1. Introduction
In general, the plant leaf is an important organ for photosynthesis and organic compound production [
1,
2]. To secure their function and orient themselves toward the light, leaves must be able to carry their own weight and twist and bend under external forces without being damaged [
3]. Many of the diverse leaf shapes and forms can be mechanically described as a cantilevered beam (the petiole), fixed at the shoot supporting the lamina on the other side [
3]. The stability and flexibility of the leaf are defined by the combination and spatial arrangement of tissue types within the petiole and lamina and their different mechanical properties [
3,
4]. The mechanical behavior of hydrostatic tissues, like the viscoelastic parenchyma and collenchyma, depends on turgor pressure [
4]. The thin cell walls of parenchymatic tissue alone are mechanically of no great relevance, but in combination with turgor pressure internal and external loads are transferred as tensile stresses to the cell walls resulting in stiffening the tissue as a whole [
4]. Not only turgor pressure affects the mechanical properties of parenchyma tissue, but also density of cell packaging [
4]. Collenchyma tissue is found in growing organs, stems, and leaves and characterized by partially thickened cell walls and elongated cell shape [
5]. Its elastic modulus is higher compared to parenchyma and additionally, its mechanical properties are affected by tissue age [
4,
6,
7]. In contrast to hydrostatic tissues, sclerenchymatous tissue, such as fibers, xylem elements, and sclereids, have lignified, thickened cell walls [
8]. The polymer lignin reinforces cell walls resulting in a higher elastic moduli and thus, is essential for the stiffness of organs [
4,
9].
While anatomy and biomechanics of leaves are generally well-studied so far, only few studies focused on peltate-shaped leaves and their properties. Peltate leaves are characterized by the petiole attaching to the lamina on the abaxial side of the leaf [
10]. The petiole insertion point can be in the center of the leaf or closer to the leaf margin [
10,
11]. The petioles of peltate leaves are characterized by their unifacial anatomy [
10,
12,
13]. Wilhelm Troll was one of the first to provide an explanation and definition of the peltate leaf shape and studied the morphology and anatomy of these leaves and their petioles [
10]. In the 1950s, first studies on the development of peltate leaves and unifacial petioles were conducted [
10,
12]. In 1998, Friedrich Ebel followed with a detailed analysis regarding the geographical and habitat-specific distribution of peltate-leaved species [
14]. After early attempts, a study among vascular plants, comprising approximately 350 peltate-leaved taxa representing 40 families and 99 genera was compiled [
11]. Compared to the estimated number of vascular plants exceeding 380,000 species, peltate leaves are not very common [
11,
15]. Nonetheless, appearance and dimensions of peltate leaves are very diverse [
10,
11,
14].
Begonia L. is one of the two genera of Begoniaceae and one of the largest and diverse genera of flowering plants, exceeding 1800 species in 70 sections [
16,
17]. The number of published species of the pantropical genus has increased considerably in the last decades and is estimated well over 2000 [
16,
17]. Begonias are terrestrial or epiphytic, perennial (rarely annual) herbs or shrubs [
16] having herbaceous (often succulent) or woody stems or are tuberous and acaulescent. The leaves are arranged spirally and petiolate, typically asymmetric and diverse in shape and geometry [
16]. In 21 of 70 sections peltate leaves can be found [
16,
17]. While there are some early general anatomical studies on
Begonia [
18,
19], no detailed analysis has been conducted on the anatomy and biomechanical properties of peltate
Begonia leaves in comparison to non-peltate
Begonia species.
