Plant-Community Vulnerability in Highly Fragmented Landscapes Is Higher in Secondary Forests Than in Old Growth Forests in the Andean–Amazonian Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Species Selection
2.3. Traits Sampling
2.4. Measurements of Functional Composition, Diversity and Vulnerability
2.5. Environmental Parameters
2.6. Data Analysis
3. Results
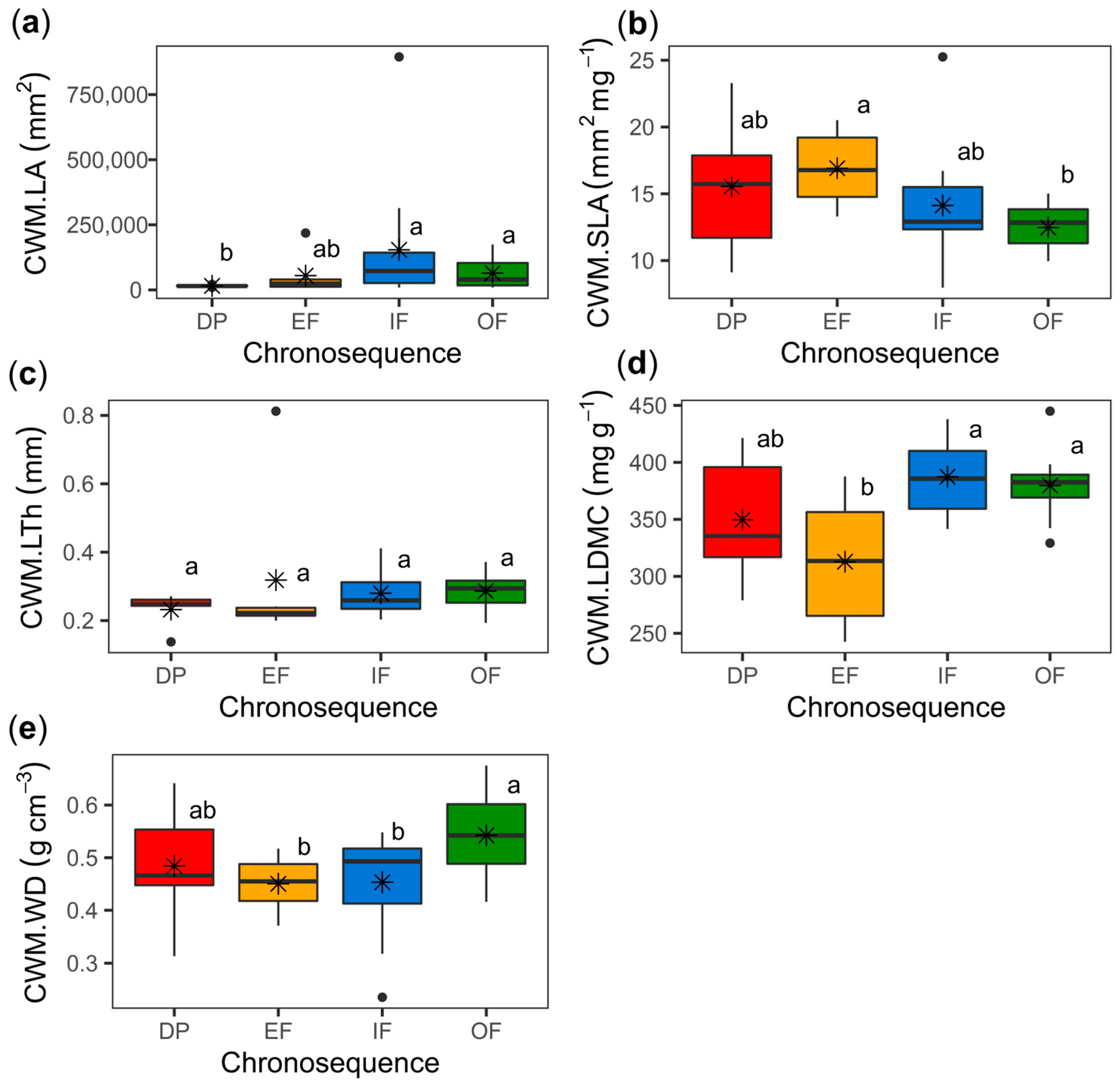
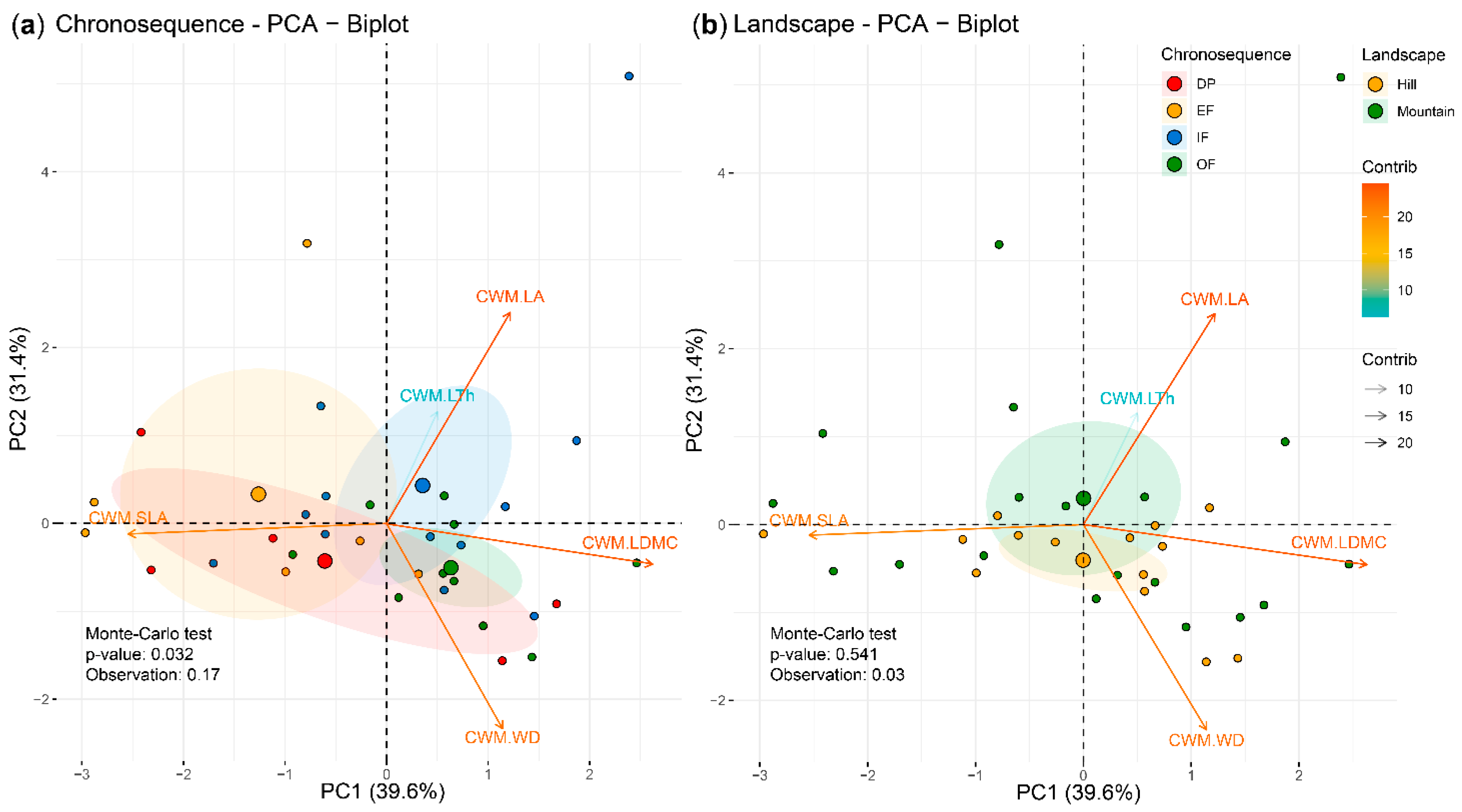
3.1. Functional Composition
3.2. Functional Diversity
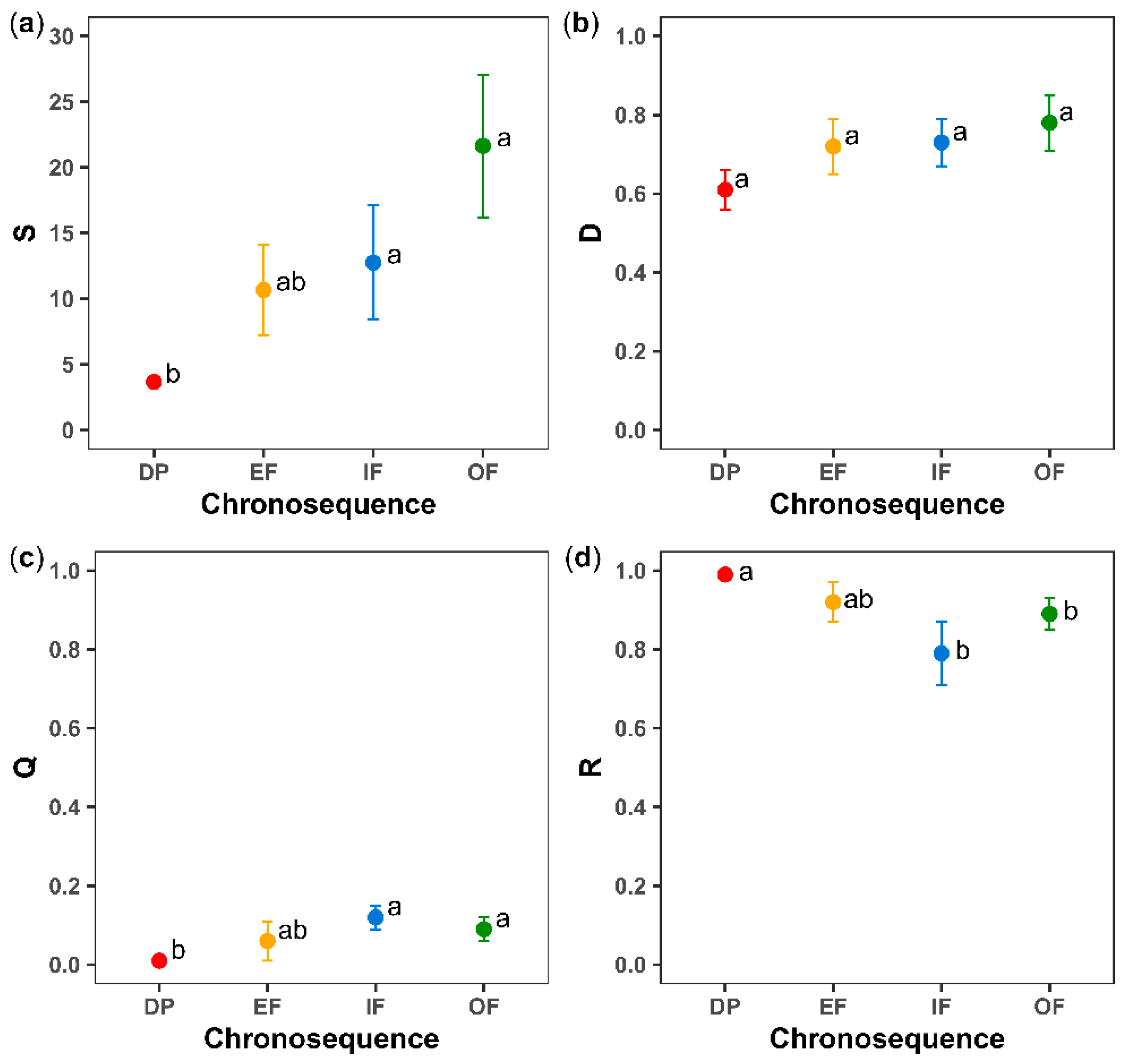
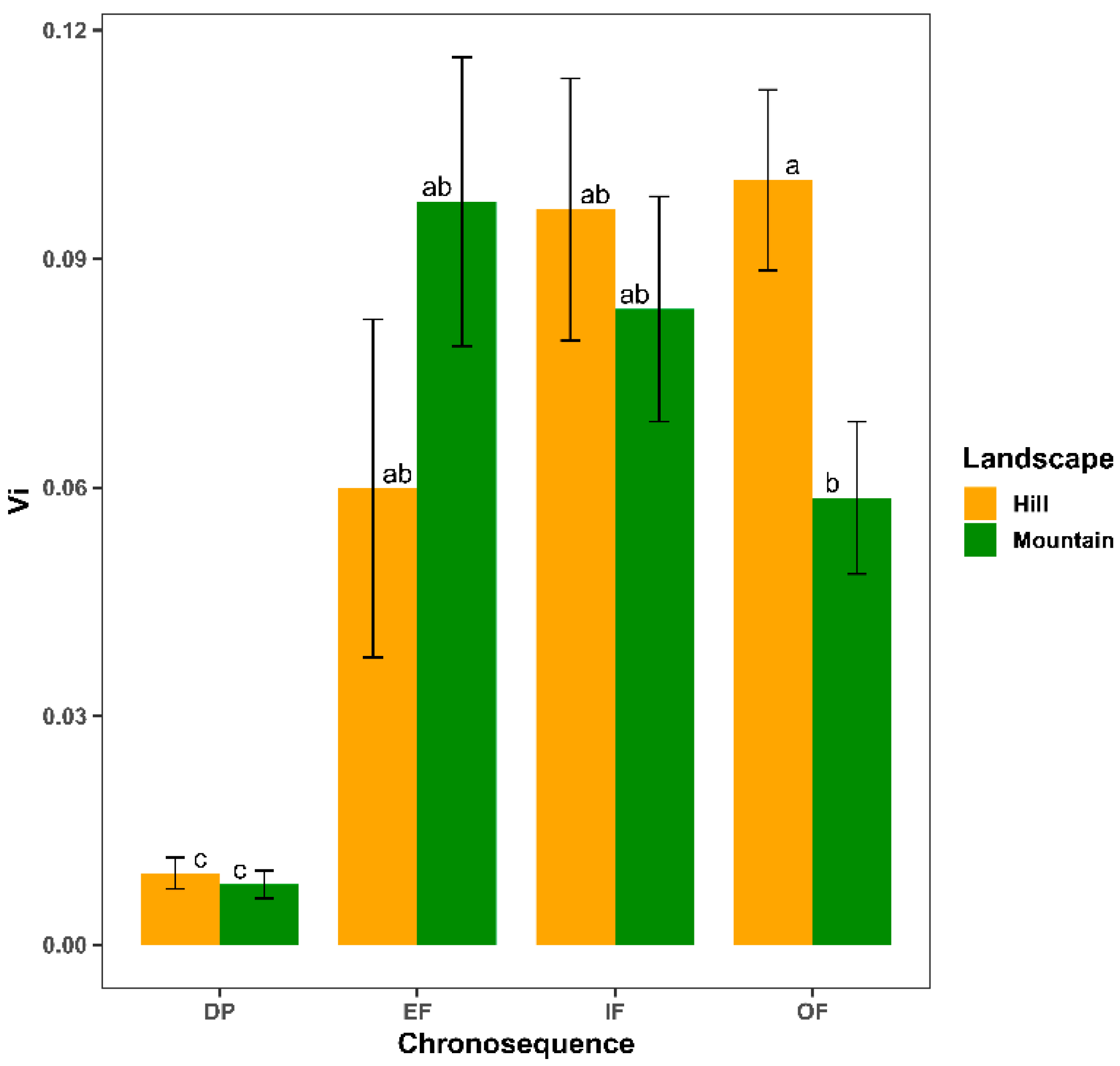
3.3. Functional-Vulnerability
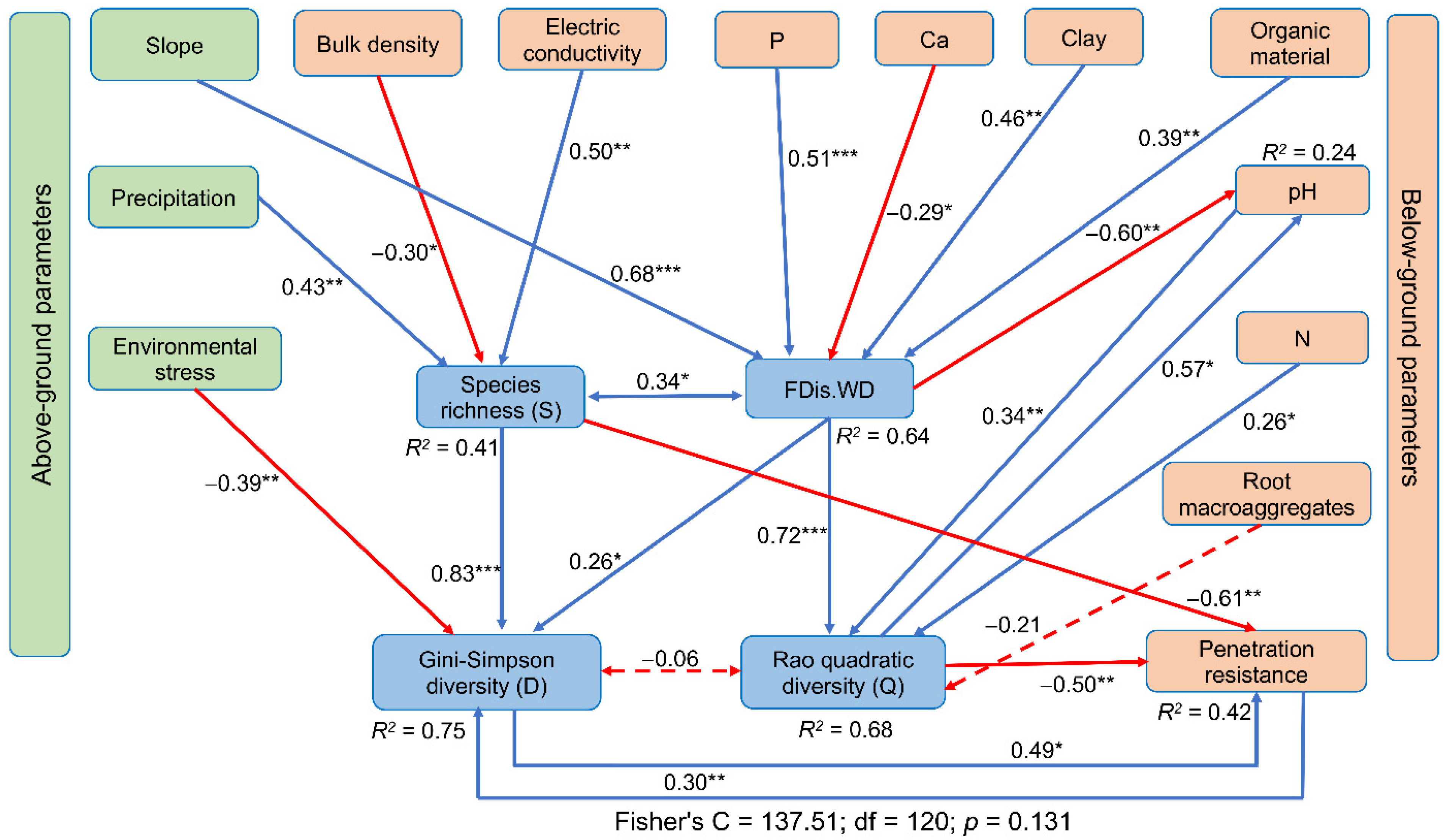
3.4. Environmental Filters and Their Relationships with Functional Attributes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating Plant Functional Diversity Effects in Ecosystem Service Assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive La Différence: Plant Functional Diversity Matters to Ecosystem Processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Peco, B.; Navarro, E.; Carmona, C.P.; Medina, N.G.; Marques, M.J. Effects of Grazing Abandonment on Soil Multifunctionality: The Role of Plant Functional Traits. Agric Ecosyst. Environ. 2017, 249, 215–225. [Google Scholar] [CrossRef]
- Becknell, J.M.; Powers, J.S. Stand Age and Soils as Drivers of Plant Functional Traits and Aboveground Biomass in Secondary Tropical Dry Forest. Can. J. For. Res. 2014, 44, 604–613. [Google Scholar] [CrossRef]
- Purschke, O.; Schmid, B.C.; Sykes, M.T.; Poschlod, P.; Michalski, S.G.; Durka, W.; Kühn, I.; Winter, M.; Prentice, H.C. Contrasting Changes in Taxonomic, Phylogenetic and Functional Diversity during a Long-Term Succession: Insights into Assembly Processes. J. Ecol. 2013, 101, 857–866. [Google Scholar] [CrossRef]
