Responses of Spring Barley to Zn- and Cd-Induced Stress: Morphometric Analysis and Cytotoxicity Assay
Abstract
:1. Introduction
2. Results
2.1. Total Content and Exchangeable Forms of Metals in the Soil
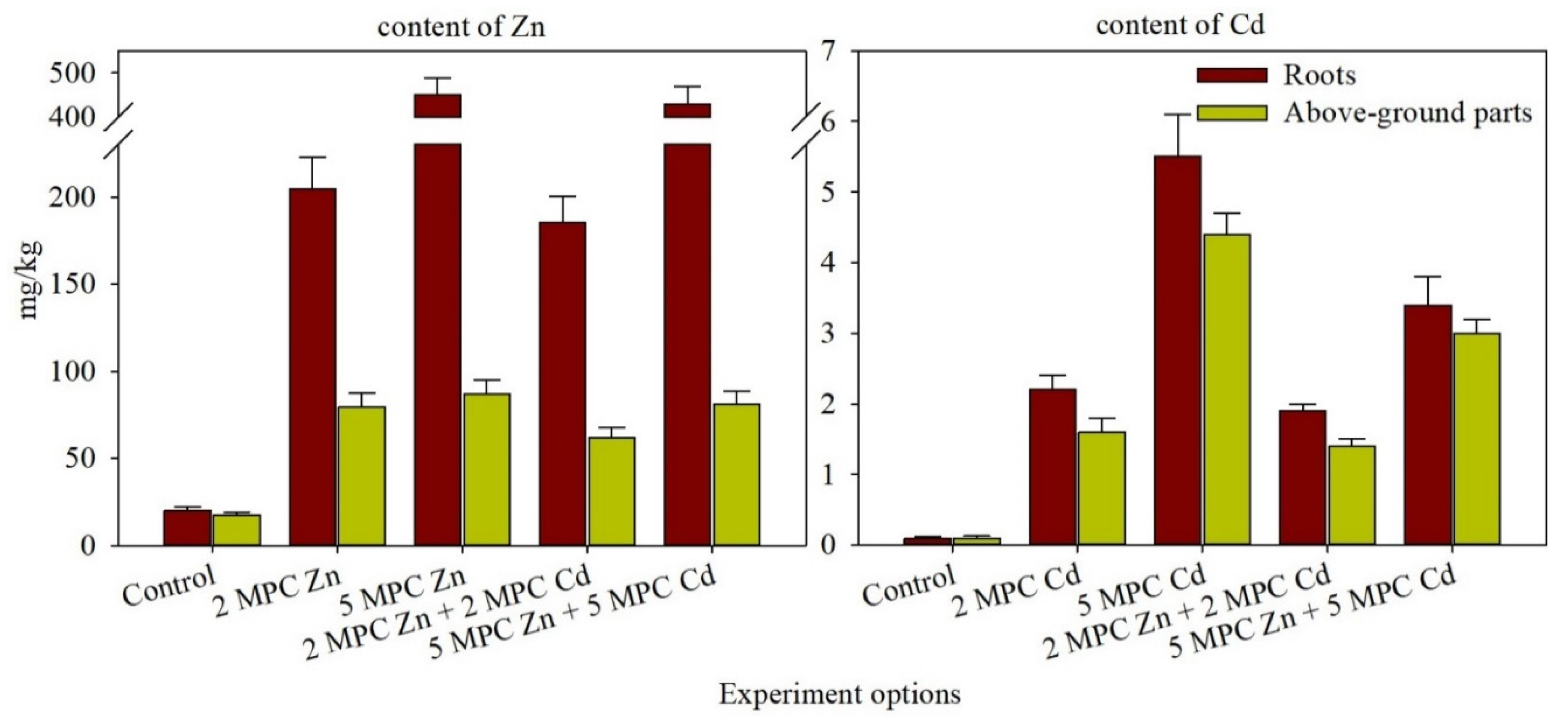