Contrary to the 2-dimensional architecture of leaves with a marginally attached petiole, peltate leaves are defined by a 3-dimensional spatial arrangement [
20]. In particular the transition zone from petiole to peltate lamina shows a significant change in geometry [
11,
20]. To secure the connection between petiole and lamina, the transition zone needs to provide mechanical stability while transporting water and nutrients through petiole and lamina. The important role of the transition zone of peltate leaves in handling mechanical stress and load dissipation has already been demonstrated [
21] and showed significant differences in mechanical properties of petiole and petiole-lamina transition zone indicating that petiole and transition zone are optimized to cope with different mechanical loads [
22]. Additionally, in several studies different types of fiber organization in the transition zone were revealed [
20,
21]. A following anatomical study focusing on the fiber orientation in the transition zone from petiole to lamina in a variety of peltate plant species identified seven types of strengthening structures [
11]. The transition zones of three
Begonia species analyzed in this study were described as net-like structures.
In this case study, we aim to provide an analysis of morphology, anatomy, and biomechanics of the leaves of four Begonia species focusing on the petiole and the petiole-lamina transition zone to understand the principles behind tensile stiffness, tensile fracture mechanics and damage prevention of the leaf. The overall motivation for our studies of the transition zones of peltate leaves is the transfer of the obtained results into carbon-fiber reinforced concrete components. In a leaf, the fibers run through the petiole and into the lamina with the transition zone securely connecting these two leaf parts. This connection is load adapted and differently organized in different species. The peltate leaf can be seen as a model for future biomimetic junctions between a concrete column and a plate, such as a ceiling of a room. For a secure connection between concrete column and plate, a load-adapted structure is needed where the carbon-fibers are arranged in a way that helps load dissipation from the plate into the column. The differently organized petiole-lamina transition zones can be described anatomically and biomechanically and transferred into models that serve as inspiration for the carbon-fiber arrangement in column-plate transitions.
3. Discussion
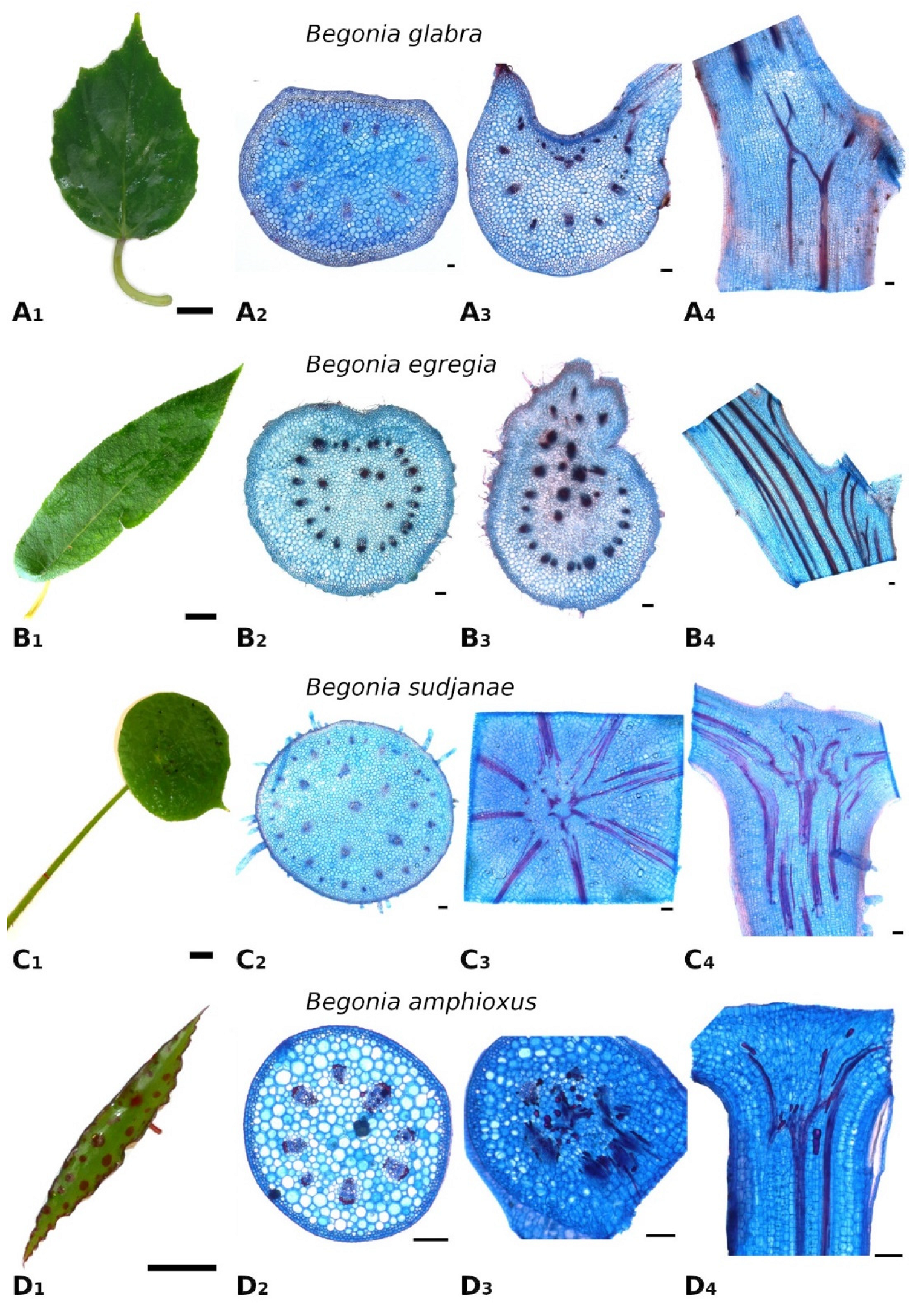
As one of the largest genera of the flowering plants,
Begonia shows a huge diversity in growth forms, sizes, and leaf geometries [
17]. The four
Begonia species analyzed in this comparative case study represent only a small fraction of this diversity and differ in growth habit (climbing, shrub-like, herbaceous), leaf size (few cm