- Kelemen, A.; Tóthmérész, B.; Valkó, O.; Miglécz, T.; Deák, B.; Török, P. New Aspects of Grassland Recovery in Old-Fields Revealed by Trait-Based Analyses of Perennial-Crop-Mediated Succession. Ecol. Evol. 2017, 7, 2432–2440. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Paz, H.; Pla, L.; van Breugel, M.; Martínez-Ramos, M.; Bongers, F. Functional Diversity Changes during Tropical Forest Succession. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 89–96. [Google Scholar] [CrossRef]
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Diversity of Plant Nutrient-Acquisition Strategies Increases during Long-Term Ecosystem Development. Nat. Plants 2015, 1, 15050. [Google Scholar] [CrossRef]
- Craven, D.; Hall, J.S.; Berlyn, G.P.; Ashton, M.S.; van Breugel, M. Environmental Filtering Limits Functional Diversity during Succession in a Seasonally Wet Tropical Secondary Forest. J. Veg. Sci. 2018, 29, 511–520. [Google Scholar] [CrossRef]
- Wassenaar, T.; Gerber, P.; Verburg, P.H.; Rosales, M.; Ibrahim, M.; Steinfeld, H. Projecting Land Use Changes in the Neotropics: The Geography of Pasture Expansion into Forest. Glob. Environ. Chang. 2007, 17, 86–104. [Google Scholar] [CrossRef]
- Ricotta, C.; de Bello, F.; Moretti, M.; Caccianiga, M.; Cerabolini, B.E.L.; Pavoine, S. Measuring the Functional Redundancy of Biological Communities: A Quantitative Guide. Methods Ecol. Evol. 2016, 7, 1386–1395. [Google Scholar] [CrossRef]
- Mouillot, D.; Bellwood, D.R.; Baraloto, C.; Chave, J.; Galzin, R.; Harmelin-Vivien, M.; Kulbicki, M.; Lavergne, S.; Lavorel, S.; Mouquet, N.; et al. Rare Species Support Vulnerable Functions in High-Diversity Ecosystems. PLoS Biol. 2013, 11, e1001569. [Google Scholar] [CrossRef] [PubMed]