2.2. Zn and Cd Accumulation and Distribution in H. vulgare Tissues
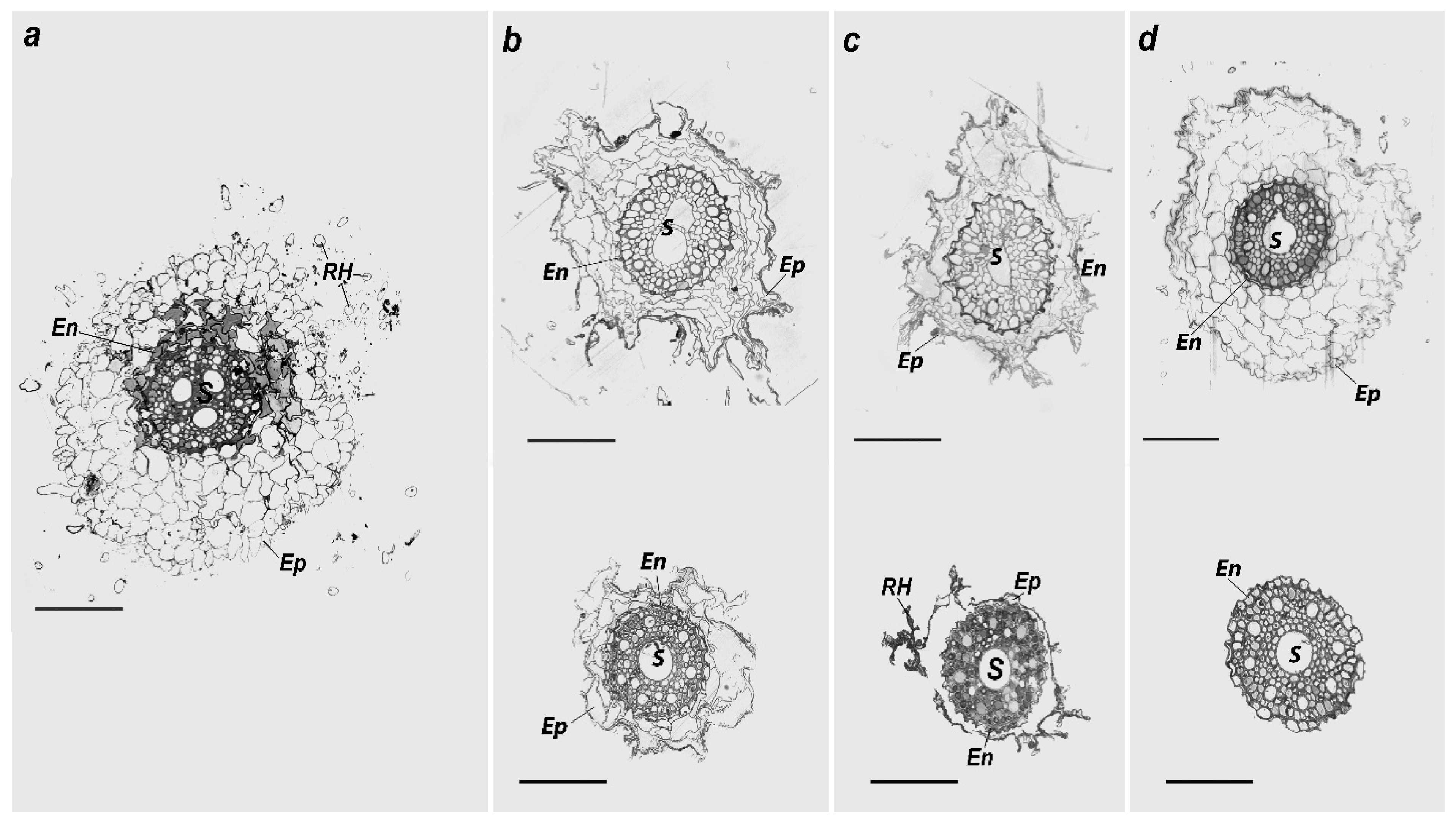
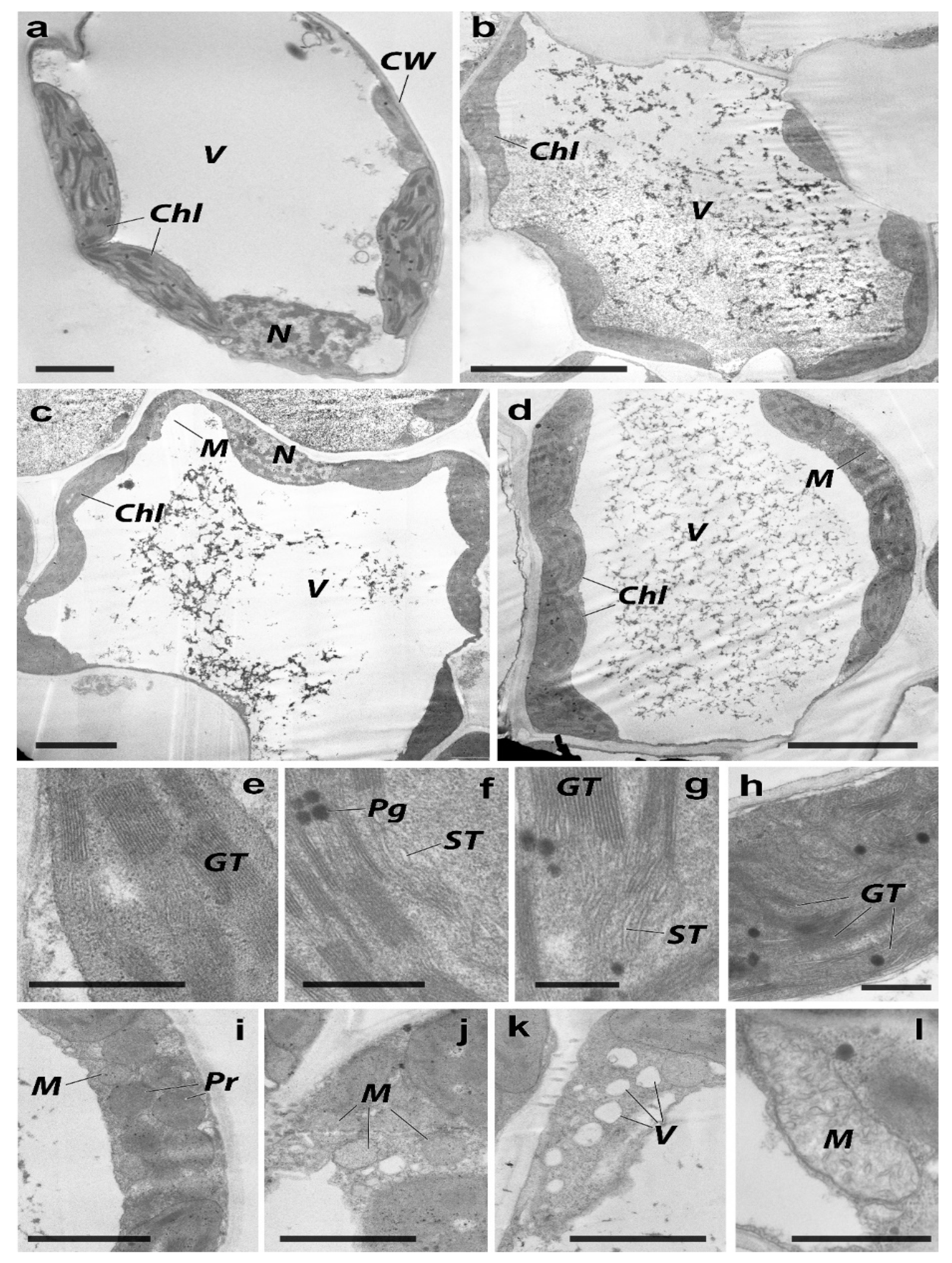
2.3. Morphological and Ultrastructural Changes in the H. vulgare
3. Discussion
4. Materials and Methods
4.1. Model Experiment
- (1)
- Control;
- (2)
- 2 MPC: 440 mg/kg Zn;
- (3)
- 2 MPC: 4 mg/kg Cd;
- (4)
- 2 MPC: 440 mg/kg Zn + 4 mg/kg Cd;
- (5)
- 5 MPC: 1100 mg/kg Zn;
- (6)
- 5 MPC: 10 mg/kg Cd;
- (7)
- 5 MPC: 1100 mg/kg Zn + 10 mg/kg Cd.