2 to over 200 cm
2 lamina area,
Figure 3A), and geometry. When comparing the cross-sectional geometry of the petiole, two groups are apparent. While non-peltate
B. glabra and eccentric peltate
B. egregia are characterized by a mirror-symmetrical petiole cross-section, the more central peltate
B. sudjanae and
B. amphioxus show radially symmetrical, circular petioles. The circular petiole geometry can also be found in other peltate Begonias, e.g.,
B. nelumbiifolia and
B. peltata [
11]. The shape and geometry of the petiole affect the mechanical properties, more precisely the resistance to bending and twisting and thus, the resistance to damage. The leaves of
B. glabra and
B egregia are horizontally oriented. A D-shaped cross section (
B. glabra) or an elliptical cross section with an indentation (
B. egregia) are more resistant to downward bending while being highly flexible in torsion and thus, are of advantage for leaves with a 2D configuration [
27,
28]. In contrast, in leaves with a 3D configuration as in
B. sudjanae and
B. amphioxus the petiole must be resistant to bending and torsion in all directions. Thus, a circular petiole cross section is beneficial as it does not have a preferred direction for bending. Regarding anatomy, petioles of all four species show the characteristic tissue arrangement of
Begonia [
19]. The vascular bundles are embedded in parenchymatous tissue and either are arranged in one ring (like in
B. glabra and
B. amphioxus,
Figure 1(A
2,D
2)), sometimes with additional scattered central bundles (
B. egregia,
Figure 1B
2) or in more rings that can appear almost scattered (
B. sudjanae,
Figure 1(C
2)). The subepidermal collenchyma present in all four studied species, is known for
Begonia petioles and generally common as reinforcement in petioles [
5,
8]. In two species, additional strengthening tissue was found. The elongated sclereids in
B. sudjanae that are located in the periphery of the petiole or associated with the vascular bundles (
Figure 2D–F) are known from several
Begonia species [
18,
19]. In close association with the vascular bundles, sclereids are also found in the lamina providing additional stiffness to the leaf [
19]. The sclerenchyma caps (
Figure 2C) found in some large leaves of
B. amphioxus are another way the
Begonia petiole is mechanically strengthened. These fibrous caps are known from
Begonia and additionally, sclerenchymatous caps as stiffening elements are common in petioles of non-peltate and peltate leaves [
11,
13,
18]. With the cross-sectional petiole geometry and arrangement of peripheral collenchyma, xylem fibers and in some species, sclerenchyma, the petioles of the four
Begonia species are optimally designed to provide the required stiffness to secure their function while being flexible enough to prevent damage by the various mechanical stresses.