- Monge-González, M.L.; Guerrero-Ramírez, N.; Krömer, T.; Kreft, H.; Craven, D. Functional Diversity and Redundancy of Tropical Forests Shift with Elevation and Forest-Use Intensity. J. Appl. Ecol. 2021, 58, 1827–1837. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Heilpern, S.A.; Weeks, B.C.; Naeem, S. Predicting Ecosystem Vulnerability to Biodiversity Loss from Community Composition. Ecology 2018, 99, 1099–1107. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Peña-Venegas, C.P.; Sterling, A.; Castro, D.; Mahecha-Virguez, L.K.; Virguez-Díaz, Y.R.; Silva-Olaya, A.M. Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon. Agronomy 2021, 11, 2484. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Roa-Fuentes, L.L.; Sterling, A.; Suárez, J.C. Plant Biodiversity Homogenization across the Chronosequence in Highly Fragmented Landscapes in the Colombian Andean–Amazonian Transition. Forests 2022, 13, 1422. [Google Scholar] [CrossRef]
- Instituto Geográfico Agustin Codazzi (IGAC). Caquetá, Características Geográficas; IGAC: Bogotá, Colombia, 2010; 376p. [Google Scholar]
- Baraloto, C.; Timothy Paine, C.E.; Patiño, S.; Bonal, D.; Hérault, B.; Chave, J. Functional Trait Variation and Sampling Strategies in Species-Rich Plant Communities. Funct. Ecol. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-K.; Pan, X.; Liu, X.-Y.; Fu, Z.-X.; Zhang, M.-Y. Above- and Belowground Plant Functional Composition Show Similar Changes During Temperate Forest Swamp Succession. Front. Plant Sci. 2021, 12, 658883. [Google Scholar] [CrossRef]
