Effect of Rhizome Fragment Length and Burial Depth on the Emergence of a Tropical Invasive Weed Cyperus aromaticus (Navua Sedge)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Collection and Classification of Plant Material
4.2. Experimental Set Up
4.3. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chadha, A.; Florentine, S.K.; Dhileepan, K.; Dowling, K.; Turville, C. Germination biology of three populations of Navua sedge (Cyperus aromaticus). Weed Sci. 2021, 69, 69–81. [Google Scholar] [CrossRef]
- Vitelli, J.S.; Madigan, B.A.; van Haaren, P.E. Control techniques and management strategies for the problematic Navua sedge (Cyperus aromaticus). Invasive Plant Sci. Manag. 2010, 3, 315–326. [Google Scholar] [CrossRef]
- Shi, B.; Osunkoya, O.O.; Chadha, A.; Florentine, S.K.; Dhileepan, K. Biology, Ecology and Management of the Invasive Navua Sedge (Cyperus aromaticus)—A Global Review. Plants 2021, 10, 1851. [Google Scholar] [CrossRef] [PubMed]
- Black, I. Navua sedge in pastures in Fiji. Aust. Weeds 1984, 3, 16–19. [Google Scholar]
- Klimešová, J.; Klimeš, L. Bud banks and their role in vegetative regeneration—A literature review and proposal for simple classification and assessment. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 115–129. [Google Scholar] [CrossRef]
- van Evert, F.K.; Cockburn, M.; Beniers, J.E.; Latsch, R. Weekly defoliation controls, but does not kill broad-leaved dock (Rumex obtusifolius). Weed Res. 2020, 60, 161–170. [Google Scholar] [CrossRef]
- Vogler, W.D.; Carlos, E.H.; Setter, S.D.; Roden, L.; Setter, M.J. Halosulfuron-methyl: A selective herbicide option for the control of the invasive Cyperus aromaticus (Ridley) Mattf. and Kukenth (Navua sedge). Plant Prot. Q 2015, 30, 61–66. [Google Scholar] [CrossRef]
- Chadha, A.; Florentine, S.K.; Dhileepan, K.; Turville, C.; Dowling, K. Efficacy of halosulfuron-methyl in the management of Navua sedge (Cyperus aromaticus): Differential responses of plants with and without established rhizomes. Weed Technol. 2022, 36, 397–402. [Google Scholar] [CrossRef]