4.2. Quantitative Analysis of Zn and Cd in Soil
4.3. Quantitative Analysis of Zn and Cd in Plants
4.4. Morphometric, Microscopy, and Cytomorphometric Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, L.; Li, Y.; Zhu, Z.; Wang, F.; Liu, X.; Zhang, W.; Xiao, M.; Li, G.; Ding, J.; Chen, J.; et al. Soil health evaluation approaches along a reclamation consequence in Hangzhou Bay, China. Agric. Ecosyst. Environ. 2022, 337, 108045. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011. [Google Scholar]
- Zhang, C.; Yu, Z.-G.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Cui, F.; Zhu, M.-Y.; Shen, L.-Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bharagava, R.N.; More, N.S.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2018. [Google Scholar]
- Adil, M.F.; Sehar, S.; Chen, G.; Chen, Z.-H.; Jilani, G.; Chaudhry, A.N.; Shamsi, I.H. Cadmium-zinc cross-talk delineates toxicity tolerance in rice via differential genes expression and physiological / ultrastructural adjustments. Ecotoxicol. Environ. Saf. 2020, 190, 110076. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y. Spend more on soil clean-up in China. Nature 2016, 533, 469. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- DalCorso, G.; Farinati, S.; Maistri, S.; Furini, A. How Plants Cope with Cadmium: Staking All on Metabolism and Gene Expression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Sterckeman, T.; Thomine, S. Mechanisms of Cadmium Accumulation in Plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar] [CrossRef]
- Tezuka, K.; Miyadate, H.; Katou, K.; Kodama, I.; Matsumoto, S.; Kawamoto, T.; Masaki, S.; Satoh, H.; Yamaguchi, M.; Sakurai, K.; et al. A single recessive gene controls cadmium translocation in the cadmium hyperaccumulating rice cultivar Cho-Ko-Koku. TAG. Theor. Appl. Genetics. Theor. Und Angew. Genet. 2010, 120, 1175–1182. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ae, N.; Sugiyama, M.; Murakami, M.; Arao, T. Genotypic Variation in Shoot Cadmium Concentration in Rice and Soybean in Soils with Different Levels of Cadmium Contamination. Soil Sci. Plant Nutr. 2005, 51, 101–108. [Google Scholar] [CrossRef]
- Shao, J.F.; Xia, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. Effective reduction of cadmium accumulation in rice grain by expressing OsHMA3 under the control of the OsHMA2 promoter. J. Exp. Bot. 2018, 69, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Feng, Y.X.; Li, C.Z.; Zhang, P.; Yu, X.Z. Transcriptional analysis of heavy metal P(1B)-ATPases (HMAs) elucidates competitive interaction in metal transport between cadmium and mineral elements in rice plants. Env. Sci. Pollut Res. Int. 2022, 1–11. [Google Scholar] [CrossRef]
- Chaplygin, V.A.; Minkina, T.M.; Mandzhieva, S.S.; Nazarenko, O.G.; Zimulina, I.V.; Bauer, T.V.; Litvinov, Y.A.; Rajput, V. Heavy metals in agricultural crops of Rostov region through the example of soft wheat (Triticum aestivum). IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012204. [Google Scholar] [CrossRef]
- Abedi, T.; Gavanji, S.; Mojiri, A. Lead and Zinc Uptake and Toxicity in Maize and Their Management. Plants 2022, 11, 1922. [Google Scholar] [CrossRef]
- Awasthi, G.; Nagar, V.; Mandzhieva, S.; Minkina, T.; Sankhla, M.S.; Pandit, P.P.; Aseri, V.; Awasthi, K.K.; Rajput, V.D.; Bauer, T.; et al. Sustainable Amelioration of Heavy Metals in Soil Ecosystem: Existing Developments to Emerging Trends. Minerals 2022, 12, 85. [Google Scholar] [CrossRef]