- Pillar, V.D.; Blanco, C.C.; Müller, S.C.; Sosinski, E.E.; Joner, F.; Duarte, L.D.S. Functional Redundancy and Stability in Plant Communities. J. Veg. Sci. 2013, 24, 963–974. [Google Scholar] [CrossRef]
- Rao, C.R. Diversity and Dissimilarity Coefficients: A Unified Approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Pieri, C.J.M.G. Fertility of Soils: A Future for Farming in the West African Savannah; Springer: Berlin/Heidelberg, Germany, 1992; Volume 10. [Google Scholar]
- Zamudio, A.M.; Carrascal Carrascal, M.L.; Pulido Roa, C.E.; Gallardo, J.F.; Gómez Guzmán, I.D. Métodos Analíticos del Laboratorio de Suelos; Instituto Geografico Agustin Codazzi: Bogotá, Colombia, 2006.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2; Academic Press: Cambridge, MA, USA, 1996; pp. 961–1010. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Velasquez, E.; Lavelle, P.; Andrade, M. GISQ, a Multifunctional Indicator of Soil Quality. Soil Biol. Biochem. 2007, 39, 3066–3080. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-131.1; The Comprehensive R Archive Network: Vienna, Austria, 2018. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat v. 2020; Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Dray, S.; Dufour, A.-B.; Thioulouse, J. Ade4: Analysis of Ecological Data: Exploratory and Euclidean Methods in Environmental Sciences; R Package Version 1.7-16; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Package ‘Vegan’: Community Ecology; Package Version 2.5-7; The Comprehensive R Archive Network: Vienna, Austria, 2018. [Google Scholar]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package: “Corrplot”: Visualization of a Correlation Matrix; Version 