The high amount of hydrostatic tissue, like parenchyma and collenchyma, visible in the petiole and lamina cross-sections is reflected in the low percentage of leaf dry mass and percentage of lignified tissue in three out of four species (
Table 1,
Figure 3B,C). Not surprisingly, these low amounts of lignified tissue point toward turgor pressure as crucial for the stability of the leaf. In hydrostatic tissues, such as parenchyma and collenchyma representing more than 98% of petiole tissue of the studied species, turgor pressure defines the mechanical properties of the whole structure [
3,
4]. At full turgor, external stresses are redistributed across the cell walls which results in stiffening of the tissue as a whole [
4]. When deprived of water,
E values of parenchyma and collenchyma decrease and show a higher flexibility and lower flexural rigidity of the petiole [
29]. The impact of the above-mentioned fiber bundles in the plant individual of
B. amphioxus now becomes apparent as this species shows higher percentages of leaf dry mass and lignified petiolar tissue than the other analyzed species. In comparison to other
Begonia species, the dry mass values correspond well with measured dry masses from
B. peltata and
B. nelumbiifolia [
11]. In contrast, the amount of lignified tissue measured in this study is lower than the values in the study of Wunnenberg et al. [
11] who measured between 4 and 8% of lignified strengthening tissue (in comparison to 0.9 to 1.8% in this case study). This difference might be explained by the low sample size in Wunnenberg et al. [
11], but it must also be considered that these few analyzed plants of four species represent only a small fraction of the large genus of
Begonia. Thus, variations from species to species in amount of lignified tissue are most likely.
Comparing the petiole-lamina transition zones in this case study of the four species, two groups are apparent. While both species with marginal and eccentric petiole insertion can be classified into the simple branching type, the two species with a more central petiole insertion can be sorted into the net-like structure type (according to Wunnenberg et al. [
11]). This net-like structure was also detected in other
Begonia species with a more central petiole insertion [
11] indicating a change and increase in complexity in fiber orientation and branching from non-peltate- to peltate-leaved species. Especially the intermediate leaf shape of
B. egregia with a slightly more complex transition zone than the non-peltate
B. glabra seems to support this hypothesis (
Figure 1A,B). In non-peltate leaves the vascular bundles mostly run in one direction into the lamina, while in peltate leaves, the bundles run radially in several directions. Therefore, the increase of complexity in the transition zone could simply be explained by the change of spatial configuration (from 2D to 3D) and the need of vascular bundles sustaining all parts of the lamina. Nonetheless, the organization of the petiole-lamina transition zone plays an important role in load-dissipation and, as a damage-resistant structure, is optimized to secure the connection between petiole and lamina [
21,
22]. While the mechanical testing of only the transition zone is challenging, there are several approaches for tensile and torsion tests, as well as deformation tests that could shed further light onto the mechanical properties of the petiole-lamina transition zone [
21,
22].