- Bonnet, M.; Camares, O.; Veisseire, P. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.J.; Lombi, E.; McGrath, S.P. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator Thlaspi caerulescens. Plant Soil 2003, 249, 37–43. [Google Scholar] [CrossRef]
- Lagerwerff, J.V.; Brower, D.L. Exchange Adsorption of Trace Quantities of Cadmium in Soils Treated with Chlorides of Aluminum, Calcium and Sodium. Soil Sci. Soc. Am. J. 1972, 36, 734–737. [Google Scholar] [CrossRef]
- Parr, J.F. Heavy Metal Pollution in Soils of Japan. J. Environ. Qual. 1983, 12, 426. [Google Scholar] [CrossRef]
- Cullen, J.T.; McAlister, J. Biogeochemistry of Lead. Its Release to the Environment and Chemical Speciation. Met. Ions Life Sci. 2017, 17, 21–48. [Google Scholar] [CrossRef]
- Lilay, G.H.; Castro, P.H.; Campilho, A.; Assunção, A.G.L. The Arabidopsis bZIP19 and bZIP23 Activity Requires Zinc Deficiency – Insight on Regulation From Complementation Lines. Front. Plant Sci. 2019, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-Ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Yu, H.T.; Zhen, J.; Leng, J.Y.; Cai, L.; Ji, H.L.; Keller, B.B. Zinc as a countermeasure for cadmium toxicity. Acta Pharmacol. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Tao, J.; Lu, L. Advances in Genes-Encoding Transporters for Cadmium Uptake, Translocation, and Accumulation in Plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.E.; Dick, D.G.; Fredeen, A.L. Heavy Metal (Pb, Zn, Cd, Fe, and Cu) Contents of Plant Foliage near the Anvil Range Lead/Zinc Mine, Faro, Yukon Territory. Ecotoxicol. Environ. Saf. 2002, 52, 273–279. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; Mzibri, M.E.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front Plant Sci. 2013, 4, 175. [Google Scholar] [CrossRef] [Green Version]
- Kevresan, S.; Kirsek, S.; Kandrac, J.; Petrovic, N.; Kelemen, D. Dynamics of Cadmium Distribution in the Intercellular Space and Inside Cells in Soybean Roots, Stems and Leaves. Biol. Plant. 2003, 46, 85–88. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. Rev. Env. Contam Toxicol 2017, 241, 73–137. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. (Eds.) 4—Barley. In Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Sawston, UK, 2013; pp. 155e–201e. [Google Scholar]
- Ebbs, S.D.; Kochian, L.V. Phytoextraction of Zinc by Oat (Avena sativa), Barley (Hordeum vulgare), and Indian Mustard (Brassica juncea). Environ. Sci. Technol. 1998, 32, 802–806. [Google Scholar] [CrossRef]
- Minkina, T.; Rajput, V.; Fedorenko, G.; Fedorenko, A.; Mandzhieva, S.; Sushkova, S.; Morin, T.; Yao, J. Anatomical and ultrastructural responses of Hordeum sativum to the soil spiked by copper. Environ. Geochem. Health 2020, 42, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.; Khandelwal, A.; Srivastava, S. Heavy Metal Hyperaccumulator Plants: The Resource to Understand the Extreme Adaptations of Plants Towards Heavy Metals. In Plant-Metal Interactions; Srivastava, S., Srivastava, A.K., Suprasanna, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 79–97. [Google Scholar]
- Caporale, A.G.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of heavy metals in soils and their immobilization at micro-scale interfaces among diverse soil components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef] [PubMed]