- Heap, I. Herbicide resistant weeds. In Integrated Pest Management; Pimental, D., Peshin, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 281–301. [Google Scholar]
- Powles, S.B.; Gaines, T.A. Exploring the Potential for a Regulatory Change to Encourage Diversity in Herbicide Use. Weed Sci. 2016, 64, 649–654. [Google Scholar] [CrossRef]
- Fernandez, O.N. Establishment of Cynodon dactylon from stolon and rhizome fragments. Weed Res. 2003, 43, 130–138. [Google Scholar] [CrossRef]
- Sakamaki, Y.; Ino, Y. Tubers and rhizome fragments as propagules: Competence for vegetative reproduction in Equisetum arvense. J. Plant Res. 2006, 119, 677–683. [Google Scholar] [CrossRef]
- Anderson, J.V.; Horvath, D.P.; Chao, W.S.; Foley, M.E. Bud dormancy in perennial plants: A mechanism for survival. In Dormancy and Resistance in Harsh Environments; Lubzens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 69–90. [Google Scholar]
- Brandsaeter, L.O.; Fogelfors, H.; Fykse, H.; Graglia, E.; Jensen, R.K.; Melander, B.; Salonen, J.; Vanhala, P. Seasonal restrictions of bud growth on roots of Cirsium arvense and Sonchus arvensis and rhizomes of Elymus repens. Weed Res. 2010, 50, 102–109. [Google Scholar] [CrossRef]
- Hǻkansson, S. Weeds and Weed Management on Arable Land: An Ecological Approach; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Hakansson, S. Multiplication, growth and persistence of perennial weeds. In Biology and Ecology of Weeds; Holzner, W., Numata, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 2, pp. 123–135. [Google Scholar]
- Suzuki, J.-I.; Stuefer, J. On the ecological and evolutionary significance of storage in clonal plants. Plant Species Biol. 1999, 14, 11–17. [Google Scholar] [CrossRef]
- Johnson, W.G.; Li, J.; Wait, J.D. Johnsongrass control, total nonstructural carbohydrates in rhizomes, and regrowth after application of herbicides used in herbicide-resistant corn (Zea mays). Weed Technol. 2003, 17, 36–41. [Google Scholar] [CrossRef]
