ± 0.16 | 41.16 ± 1.67 | 3.11 ± 0.91 |
| 2015 | 3 | 17.78 ± 0.57 | 4.14 ± 0.19 | 33.36 ± 1.45 | 2.84 ± 0.38 | |
| Mean for 2 years | 18.65 | 4.09 | 37.26 | 2.97 | ||
| A. byzantina Coch. | 2014 | 20 | 14.65 ± 1.92 | 4.42 ± 0.75 | 36.36 ± 3.35 | 3.46 ± 0.26 |
| 2015 | 20 | 12.20 ± 1.15 | 4.70 ± 0.79 | 38.68 ± 4.48 | 3.35 ± 0.58 | |
| Mean for 2 years | 13.42 | 4.56 | 37.52 | 3.40 | ||
| A. sativa L. (reference) | 2014 | 1 | 12.51 ± 0.39 | 4.91 ± 0.03 | 37.88 ± 0.22 | 3.03 ± 0.11 |
| 2015 | 1 | 11.80 ± 0.28 | 4.99 ± 0.04 | 38.90 ± 0.04 | 2.87 ± 0.00 | |
| Mean for 2 years | 12.15 | 4.95 | 38.39 | 2.95 |
| Variable | Protein * | Oil * | Starch * | β-Glucans ** | ||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | |
| Protein * | – | – | 0.08 | 0.14 | 0.42 | −0.61 | −0.53 | −0.55 |
| Oil * | 0.08 | 0.14 | – | – | 0.15 | −0.23 | −0.38 | −0.21 |
| Starch * | 0.42 | −0.61 | 0.15 | −0.23 | – | – | −0.58 | 0.35 |
| β-Glucans ** | −0.53 | −0.55 | −0.38 | −0.21 | −0.58 | 0.35 | – | – |
| Indicators | PH * | LP * | NSP * | NG * | W1000 * | SP * | Protein ** | Oil ** | Starch ** |
|---|---|---|---|---|---|---|---|---|---|
| 2014 | |||||||||
| LP * | 0.57 | ||||||||
| NSP * | 0.44 | 0.58 | |||||||
| NG * | 0.41 | 0.62 | 0.61 | ||||||
| W1000 * | −0.29 | −0.49 | −0.65 | −0.34 | |||||
| SP * | 0.17 | −0.03 | −0.16 | 0.24 | 0.50 | ||||
| Protein ** | 0.01 | −0.14 | 0.10 | −0.32 | −0.35 | -0.57 | |||
| Oil ** | −0.20 | −0.05 | −0.28 | −0.35 | 0.05 | −0.05 | 0.08 | ||
| Starch ** | 0.03 | −0.18 | −0.21 | −0.22 | 0.01 | −0.12 | 0.42 | 0.15 | |
| 2015 | |||||||||
| LP * | 0.64 | ||||||||
| NSP * | 0.70 | 0.69 | |||||||
| NG * | 0.39 | 0.55 | 0.83 | ||||||
| W1000 * | −0.62 | −0.75 | −0.70 | −0.39 | |||||
| SP * | −0.23 | −0.21 | 0.06 | 0.41 | 0.26 | ||||
| Protein ** | 0.53 | 0.43 | 0.39 | −0.07 | −0.66 | −0.51 | |||
| Oil ** | −0.13 | 0.10 | −0.23 | −0.20 | −0.04 | −0.19 | 0.14 | ||
| Starch ** | −0.01 | −0.06 | 0.08 | 0.32 | 0.24 | 0.37 | −0.61 | −0.23 | |
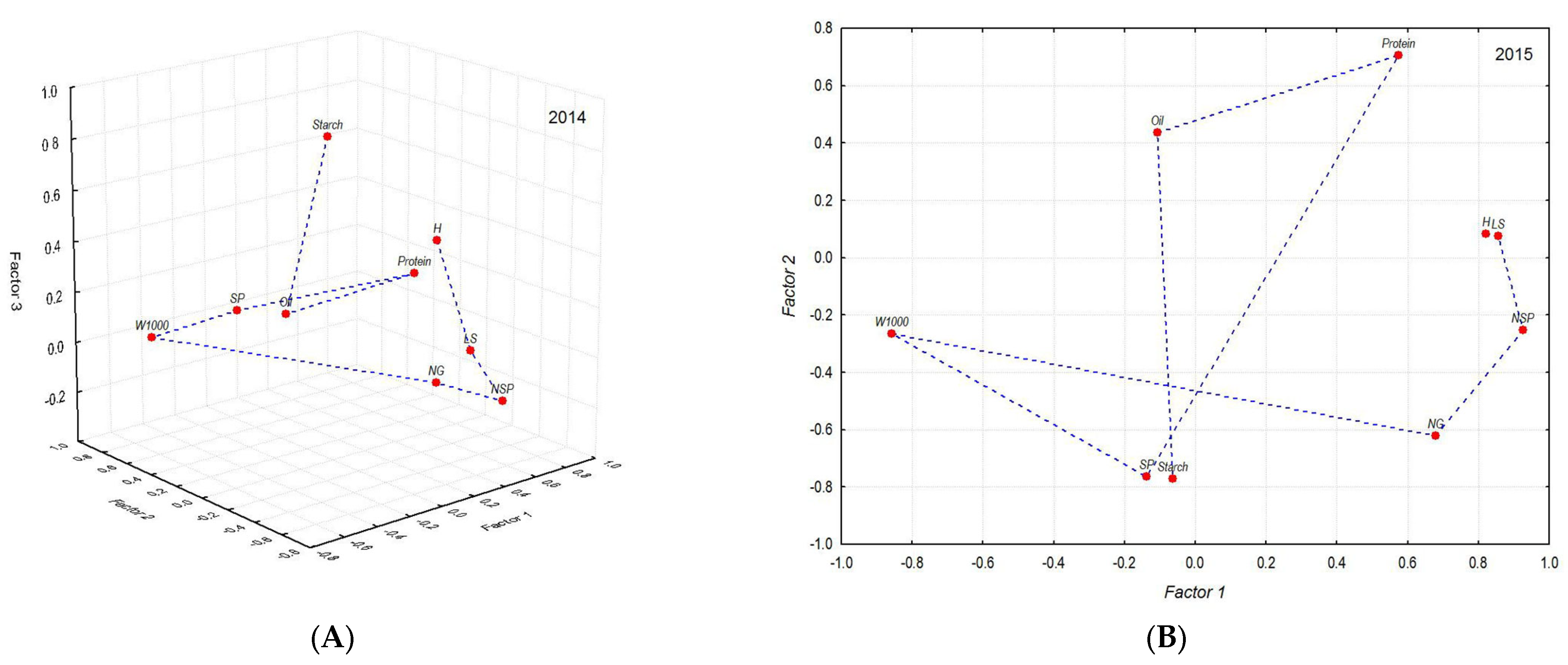
| Indicators | 2014 | 2015 | |||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | |
| PH * | 0.785 | 0.134 | 0.322 | 0.822 | 0.084 |
| LP * | 0.824 | −0.080 | −0.088 | 0.857 | 0.076 |
| NSP * | 0.776 | −0.371 | −0.223 | 0.925 | −0.252 |
| NG * | 0.811 | 0.162 | −0.267 | 0.680 | −0.622 |
| W1000 * | −0.551 | 0.713 | 0.038 | −0.859 | −0.265 |
| SP * | 0.129 | 0.904 | −0.004 | −0.137 | −0.764 |
| Protein ** | −0.108 | −0.719 | 0.494 | 0.574 | 0.706 |
| Oil ** | −0.332 | −0.054 | 0.244 | −0.108 | 0.437 |
| Starch ** | −0.094 | −0.077 | 0.892 | −0.064 | −0.772 |
| Factor share | 33.4 | 22.7 | 14.8 | 42.6 | 26.7 |
| Avena L. spp. | Ploidy | Origin | Quantity |
|---|---|---|---|
| A. sativa L. | 2 n = 42 | Russia, Brazil, Germany, Belarus, USA | 23 |
| A. byzantina Coch. | 2 n = 42 | Finland, Ukraine, Great Britain, Portugal, USA, Kazakhstan, Germany, Brazil, Ethiopia, Russia | 20 |
| A. abyssinica Hochst. | 2 n = 28 | Ethiopia | 3 |
| A. strigosa Schreb. | 2 n = 14 | Brazil | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, V.S.; Khoreva, V.I.; Konarev, A.V.; Shelenga, T.V.; Blinova, E.V.; Malyshev, L.L.; Loskutov, I.G. Evaluating Germplasm of Cultivated Oat Species from the VIR Collection under the Russian Northwest Conditions. Plants 2022, 11, 3280. https://doi.org/10.3390/plants11233280
Popov VS, Khoreva VI, Konarev AV, Shelenga TV, Blinova EV, Malyshev LL, Loskutov IG. Evaluating Germplasm of Cultivated Oat Species from the VIR Collection under the Russian Northwest Conditions. Plants. 2022; 11(23):3280. https://doi.org/10.3390/plants11233280
Chicago/Turabian StylePopov, Vitaliy S., Valentina I. Khoreva, Alexei V. Konarev, Tatyana V. Shelenga, Elena V. Blinova, Leonid L. Malyshev, and Igor G. Loskutov. 2022. "Evaluating Germplasm of Cultivated Oat Species from the VIR Collection under the Russian Northwest Conditions" Plants 11, no. 23: 3280. https://doi.org/10.3390/plants11233280
APA StylePopov, V. S., Khoreva, V. I., Konarev, A. V., Shelenga, T. V., Blinova, E. V., Malyshev, L. L., & Loskutov, I. G. (2022). Evaluating Germplasm of Cultivated Oat Species from the VIR Collection under the Russian Northwest Conditions. Plants, 11(23), 3280. https://doi.org/10.3390/plants11233280