0.84; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- Wheeler, B.; Torchiano, M. Package ‘LmPerm’: Permutation Tests for Linear Models; Version 2.1.0; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- Li, S.; Liu, W.; Lang, X.; Huang, X.; Su, J. Species Richness, Not Abundance, Drives Ecosystem Multifunctionality in a Subtropical Coniferous Forest. Ecol. Indic. 2021, 120, 106911. [Google Scholar] [CrossRef]
- Feizabadi, M.F.; Tahmasebi, P.; Broujeni, E.A.; Ebrahimi, A.; Omidipour, R. Functional Diversity, Functional Composition and Functional β Diversity Drive Aboveground Biomass across Different Bioclimatic Rangelands. Basic Appl. Ecol. 2021, 52, 68–81. [Google Scholar] [CrossRef]
- Bonilla-Valencia, L.; Castillo-Argüero, S.; Martínez-Orea, Y.; Espinosa García, F.J.; Lindig-Cisneros, R.; Alvarez-Añorve, M.Y.; Avila-Cabadilla, L.D. Predictions of the Community Assemblage in a Temperate Forest through Indicators That Evaluate the Anthropogenic Disturbance Effect on Natural Regeneration. Flora 2021, 275, 151764. [Google Scholar] [CrossRef]
- Allan, E.; Manning, P.; Alt, F.; Binkenstein, J.; Blaser, S.; Blüthgen, N.; Böhm, S.; Grassein, F.; Hölzel, N.; Klaus, V.H.; et al. Land Use Intensification Alters Ecosystem Multifunctionality via Loss of Biodiversity and Changes to Functional Composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The Coefficient of Determination R2 and Intra-Class Correlation Coefficient from Generalized Linear Mixed-Effects Models Revisited and Expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Shipley, B. The AIC Model Selection Method Applied to Path Analytic Models Compared Using a D-Separation Test. Ecology 2013, 94, 560–564. [Google Scholar] [CrossRef]