- Chicouene, D. Mechanical destruction of weeds. A review. Agron. Sustain. Dev. 2007, 27, 19–27. [Google Scholar] [CrossRef]
- Batson, M.-G. The Ecology of Agrostis spp. (Bent Grass) Invasion into Temperate Pastures. Doctoral Dissertation, University of Melbourne, Melbourne, VIC, Australia, 1996. [Google Scholar]
- Vengris, J. The effect of rhizome length and depth of planting on the mechanical and chemical control of Quackgrass. Weeds 1962, 10, 71–74. [Google Scholar] [CrossRef]
- Håkansson, S. Experiments with Agropyron repens (L.) Beauv. II. Production from rhizome pieces of different sizes and from seeds. Various environmental conditions compared. Ann. Agric. Coll. Swed. 1968, 34, 3–29. [Google Scholar]
- Håkansson, S. Experiments with Agropyron repens (L.) Beauv. III. Production of aerial and underground shoots after planting rhizome pieces of different lengths at varying depths. Ann. Agric. Coll. Swed. 1968, 34, 31–51. [Google Scholar]
- Swanton, C.J.; Cavers, P.B. Regenerative capacity of rhizomes and tubers from two populations of Helianthus tuberosus L. (Jerusalem artichoke). Weed Res. 1988, 28, 339–345. [Google Scholar] [CrossRef]
- Batson, M.G. Length of rhizome and depth of burial affects the regeneration of bent grass (Agrostis castellana). Aust. J. Agric. Res. 1998, 49, 1141–1146. [Google Scholar] [CrossRef]
- Dalbato, A.L.; Alfredsson, T.; Karlsson, L.M.; Andersson, L. Effect of rhizome fragment length and burial depth on emergence of Tussilago farfara. Weed Res. 2014, 54, 347–355. [Google Scholar] [CrossRef]
- Faulkner, S.; Young, K.; Preston, C.; Watts, J.; Crossman, N. The effect of rhizome fragment length and burial depth on Physalis viscosa L. survival. In Proceedings of the 15th Australian Weeds Conference Adelaide: Weed Management Society of South Australia, Adelaide, SA, Australia, 24–28 September 2006; pp. 130–132. [Google Scholar]
