On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) Cultivars in South Africa
Abstract
:1. Introduction
2. Results
2.1. Pooled Analysis of Variance (ANOVA)
2.2. Evaluation of Genotype and Environment Performances
2.3. Mean Performance of the Genotypes
2.4. Trait Association
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
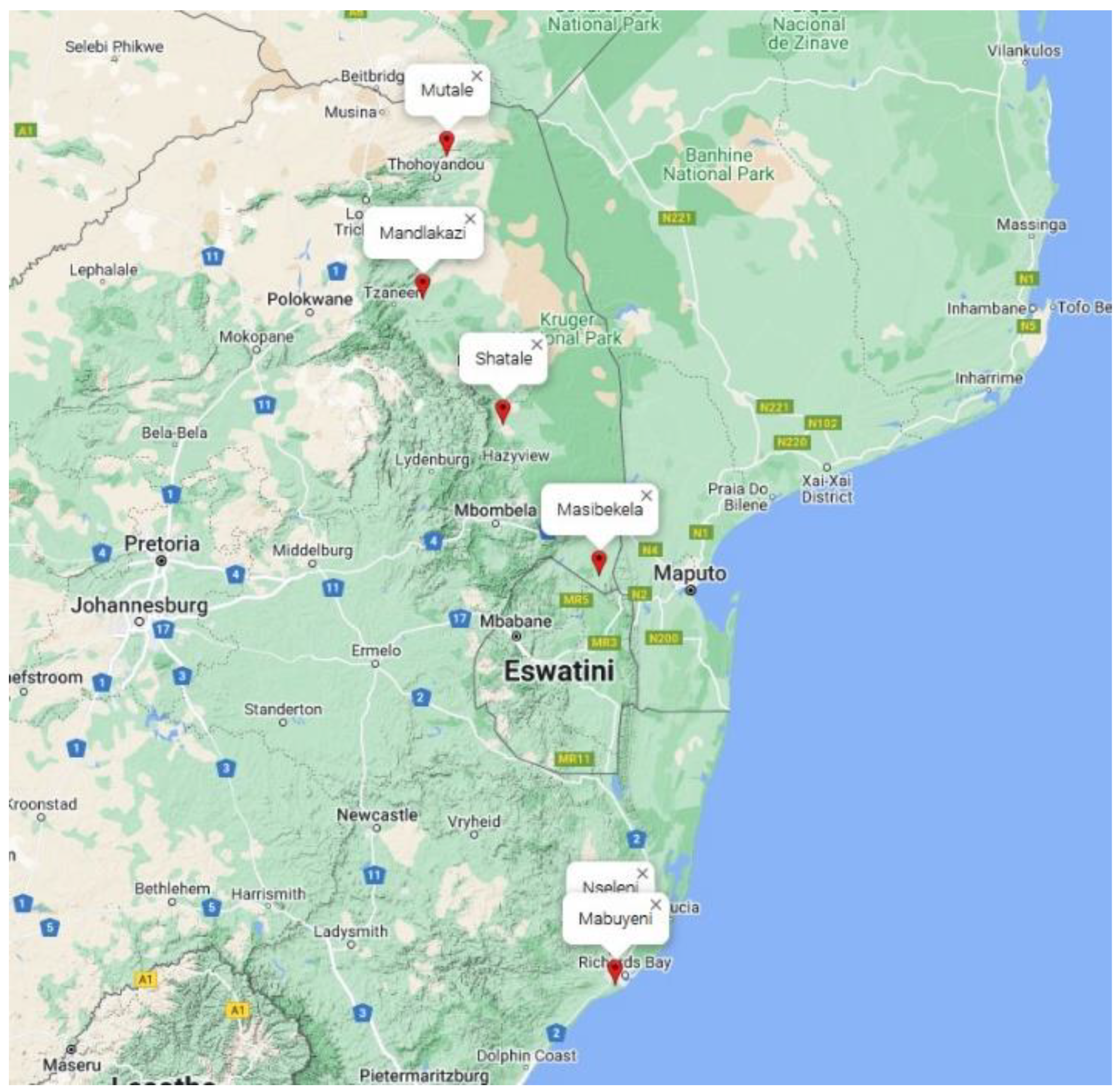
4.1. Testing Environment
4.2. Planting Material and Experimental Design
4.3. Data Collected
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdu-Raheem, K.A.; Worth, S.H. Household Food Security in South Africa: Evaluating Extension’s Paradigms Relative to the Current Food Security and Development Goals. S. Afr. J. Agric. Exten. 2011, 39, 91–103. Available online: https://hdl.handle.net/10520/EJC120889 (accessed on 7 June 2022).