- Lefcheck, J.; Byrnes, J.; Grace, J. Package ‘PiecewiseSEM’: Piecewise Structural Equation Modeling; Version 2.1.2; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; Version 4.0.3; The Comprehensive R Archive Network: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio v.1.3.1093; RStudio—Open Source & Professional Software for Data Science: Boston, MA, USA, 2020. [Google Scholar]
- Warring, B.; Cardoso, F.C.G.; Marques, M.C.M.; Varassin, I.G. Functional Diversity of Reproductive Traits Increases across Succession in the Atlantic Forest. Rodriguesia 2016, 67, 321–333. [Google Scholar] [CrossRef]
- Umaña, M.N.; Zhang, C.; Cao, M.; Lin, L.; Swenson, N.G. Commonness, Rarity, and Intraspecific Variation in Traits and Performance in Tropical Tree Seedlings. Ecol. Lett. 2015, 18, 1329–1337. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, R. Tropical Forests Are Vulnerable in Terms of Functional Redundancy. Biol. Conserv. 2021, 262, 109326. [Google Scholar] [CrossRef]
- Mi, X.; Sun, Z.; Song, Y.; Liu, X.; Yang, J.; Wu, J.; Ci, X.; Li, J.; Lin, L.; Cao, M.; et al. Rare Tree Species Have Narrow Environmental but Not Functional Niches. Funct. Ecol. 2020, 35, 511–520. [Google Scholar] [CrossRef]
- Marriott, C.A.; Fisher, J.M.; Hood, K.; Pakeman, R.J. Impacts of Extensive Grazing and Abandonment on Grassland Soils and Productivity. Agric. Ecosyst. Environ. 2010, 139, 476–482. [Google Scholar] [CrossRef]
- Pinho, B.X.; de Melo, F.P.L.; Arroyo-Rodríguez, V.; Pierce, S.; Lohbeck, M.; Tabarelli, M. Soil-Mediated Filtering Organizes Tree Assemblages in Regenerating Tropical Forests. J. Ecol. 2018, 106, 137–147. [Google Scholar] [CrossRef]