Comparing the mechanical properties, all analyzed species of this case study show tensile
E values in a similar range in venation (21.38 MPa to 27.90 MPa,
Table 1,
Figure 4D) and intercostal areas (4.51 to 8.48 MPa,
Table 1,
Figure 4E), respectively. The intercostal areas mostly comprise of upper and lower epidermis and a parenchymatous mesophyll with large air spaces in between. Additionally, parenchyma layers or an epidermis with large cells are prominent in all four species. The mechanical support of parenchyma depends on turgor and packaging density [
4]. Densely packed parenchyma cells (e.g., in petioles) provide much more mechanical support than the spongy mesophyll found in the intercostal areas of the leaf. Thus, lower
E values are expected. In contrast, the venation is composed of vascular tissue including the lignified xylem elements surrounded by parenchyma and, in some species, collenchyma, and is a critical component in ensuring the stiffness of the lamina [
1,
4,
30]. Thus, to support the lamina, higher
E values in the scaffolding (venation) are advantageous. Whereas tensile
E values in intercostal areas and venation are similar across the four studied species, differences in the petioles are apparent (
Table 1,
Figure 4A). In non-peltate
B. glabra and eccentric peltate
B. egregia, tensile
E averages at 14.72 and 14.70 MPa, respectively. The higher
E values in
B. sudjanae (20.79 MPa) and
B. amphioxus (25.81 MPa) can be attributed to the additional strengthening tissues in the petioles. Especially lignified tissue such as the single cell sclereids and sclerenchyma strands increase stiffness significantly resulting in higher Young’s moduli [
3,
4]. The impact of the amount of lignified tissue in the petiole can be seen in the simultaneous increase of lignified petiolar tissue and Young’s modulus (
Figure 3C and
Figure 4A). In comparison to tensile testing, the values for flexural Young’s modulus are higher in all three tested species (
Figure 4A,B,
Table 1). While under tensile loads only the amount of strengthening tissues affect the petiole stiffness, under bending loads the stiffness is additionally affected by the position of these strengthening tissues within the petiole cross-section resulting in higher flexural
E values. The quite similar
E values across all three species (
B. glabra: 50.12 MPa,
B. egregia: 39.94 MPa and
B. sudjanae: 48.80 MPa) could be explained by the similar arrangement of xylem fibers (in one or two rings,
Figure 1) and collenchyma (below the epidermis) in all species analyzed in this case study resulting in a similar stiffness. The lower tensile
E values might also be explained by the difficulty of sufficiently clamping the petiole samples in tensile tests without damaging them. Thus, the softer polyurethane layer on the clamps could influence the measurements and slippage of the samples might occur. When comparing the flexural rigidity of the petioles,
B. sudjanae shows significant higher values (2351.98 Nmm
2,
Figure 4C) than
B. glabra (428.70 Nmm
2) and
B. egregia (456.40 Nmm
2). This difference can be attributed to the higher petiole diameters and second moments of area in
B. sudjanae in general and especially in basal parts of the petiole. To fully characterize and compare the biomechanical properties of the
Begonia, petioles compression and torsional testing are required.
The percentage of lignified tissue in the petiole cross-section increases significantly in most species analyzed in this case study from base to apex. Since the types of stresses that affect the petiole are different depending on the part of the petiole and since tissue composition and arrangement define the mechanical properties, differences in anatomy are expected [
3,
4]. At the base of the petiole the highest bending stresses occur, whereas the apex is exposed to high torsional shear stresses [
3]. The parenchyma in the center of
Begonia petioles can deal with shear stresses from bending of the petiole. The subepidermal collenchyma layers can deal with torsional shear stresses [
3]. In contrast, sclerenchymatous tissue below the epidermis (vascular bundles or sclereids/sclerenchyma caps in
Begonia) stiffens the petiole. Since twisting and bending stresses are especially high at the base of the petiole, a high proportion of parenchyma and collenchyma is optimal, whereas at the apex, higher amounts of lignified tissue additionally help to cope with higher torsional shear stresses [
3]. These tissue proportions and arrangements result in higher flexural rigidity and lower flexural Young’s modulus at the base of the petiole, where the highest bending stresses occur, as can be seen in