- Minkina, T.M.; Motuzova, G.V.; Mandzhieva, S.S.; Nazarenko, O.G. Ecological resistance of the soil–plant system to contamination by heavy metals. J. Geochem. Explor. 2012, 123, 33–40. [Google Scholar] [CrossRef]
- Minkina, T.M.; Motuzova, G.V.; Mandzhieva, S.S.; Nazarenko, O.G.; Burachevskaya, M.V.; Antonenko, E.M. Fractional and group composition of the Mn, Cr, Ni, and Cd compounds in the soils of technogenic landscapes in the impact zone of the Novocherkassk Power Station. Eurasian Soil Sci. 2013, 46, 375–385. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Ippolito, J.A.; Xing, W.; Qiu, K.; Wang, Y. Cadmium foliar application affects wheat Cd, Cu, Pb and Zn accumulation. Environ. Pollut. 2020, 262, 114329. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol Env. Saf 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.; Apostolova, E.; Adamakis, I.-D.S.; Hanć, A.; Sperdouli, I.; Moustakas, M. Combined Impact of Excess Zinc and Cadmium on Elemental Uptake, Leaf Anatomy and Pigments, Antioxidant Capacity, and Function of Photosynthetic Apparatus in Clary Sage (Salvia sclarea L.). Plants 2022, 11, 2407. [Google Scholar] [CrossRef]
- Sperdouli, I.; Adamakis, I.-D.S.; Dobrikova, A.; Apostolova, E.; Hanć, A.; Moustakas, M. Excess Zinc Supply Reduces Cadmium Uptake and Mitigates Cadmium Toxicity Effects on Chloroplast Structure, Oxidative Stress, and Photosystem II Photochemical Efficiency in Salvia sclarea Plants. Toxics 2022, 10, 36. [Google Scholar] [CrossRef]
- Detterbeck, A.; Pongrac, P.; Persson, D.P.; Vogel-Mikuš, K.; Kelemen, M.; Vavpetič, P.; Pelicon, P.; Arčon, I.; Husted, S.; Kofod Schjoerring, J.; et al. Temporal and Spatial Patterns of Zinc and Iron Accumulation during Barley (Hordeum vulgare L.) Grain Development. J. Agric. Food Chem. 2020, 68, 12229–12240. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Gil-Díaz, M.M.; Pinilla, P.; Lobo, M.C. Impact of Cr and Zn on Growth, Biochemical and Physiological Parameters, and Metal Accumulation by Wheat and Barley Plants. Water Air Soil Pollut. 2017, 228, 419. [Google Scholar] [CrossRef]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and Function of Zinc Plants. In Zinc in Soils and Plants: Proceedings of the International Symposium on ‘Zinc in Soils and Plants’ Held at The University of Western Australia, 27–28 September 1993; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 93–106. [Google Scholar]
- Zolotova, E.; Ryabinin, V. Elements Distribution in Soil and Plants of an Old Copper Slag Dump in the Middle Urals, Russia. Ecol. Quest. 2019, 30, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Dikarev, A.V.; Dikarev, V.G.; Dikareva, N.S. The assessment of cadmium nitrate effect on morphological and cytogenetic indices of spring barley (Hordeum vulgare) seedlings. Braz. J. Bot. 2021, 44, 43–56. [Google Scholar] [CrossRef]
- Sabella, E.; Aprile, A.; Tenuzzo, B.A.; Carata, E.; Panzarini, E.; Luvisi, A.; De Bellis, L.; Vergine, M. Effects of Cadmium on Root Morpho-Physiology of Durum Wheat. Front. Plant Sci. 2022, 13, 936020. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.S.; Wang, Y.J.; Ding, G.; Ma, H.L.; Zhang, Y.J.; Gong, J.M. A Pivotal Role of Cell Wall in Cadmium Accumulation in the Crassulaceae hyperaccumulator Sedum plumbizincicola. Mol. Plant 2017, 10, 771–774. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron alleviates cadmium toxicity in Brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci. Total Environ. 