- Donnenfeld, Z.; Crookes, C.; Hedden, S. A Delicate Balance: Water Scarcity in South Africa. In Southern Africa Report 13. Institute for Security Studies and Frederick S. Pardee Center for International Futures; Josef Korbel School of International Studies, University of Denver: Denver, CO, USA, 2018; p. 23. [Google Scholar]
- Baleta, H.; Pegram, G. Water as an Input in the Food Value Chain. In Understanding the Food Energy Water Nexus; WWF South Africa: Johannesburg, South Africa, 2014. [Google Scholar]
- Prochnik, S.; Marri, P.R.; Desany, B.; Rabinowicz, P.D.; Kodira, C.; Mohiuddin, M.; Rodriguez, F.; Fauquet, C.; Tohme, J.; Harkins, T.; et al. The cassava genome: Current progress, future directions. Trop. Plant Biol. 2012, 5, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2004, 56, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations Statistics. Food and Agriculture Organization of the United Nations statistics. Available online: http://faostat.fao.org/default.htm (accessed on 7 June 2022).
- Giller, K.E.; Wilson, K.J. Nitrogen Fixation in Tropical Cropping Systems; CABI: Wallingford, UK, 1991. [Google Scholar]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant Mol. Biol. 2003, 53, 621–641. [Google Scholar] [CrossRef]
- Hillocks, R.J. Cassava in Africa. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI: Wallingford, UK, 2002; pp. 41–54. [Google Scholar]
- United Nations Commodity Trade Statistics Database. Available online: http://comtrade.un.org/ (accessed on 24 July 2019).
- Amelework, A.B.; Bairu, M.W.; Maema, O.; Venter, S.L.; Laing, M. Adoption and promotion of resilient crops for climate risk mitigation and import substitution: A case analysis of cassava for South African agriculture. Front. Sustain. Food Sys. 2021, 5, 617783. [Google Scholar] [CrossRef]
- Hu, X.; Yan, S.; Li, S. The influence of error variance variation on analysis of genotype stability in multi-environment trials. Field Crops Res. 2014, 156, 84–90. [Google Scholar] [CrossRef]
- Hu, X.; Yan, S.; Shen, K. Heterogeneity of error variance and its influence on genotype comparison in multi-environment trials. Field Crops Res. 2013, 149, 322–328. [Google Scholar] [CrossRef]
- Egesi, C.N.; Ilona, P.; Ogbe, F.O.; Akoroda, M.; Dixon, A. Genetic variation and genotype x environment interaction for yield and other agronomic traits in cassava in Nigeria. Agron. J. 2007, 99, 1137–1142. [Google Scholar] [CrossRef]
- Nduwumuremyi, A.; Melis, R.; Shanahan, P.; Theodore, A. Interaction of genotype and environment effects on important traits of cassava (Manihot esculenta Crantz). Crop J. 2017, 5, 373–386. [Google Scholar] [CrossRef]
- Mtunguja, M.K.; Laswai, H.S.; Kanju, E.; Ndunguru, J.; Muzanila, Y.C. Effect of genotype and genotype by environment interaction on total cyanide content, fresh root, and starch yield in farmer-preferred cassava landraces in Tanzania. Food Sci. Nut. 2016, 4, 791–801. [Google Scholar] [CrossRef]
- Ntawuruhunga, P.; Dixon, A.G.O. Quantitative variation and interrelationship between factors influencing cassava yield. J. Appl. Bioscien. 2010, 26, 1594–1602. [Google Scholar]
- Adjebeng-Danquah, J.; Manu-Aduening, J.; Gracen, V.E.; Asante, I.K.; Offei, S.K. AMMI Stability Analysis and Estimation of Genetic Parameters for Growth and Yield Components in Cassava in the Forest and Guinea Savannah Ecologies of Ghana. Hindawi Inter. J. Agron. 2017, 2017, 8075846. [Google Scholar] [CrossRef]
- Sabesan, T.; Suresh, R.; Saravanan, K. Genetic variability and correlation for yield and grain quality characters of rice grown in coastal saline low land of Tamil Nadu. J. Plant Breed. 2009, 1, 56–59. [Google Scholar]