- Melo, V.F.; Orrutéa, A.G.; Motta, A.C.V.; Testoni, S.A. Land Use and Changes in Soil Morphology and Physical-Chemical Properties in Southern Amazon. Rev. Bras. De Ciência Do Solo 2017, 41. [Google Scholar] [CrossRef]
- Holp, K.D. Factors Limiting Tropical Rain Forest Regeneration in Abandoned Pasture: Seed Rain, Seed Germination, Microclimate, and Soil. Biotropica 1999, 31, 229–242. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant Diversity in a Changing World: Status, Trends, and Conservation Needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef]
- Olivares, I.; Svenning, J.-C.; van Bodegom, P.M.; Balslev, H. Effects of Warming and Drought on the Vegetation and Plant Diversity in the Amazon Basin. Bot. Rev. 2015, 81, 42–69. [Google Scholar] [CrossRef]

| Factor | Chronosequence | Landscape | Chronosequence × Landscape | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | df | F | p | df | F | p | Df | F | p |
| CWM.LA | 3 | 3.37 | 0.034 | 1 | 2.09 | 0.161 | 3 | 0.79 | 0.513 |
| CWM.LTh | 3 | 1.03 | 0.397 | 1 | 0.99 | 0.330 | 3 | 0.91 | 0.448 |
| CWM.SLA | 3 | 3.27 | 0.038 | 1 | 0.25 | 0.625 | 3 | 0.15 | 0.928 |
| CWM.LDMC | 3 | 3.03 | 0.046 | 1 | 0.04 | 0.845 | 3 | 0.19 | 0.900 |
| CWM.WD | 3 | 3.09 | 0.045 | 1 | 3.21 | 0.085 | 3 | 0.12 | 0.948 |
| FDis.LA | 3 | 5.87 | 0.004 | 1 | 1.10 | 0.305 | 3 | 0.73 | 0.544 |
| FDis.LTh | 3 | 0.66 | 0.583 | 1 | 1.40 | 0.248 | 3 | 0.67 | 0.577 |
| FDis.SLA | 3 | 0.43 | 0.733 | 1 | 0.21 | 0.651 | 3 | 0.81 | 0.501 |
| FDis.LDMC | 3 | 0.22 | 0.882 | 1 | 0.79 | 0.384 | 3 | 0.16 | 0.923 |
| FDis.WD | 3 | 1.43 | 0.257 | 1 | 5.72 | 0.025 | 3 | 0.45 | 0.720 |
| FRic | 3 | 4.41 | 0.013 | 1 | 0.30 | 0.588 | 3 | 0.56 | 0.644 |
| FEve | 3 | 0.54 | 0.657 | 1 | 0.52 | 0.477 | 3 | 3.32 | 0.036 |