B. sudjanae, and lower flexural rigidity and higher Young’s modulus in the apical part of the petiole.
4. Materials and Methods
4.1. Plant Material
Samples of Begonia glabra Aubl., B. egregia N.E.Br., B. sudjanae C.-A. Jansson and B. amphioxus Sands were provided by the Botanical Garden of Technische Universität Dresden, Germany. All plants were cultivated in the greenhouse under tropical conditions (20 ± 5 °C, humidity: 70%, no artificial lighting). The selection of the plant species included one non-peltate leaved species (B. glabra), one peltate species with the petiole insertion point close to the leaf margin (B. egregia), and two peltate species with a more central petiole insertion point (B. sudjanae and B. amphioxus). One plant individual per species (two for B. sudjanae) was available for sampling. Before sampling, lamina lengths were measured. Only intact, undamaged leaves were taken and collected between March 2021 and March 2022. Young and growing leaves were excluded from sampling but no detailed maturity analysis was conducted. For caulescent species, no samples were taken from the first two to three leaves/pairs of leaves of a growing shoot. For the acaulescent species B. sudjanae, samples were taken only by visual estimate. Young, growing leaves of B. sudjanae showed lighter leaf color, denser pubescence, and often a not fully unfolded lamina in comparison to adult leaves. In total, 58 leaves of B. glabra, 61 leaves of B. egregia, 34 leaves of B. sudjanae, and 35 leaves of B. amphioxus were sampled. Fresh leaves were transported in airtight containers and scanned (Ricoh MP C3004ex, Ricoh Company, Ltd., Chuo, Tokyo, Japan) or photographed (Lumix DMC-G81, Panasonic, Kodama, Osaka, Japan) from both sides and subsequently processed within a few hours or preserved in ethanol (70%) for later analysis.
4.2. Anatomy and Morphology
Leaf and petiole dimensions for all samples (taken from one to two plants per species) were measured based on scans or photographs using the software ImageJ (National Institutes of Health, Bethesda, MD, USA). Lamina length was determined at the longest point along the midrib, leaf width perpendicular to the midrib at the widest part. Compilation of measured variables was made in MS Excel (Microsoft Corporation, Redmont, WA, USA).
For the measurement of fresh and dry weight, petioles, venation, and intercostal areas of nine to 15 leaves of different sizes were separated using a scalpel or razor blade. To minimize water loss during cutting, the leaves were stored in water. Fresh and dry weights of the structural elements were measured with a precision scale (Mettler Toledo XA205DU, Columbus, OH, USA). To determine the dry weight, the fresh leaf samples were dried to constant mass at 60 °C in a drying cabinet (Heraeus T12, Heraeus Instruments, Hanau, Germany).
Fresh, non-embedded cross sections of the petiole, midrib, and intercostal area and cross and longitudinal sections of the petiole-lamina transition zone were prepared with a razor blade or vibratome (Hyrax V50, Carl Zeiss AG, Jena, Germany). Petiole cross sections were taken from basal, central, and apical parts of the petioles of differently sized leaves. Sections were bleached in sodium hypochlorite solution (2.8%), washed in water, stained with astrablue (1% in 2% acetic acid)/safranin (1% in H
2O) and differentiated in ethanol (70%). Astrablue stains non-lignified tissue blue, safranin stains lignified tissue red. The stained sections were photographed using a light microscope (VHX-970F, Keyence AG, Osaka, Japan) and integrated camera (VHX-970F, CMOS-image sensor, Keyence AG, Osaka, Japan). The amount of lignified strengthening tissue in the petiole was measured for at least nine leaves per species. For each of these leaves, three to five sections from each basal, central, and apical part of the petiole were analyzed using the software ImageJ (National Institutes of Health, Bethesda, MD, USA). First, the area of the cross section was measured using the binary function. For the area measurement of the lignified tissue, the colored images were split into RGB channels and the blue channel was used for further analysis (
Figure 5A,B). In the blue channel image, red/pink stained tissues appear dark and blue stained tissues appear light. With the threshold function all darker areas up to a certain value could be highlighted. These highlighted areas could either be measured directly via the measurement function (setting the measurement to “limit to threshold” in the settings), or were added to Regions of Interest. Subsequently, these Regions of Interest could be edited with the brush selection tool, if needed, combined (
Figure 5C) in the ROI manager and measured via the measurement function.