- Singh, A.K.; Sharma, P.; Singh, P.K. Studies on genetic characteristic of upland rice (Oryza sativa L.). Intern. J. Agricult. Environ. Biotechn. 2013, 6, 515–520. [Google Scholar] [CrossRef]
- Kaluba, P.; Mwamba, S.; Moualeu-Ngangue, D.P.; Chiona, M.; Munyinda, K.; Winter, E.; Stützel, H.; Chishala, B.H. Cropping practices and effects on soil nutrient adequacy levels and cassava yield of smallholder farmers in northern Zambia. Intern. J. Agron. 2021, 2021, 1325964. [Google Scholar] [CrossRef]
- Maur, R.S.S.; Neto, A.C.A.; Josa, A.R.S.; Lima, R.S.; Moreira, E.S.; Prado, T.R.; Silva, R.A.; Moreira, G.L.P. Effect of weeds on yield loss of cassava plants in response to NPK fertilization. Afr. J. Agric. Res. 2016, 5, 356–370. [Google Scholar] [CrossRef] [Green Version]
- Ebah-Djedi, B.C.; Dje, K.M.; Zue, B.N.; Zohouri, G.P.; Amani, N.G. Effect of harvest period on starch yield and dry matter content from the tuberous roots of improved cassava (Manihot esculenta Crantz) varieties. Pakistan J. Nut. 2012, 11, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Lebot, V. Tropical root and tuber crops: Cassava, sweet potato, yams and aroids. In Crop Production Science in Horticulture; CABI: Wallingford, UK, 2009. [Google Scholar]
- Carvalho, L.; Filho, J.F.; Anderson, J.V.; Figueiredo, P.G.; Chen, S. Storage root of cassava: Morphological types, anatomy, formation, growth, development and harvest time. In Cassava; Waisundara, V., Ed.; IntechOpen: London, UK, 2017; pp. 55–68. [Google Scholar] [CrossRef] [Green Version]
- Aina, O.O.; Dixon, A.G.O.; Akinrinde, E.A. Genetic variability in cassava as it influences storage root yield in Nigeria. J. Biolog. Sci. 2007, 7, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Teye, E.; Asare, A.P.; Amoah, R.S.; Tetteh, J.P. Determination of the dry matter content of cassava (Manihot esculenta Crantz) tubers using specific gravity method. ARPN J. Agric. Biol. Sci. 2011, 6, 23–28. [Google Scholar]
- Mehouenou, F.M.; Dassou, A.; Sanoussi, F.; Dansi, A.; Adjatin, A.; Dansi, M.; Assogba, P.; Ahissou, H. Physicochemical characterization of cassava (Manihot esculenta) elite cultivars of Southern Benin. Int. J. Adv. Res. Biol. Sci. 2016, 3, 190–199. [Google Scholar]
- Okechukwu, R.U.; Dixon, A.G.O. Performance of improved cassava genotypes for early bulking, disease resistance, and culinary qualities in an Inland Valley ecosystem. Agron. J. 2009, 101, 1258–1265. [Google Scholar] [CrossRef]
- Esuma, W.; Kawuki, R.S.; Herselman, L.; Labuschagne, M.T. Stability and genotype by environment interaction of provitamin a carotenoid and dry matter content in cassava in Uganda. Breed. Sci. Prev. 2016, 66, 16004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceballos, H.; Pérez, J.C.; Joaqui, B.O.; Lenis, J.I.; Morante, N.; Calle, F.; Pino, L.; Hershey, C.H. Cassava breeding I: The value of breeding value. Front. Plant Sci. 2016, 7, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitayavardhana, S.; Shrestha, P.; Rasmussen, M.L.; Lamsal, B.P.; van Leeuwen, J.H.; Khanal, S.K. Ultrasound improved ethanol fermentation from cassava chips in cassava-based ethanol plants. Bioresour. Technol. 2010, 101, 2741–2747. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pre-treatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef]
- Jansson, C.; Westerbergh, A.; Zhang, J.; Hu, X.; Sun, C. Cassava, a potential biofuel crop in People’s Republic of China. Appl. Ener. 2009, 86, S95–S99. [Google Scholar] [CrossRef]