2020, 728, 138833. [Google Scholar] [CrossRef]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Della Rovere, F.; Eiche, E.; Riemann, M.; Altamura, M.M.; Falasca, G. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- Benáková, M.; Ahmadi, H.; Dučaiová, Z.; Tylová, E.; Clemens, S.; Tůma, J. Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ. Sci. Pollut. Res. 2017, 24, 20705–20716. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z.; Clode, P.L.; Kilburn, M.R.; Guagliardo, P.; Romic, D. Zinc and cadmium mapping by NanoSIMS within the root apex after short-term exposure to metal contamination. Ecotoxicol Env. Saf 2019, 171, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.F.; Yang, X.E.; Islam, E.; Liu, D.; Mahmood, Q.; Li, H.; Li, J. Ultrastructural changes, zinc hyperaccumulation and its relation with antioxidants in two ecotypes of Sedum alfredii Hance. Plant Physiol. Biochem. 2008, 46, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Santoro, P.H.; Neves, P.M.O.J.; Alexandre, T.M.; Sartori, D.; Alves, L.F.A.; Fungaro, M.H.P. Selection of Beauveria bassiana isolates to control Alphitobius diaperinus. J. Invertebr. Pathol. 2008, 97, 83–90. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Zhao, Z.Q.; Li, H.Y.; Smith, S.E.; Smith, F.A. Effect of Zinc–Cadmium Interactions on the Uptake of Zinc and Cadmium by Winter Wheat (Triticum aestivum) Grown in Pot Culture. Bull. Environ. Contam. Toxicol. 2003, 71, 1289–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Khan, A.M.; Rengel, Z. Zinc-biofortified wheat accumulates more cadmium in grains than standard wheat when grown on cadmium-contaminated soil regardless of soil and foliar zinc application. Sci. Total Environ. 2019, 654, 402–408. [Google Scholar] [CrossRef]
- Zare, A.A.; Khoshgoftarmanesh, A.H.; Malakouti, M.J.; Bahrami, H.A.; Chaney, R.L. Root uptake and shoot accumulation of cadmium by lettuce at various Cd:Zn ratios in nutrient solution. Ecotoxicol. Environ. Saf. 2018, 148, 441–446. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Kochian, L.V. Transport interactions between cadmium and zinc in roots of bread and durum wheat seedlings. Physiol Plant 2002, 116, 73–78. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, B.; Liu, H.; Liang, X.; Ma, W.; Shi, Z.; Yang, S. Zinc effects on cadmium toxicity in two wheat varieties (Triticum aestivum L.) differing in grain cadmium accumulation. Ecotoxicol Env. Saf 2019, 183, 109562. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Z.; Li, J.; Yao, Q.; Tan, W.; Xing, W.; Lu, Z. Effects of cadmium stress on the morphology, physiology, cellular ultrastructure, and BvHIPP24 gene expression of sugar beet (Beta vulgaris L.). Int. J. Phytoremediation 2022, 1–11. [Google Scholar] [CrossRef]
- ISO10381-1; Soil Quality. Sampling. Part 1. Guidance on the Design of Sampling Programmes: Geneva, Switzerland, 2002.
- Minkina, T.M.; Linnik, V.G.; Nevidomskaya, D.G.; Bauer, T.V.; Mandzhieva, S.S.; Khoroshavin, V.Y. Forms of Cu (II), Zn (II), and Pb (II) compounds in technogenically transformed soils adjacent to the Karabashmed copper smelter. J. Soils Sediments 2018, 18, 2217–2228. [Google Scholar] [CrossRef]
- Tumanyan, A.F.; Shcherbakova, N.A.; Tusaint, F.; Seliverstova, A.P.; Tyutyuma, N.V. Heavy Metal Contents in Soils and Vegetables of Southern Russia. Chem. Technol. Fuels Oils 2019, 54, 766–770. [Google Scholar] [CrossRef]