4.3. Mechanical Testing
Individual samples (taken from one to two plants per species) of petiole, venation and intercostal areas were subjected to tensile tests until fracture using a universal testing machine (zwickiLine, Zwick/Roell GmbH &Co. KG, Ulm, Germany) and corresponding software (testXpert II V3.5, Zwick/Roell GmbH &Co. KG, Ulm, Germany). Additionally, samples of petioles were subjected to three-point bending tests using a universal testing machine (Zwick/Roell AllroundLine Z005, Zwick/Roell GmbH &Co. KG, Ulm, Germany) and corresponding software (see above). Due to small petiole dimensions of
B. amphioxus, only tensile tests were performed for this species. Samples of nine to fifteen leaves per testing method were prepared depending on the leaf size and tested. For tensile tests, petioles were cut into pieces of 10 to 40 mm. The venation was separated from intercostal areas using a razor blade and cut to a length of 10 to 40 mm. The intercostal areas were cut into samples with a width-to-length-ratio of 1:8. For three-point bending tests, petioles were tests as a whole or cut into pieces of at least 30 mm depending on the length of the petiole. Width of petiole and venation samples and thickness of intercostal area samples were measured using a digital caliper (Precise PS 7215, Burg-Wächter, Wetter-Volmarstein, Germany). Where needed, samples were fixed onto small pieces of paper using instant glue (UHU instant glue, UHU GmbH & Co. KG, Bühl, Germany) to prevent slippage of the samples during tensile testing. Approx. five samples of petiole (if possible), venation and intercostal areas per leaf were tested. To minimize water loss, the samples were stored between wet paper sheets until testing. For tensile tests, clamping length was set to 5 mm, 10 mm or 20 mm, respectively, depending on sample dimensions. Clamps with a thin layer of polyurethane were used. For three-point bending tests, supporting width was set to 20 to 50 mm. Pre-load was set to 0.01 N and testing speed to 2 mm/min. Resulting forces were recorded using a 50 N and 5 kN load cell (50 N: Type: KAP-Z, AST Angewandte Systemtechnik GmbH, Dresden, Germany, 5 kN: Zwick/Roell xforce P, Zwick/Roell GmbH & Co. KG, Ulm, Germany). The software OriginLab 2021 Pro (OriginLab Corporation, Northhampton, MA, USA) was used for further analysis. For tensile tests, stress–strain diagrams were created from measured force and displacement. Where possible, for each sample the structural Young’s modulus (
E) was determined from the linear elastic part of the stress–strain curve and the tensile strength determined as the maximum stress. For three-point bending, the Young’s modulus (
E) was calculated using the following formula:
with
b being the slope of the displacement-force diagram,
l the supporting width, and
I the second moment of area. Flexural rigidity (
EI) was calculated multiplying Young’s modulus by second moment of area.
4.4. Statistical Analysis
The statistical analysis of data was performed with OriginLab 2021 Pro (OriginLab Corporation, Northhampton, MA, USA). To check for normal distribution of the data, the Shapiro–Wilk test was used. Samples from one species were considered dependent, as leaf samples were taken mostly from one plant. In the species comparison, samples were considered independent. All data are given as median and interquartile range (IQR) with respective sample size (n). The Student’s t-test for paired samples and the Wilcoxon signed-rank test were applied to test the significance of possible differences between percentages of lignified tissue in apical and basal parts of the petiole for normally distributed and non-normally distributed data, respectively. For species comparison and comparison of biomechanical variables in relation to sample position, the Kruskal–Wallis ANOVA with Dunn’s test were used. For all tests, a significance level of 0.05 was chosen.