- Rask, A.M.; Andreasen, C. Influence of mechanical rhizome cutting, rhizome drying and burial at different developmental stages on the regrowth of Calystegia sepium. Weed Res. 2007, 47, 84–93. [Google Scholar] [CrossRef]
- Gregorio, J.; Pioto, N.; Forti, V.; Rodrigues, J.; Da Silva, P.V.; Monquero, P. Propagation and control of Anredera cordifolia (Ten.) Stennis (madeira vine). Aust. J. Crop Sci. 2021, 15, 1835–2707. [Google Scholar] [CrossRef]
- Klingeman, W.E.; Robinson, D.K.; McDaniel, G.L. Mugwort (Artemisia vulgaris) rhizome regeneration in pine bark, soil and sand substrates. HortScience 2004, 39, 746A-746. [Google Scholar] [CrossRef]
- Omezine, A.; Skhiri-Harzallah, F. Resumption and growth of Cynodon dactylon rhizome fragments. Pak. J. Weed Sci. Res. 2011, 17, 215–227. [Google Scholar]
- King, J.R.; Conway, W.C.; Rosen, D.J.; Oswald, B.P.; Williams, H.M. Total nonstructural carbohydrate trends in Deeproot Sedge (Cyperus entrerianus). Weed Sci. 2014, 62, 186–192. [Google Scholar] [CrossRef]
- Kolberg, D.; Brandsæter, L.O.; Bergkvist, G.; Solhaug, K.A.; Melander, B.; Ringselle, B. Effect of Rhizome Fragmentation, Clover Competition, Shoot-Cutting Frequency, and Cutting Height on Quackgrass (Elymus repens). Weed Sci. 2018, 66, 215–225. [Google Scholar] [CrossRef]
- Ringselle, B.; Oliver, B.W.; Berge, T.W.; Sundheim Fløistad, I.; Berge, L.; Brandsæter, L.O. Dry weight minimum in the underground storage and proliferation organs of six creeping perennial weeds. Weed Res. 2021, 61, 231–241. [Google Scholar] [CrossRef]
- Bourdot, G.W. Regeneration of yarrow (Achillea millefolium L.) rhizome fragments of different length from various depths in the soil. Weed Res. 1984, 24, 421–429. [Google Scholar] [CrossRef]


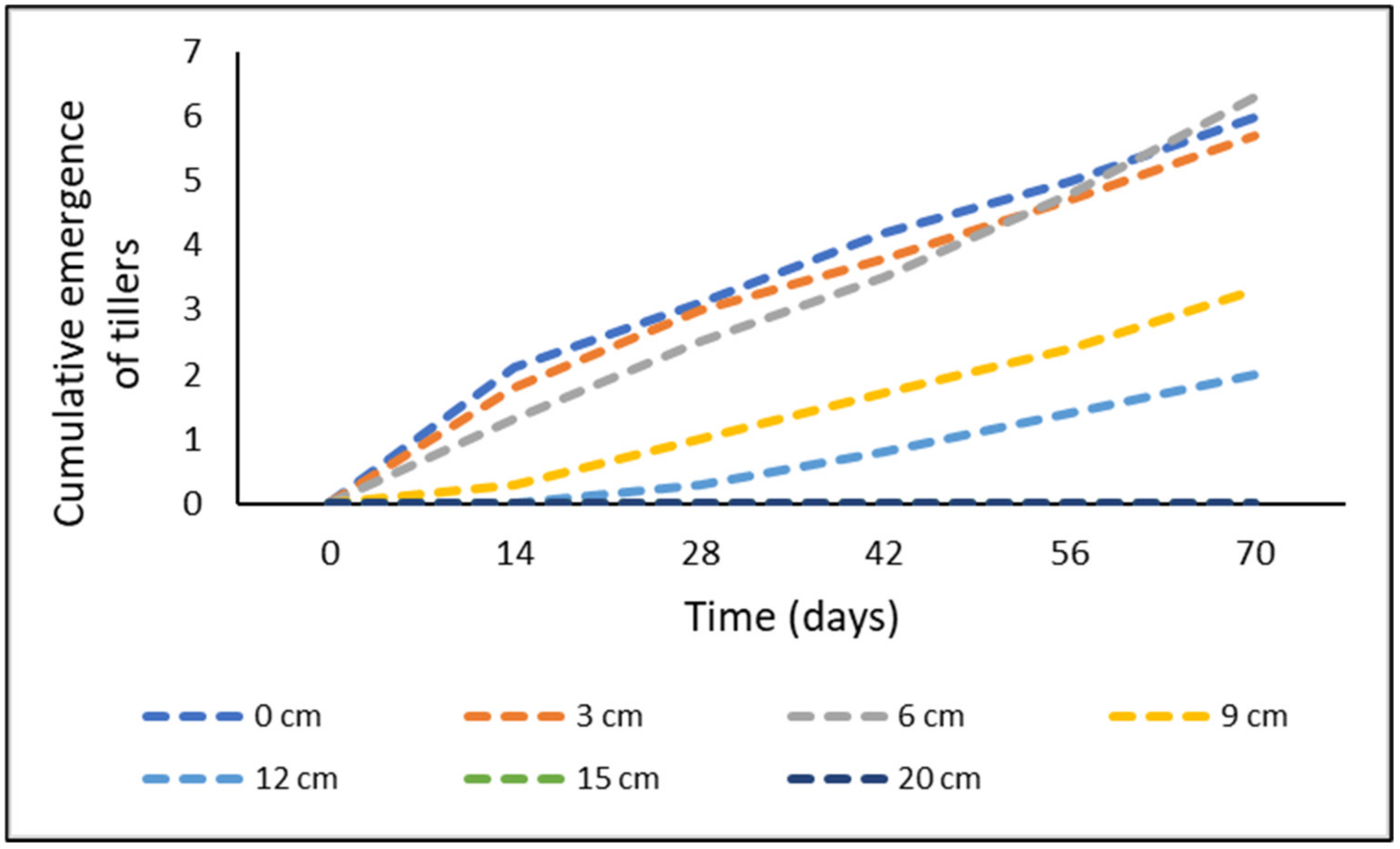
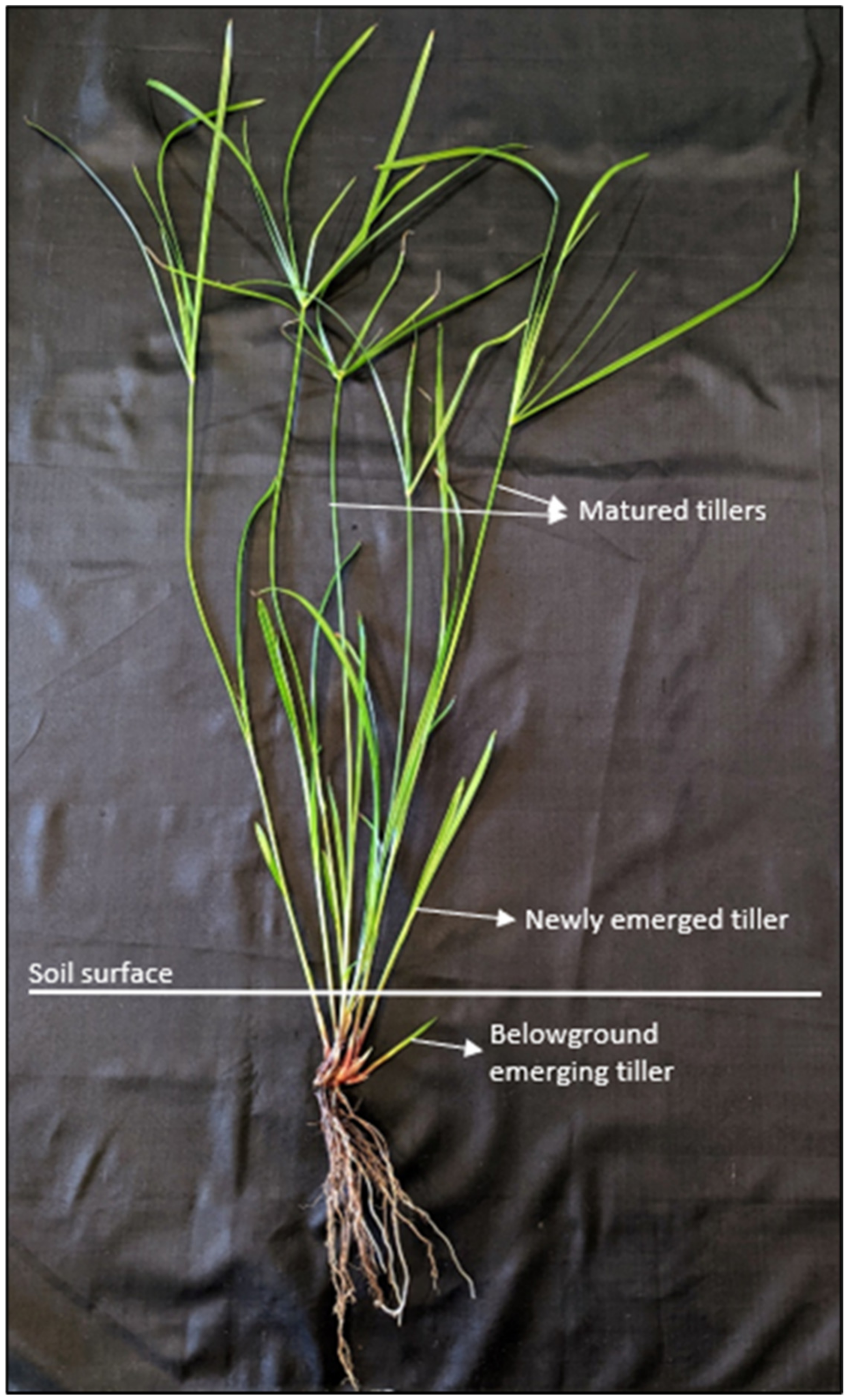
| Cumulative Emergence of Tillers | ||||
|---|---|---|---|---|
| df1 | df2 | F | p-Value | |
| Soil | 1 | 150.937 | 0.244 | 0.622 |
| Burial depth | 6 | 150.568 | 130.481 | <0.001 |
| Rhizome size | 2 | 30.318 | 7.060 | 0.003 |
| Soil*burial depth | 6 | 11.360 | 2.469 | 0.090 |