- Lukin, S.V.; Selyukova, S.V. Ecological Assessment of the Content of Cadmium in Soils and Crops in Southwestern Regions of the Central Chernozemic Zone, Russia. Eurasian Soil Sci. 2018, 51, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Minkina, T.M.; Mandzhieva, S.S.; Burachevskaya, M.V.; Bauer, T.V.; Sushkova, S.N. Method of determining loosely bound compounds of heavy metals in the soil. MethodsX 2018, 5, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Du Laing, G.L.A.; Tack, F.L.A.; Verloo, M. Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Anal. Chim. Acta 2003, 497, 191–198. [Google Scholar] [CrossRef]


| Rate of Application | Zn | Cd | ||
|---|---|---|---|---|
| Total Content | Exchangeable Form Concentration | Total Content | Exchangeable Form Concentration | |
| Control | 68 ± 7 | 0.7 ± 0.1 | 0.27 ± 0.02 | 0.02 ± 0.002 |
| 2 MPC | 512 ± 62 * | 27.1 ± 2 * | 4.18 ± 0.31 * | 0.85 ± 0.11 * |
| 5 MPC | 1154 ± 104 * | 75.4 ± 6 * | 10.05 ± 0.93 * | 2.27 ± 0.22 * |
| 2 MPC Zn + 2 MPC Cd | 487 ± 35 * | 39.0 ± 3 * | 4.23 ± 0.31 * | 1.15 ± 0.10 * |
| 5 MPC Zn + 5 MPC Сd | 1142 ± 99 * | 135.0 ± 9 * | 10.11 ± 0.92 * | 3.52 ± 0.30 * |
| Rate of HM Application, mg/кg | Root Cross-section Area | Central Cylinder Cross-Section Area | Ratio Between Root Cross-Section and Total Area | Ratio of Epidermis to Total Area | Medium Size of the Epidermis Cell |
|---|---|---|---|---|---|
| Control | 84472 ± 2254 | 13800 ± 527 | 6.1 | 5.1 | 445 ± 37 |
| 2 MPC Zn | 49211 ± 1931 * | 15535 ± 642 * | 3.2 | 2.2 | 337 ± 39 * |
| 5 MPC Zn | 29143 ± 958 * | 10744 ± 527 * | 2.7 | 1.7 | 207 ± 46 * |
| 2 MPC Cd | 28832 ± 821 * | 12361 ± 610 * | 2.3 | 1.3 | 213 ± 59 * |
| 5 MPC Cd | 22161 ± 798 * | 12253 ± 701 * | 1.8 | 0.8 | n/d |
| 2 MPC Zn + 2 MPC Cd | 56876 ± 1029 * | 10574 ± 496 * | 5.4 | 4.4 | 575 ± 81 * |
| 5 MPC Zn + 5 MPC Cd | 19130 ± 693 * | 19130 ± 783 * | 1.0 | n/d | n/d |
| Rate of Application, mg/kg | Number of Chlorenchyma Cells Per 1 μm2 of Cross-Section | Ratio Between Cells’ Cross-Section to Inter-Cell Space | The Average Chlorenchyma Size, µm2 | Number of Laminas in a Parenchyma Cell |
|---|---|---|---|---|
| Control | 2981 ± 215 | 0.6 | 124 ± 16 | 6.7 ± 0.8 |
| 2 MPC Zn | 2791 ± 186 | 0.6 | 129 ± 12 | 6.4 ± 0.8 |
| 5 MPC Zn | 2759 ± 195 | 0.5 | 122 ± 11 | 6.0 ± 0.7 |
| 2 MPC Cd | 2901 ± 208 | 0.6 | 131 ± 15 | 5.9 ± 0.9 |
| 5 MPC Cd | 2659 ± 173 | 0.5 | 129 ± 14 | 5.0 ± 0.7 * |
| 2 MPC Zn + 2 MPC Cd | 2871 ± 205 | 0.6 | 136 ± 15 | 5.6 ± 0.8 |
| 5 MPC Zn + 5 MPC Cd | 2810 ± 191 | 0.5 | 147 ± 19 | 4.4 ± 0.9 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandzhieva, S.; Chaplygin, V.; Chernikova, N.; Fedorenko, A.; Voloshina, M.; Minkina, T.; Rajput, V.D.; Elinson, M.; Wong, M.H. Responses of Spring Barley to Zn- and Cd-Induced Stress: Morphometric Analysis and Cytotoxicity Assay. Plants 2022, 11, 3332. https://doi.org/10.3390/plants11233332
Mandzhieva S, Chaplygin V, Chernikova N, Fedorenko A, Voloshina M, Minkina T, Rajput VD, Elinson M, Wong MH. Responses of Spring Barley to Zn- and Cd-Induced Stress: Morphometric Analysis and Cytotoxicity Assay. Plants. 2022; 11(23):3332. https://doi.org/10.3390/plants11233332
Chicago/Turabian StyleMandzhieva, Saglara, Victor Chaplygin, Natalia Chernikova, Aleksey Fedorenko, Marina Voloshina, Tatiana Minkina, Vishnu D. Rajput, Maria Elinson, and Ming Hung Wong. 2022. "Responses of Spring Barley to Zn- and Cd-Induced Stress: Morphometric Analysis and Cytotoxicity Assay" Plants 11, no. 23: 3332. https://doi.org/10.3390/plants11233332