| FDiv | 3 | 0.63 | 0.600 | 1 | 0.06 | 0.807 | 3 | 0.49 | 0.692 |
| FDis | 3 | 3.02 | 0.044 | 1 | 1.57 | 0.223 | 3 | 0.79 | 0.509 |
| S | 3 | 5.39 | 0.005 | 1 | 0.06 | 0.806 | 3 | 0.40 | 0.752 |
| D | 3 | 1.49 | 0.242 | 1 | 0.59 | 0.450 | 3 | 1.55 | 0.226 |
| Q | 3 | 6.24 | 0.003 | 1 | 1.04 | 0.318 | 3 | 0.74 | 0.536 |
| R | 3 | 4.30 | 0.014 | 1 | 2.21 | 0.149 | 3 | 0.86 | 0.473 |
| Vi | 3 | 50.01 | <0.001 | 1 | 0.21 | 0.644 | 3 | 2.89 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-León, C.H.; Roa-Fuentes, L.L.; Sterling, A.; Suárez, J.C. Plant-Community Vulnerability in Highly Fragmented Landscapes Is Higher in Secondary Forests Than in Old Growth Forests in the Andean–Amazonian Transition. Plants 2022, 11, 3284. https://doi.org/10.3390/plants11233284
Rodríguez-León CH, Roa-Fuentes LL, Sterling A, Suárez JC. Plant-Community Vulnerability in Highly Fragmented Landscapes Is Higher in Secondary Forests Than in Old Growth Forests in the Andean–Amazonian Transition. Plants. 2022; 11(23):3284. https://doi.org/10.3390/plants11233284
Chicago/Turabian StyleRodríguez-León, Carlos H., Lilia L. Roa-Fuentes, Armando Sterling, and Juan Carlos Suárez. 2022. "Plant-Community Vulnerability in Highly Fragmented Landscapes Is Higher in Secondary Forests Than in Old Growth Forests in the Andean–Amazonian Transition" Plants 11, no. 23: 3284. https://doi.org/10.3390/plants11233284
APA StyleRodríguez-León, C. H., Roa-Fuentes, L. L., Sterling, A., & Suárez, J. C. (2022). Plant-Community Vulnerability in Highly Fragmented Landscapes Is Higher in Secondary Forests Than in Old Growth Forests in the Andean–Amazonian Transition. Plants, 11(23), 3284. https://doi.org/10.3390/plants11233284