| Soil*rhizome size | 2 | 11.414 | 0.590 | 0.571 |
| Burial depth*rhizome size | 12 | 11.388 | 3.317 | 0.026 |
| Time | 4 | 32.138 | 126.279 | <0.001 |
| Soil*time | 4 | 32.138 | 0.816 | 0.525 |
| Burial depth*time | 24 | 32.138 | 11.281 | <0.001 |
| Rhizome size*time | 8 | 32.138 | 8.525 | <0.001 |
| Time to Emergence | Underground Emerging Tillers | |||||||
|---|---|---|---|---|---|---|---|---|
| df1 | df2 | F | p-Value | df1 | df2 | F | p-Value | |
| Soil | 1 | 72 | 0.400 | 0.529 | 1 | 108 | 0.393 | 0.532 |
| Burial depth | 4 | 72 | 120.14 | <0.001 | 5 | 108 | 9.718 | <0.001 |
| Rhizome size | 2 | 72 | 0.058 | 0.943 | 2 | 108 | 14.557 | <0.001 |
| Soil*burial depth | 4 | 72 | 1.546 | 0.198 | 5 | 108 | 0.689 | 0.633 |
| Soil*rhizome size | 2 | 72 | 1.512 | 0.227 | 2 | 108 | 0.098 | 0.906 |
| Burial depth*rhizome size | 6 | 72 | 0.872 | 0.520 | 10 | 108 | 1.220 | 0.287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chadha, A.; Florentine, S.K.; Dhileepan, K.; Turville, C. Effect of Rhizome Fragment Length and Burial Depth on the Emergence of a Tropical Invasive Weed Cyperus aromaticus (Navua Sedge). Plants 2022, 11, 3331. https://doi.org/10.3390/plants11233331
Chadha A, Florentine SK, Dhileepan K, Turville C. Effect of Rhizome Fragment Length and Burial Depth on the Emergence of a Tropical Invasive Weed Cyperus aromaticus (Navua Sedge). Plants. 2022; 11(23):3331. https://doi.org/10.3390/plants11233331
Chicago/Turabian StyleChadha, Aakansha, Singarayer K. Florentine, Kunjithapatham Dhileepan, and Christopher Turville. 2022. "Effect of Rhizome Fragment Length and Burial Depth on the Emergence of a Tropical Invasive Weed Cyperus aromaticus (Navua Sedge)" Plants 11, no. 23: 3331. https://doi.org/10.3390/plants11233331
APA StyleChadha, A., Florentine, S. K., Dhileepan, K., & Turville, C. (2022). Effect of Rhizome Fragment Length and Burial Depth on the Emergence of a Tropical Invasive Weed Cyperus aromaticus (Navua Sedge). Plants, 11(23), 3331. https://doi.org/10.3390/plants11233331