- Seiler, G.J. Root growth of interspecific sunflower seedlings derived from wild perennial sunflower species. Can. J. Plant Sci. 2008, 88, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Adu, M.O.; Yawson, D.O.; Bennett, M.J.; Broadley, M.R.; Dupuy, L.X.; White, P.J. A scanner-based rhizobox system enabling the quantification of root system development and response of Brassica rapa seedlings to external P availability. Plant Root 2017, 11, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Karim, K.Y.; Ifie, B.; Dzidzienyo, D.; Danquah, E.Y.; Blay, E.T.; Whyte, J.B.A.; Kulakow, P.; Rabbi, I.; Parkes, E.; Omoigui, L.; et al. Genetic characterization of cassava (Manihot esculenta Crantz) genotypes using agro-morphological and single nucleotide polymorphism markers. Physiol. Mol. Biol. Plants 2020, 26, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Aigbe, S.O.; Remison, S.U. The influence of root rot on dry matter partition of three cassava cultivars planted in different agro-ecological environments. Asian J. Plant Pathol. 2010, 4, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Edet, M.A.; Lagoke, S.T.O.; Tijani-Eniola, H.; Tarawali, G. Relationship of cassava growth parameters with yield, yield related components and harvest time in Ibadan, Southwestern Nigeria. J. Nat. Sci. Res. 2015, 5, 87–92. [Google Scholar]
- Tumuhimbise, R.; Shanahan, P.; Melis, R.; Kawuki, R. Genetic variation and association among factors influencing storage root bulking in cassava. J. Agric. Sci. 2015, 153, 1267–1280. [Google Scholar] [CrossRef]
- Adu, M.O.; Asare, P.A.; Asare-Bediako, E.; Amenorpe, G.; Ackah, F.K.; Afutu, E.; Amoah, M.N.; Yawson, D.O. Characterising shoot and root system trait variability and contribution to genotypic variability in juvenile cassava (Manihot esculenta Crantz) plants. Heliyon 2018, 4, e00665. [Google Scholar] [CrossRef] [PubMed]
- Okogbenin, E.; Setter, T.L.; Ferguson, M.; Mutegi, R.; Ceballos, H.; Olasanmi, B.; Fregene, M. Phenotypic approaches to drought in cassava: Review. Front. Physiol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekanayake, I.J.; Osiru, D.S.O.; Porto, M.C.M. Physiology of Cassava. IITA Research Guide No. 55, 3rd ed.; IITA: Ibadan, Nigeria, 1998; p. 32. [Google Scholar]
- Rao, B.B.; Ashok, P.; Ramanandam, G.; Sasikala, K. Trait association and path coefficient analyses in cassava. Intern. J. Veget. Sci. 2015, 21, 402–415. [Google Scholar] [CrossRef]
- Gifford, M.L.; Banta, J.A.; Katari, M.S.; Hulsmans, J.; Chen, L.; Ristova, D.; Tranchina, D.; Purugganan, M.l.D.; Coruzzi, G.M.; Birnbaum, K.D. Plasticity regulators modulate specific root traits in discrete nitrogen environments. PLoS Genet. 2013, 9, e1003760. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.; Wairegi, L.; Asadu, C.L.A.; Asawalam, D.O.; Jokthan, G.; Ugbe, U. Cassava System Cropping Guide. In Africa Soil Health Consortium, Nairobi; CAB International: Wallingford, UK, 2014. [Google Scholar]
- Fukuda, W.M.G.; Guevara, C.L.; Kawuki, R.; Ferguson, M.E. Selected Morphological and Agronomic Descriptors for the Characterization of Cassava; International Institute of Tropical Agriculture (IITA): Ibadan, Nigeria, 2010; p. 19. [Google Scholar]
- GenStat. GenStat for Windows, 9th ed.; VSN International Ltd.: Oxford, UK, 2020. [Google Scholar]
- Padi, F.K. Genotype * environment interaction and yield stability in a cowpea-based cropping system. Euphytica 2007, 158, 11–25. [Google Scholar] [CrossRef]
- Ssemakula, G.; Dixon, A. Genotype X environment interaction, stability and agronomic performance of carotenoid-rich cassava clones. Sci. Res. Ess. 2007, 2, 390–399. [Google Scholar]
- Dixon, A.G.O.; Nukenine, E.N. Genotype X environment interaction and optimum resource allocation for yield and yield componenets of cassava. Afr. Crop Sci. J. 2000, 8, 1–10. [Google Scholar] [CrossRef]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of genetic diversity assessment in crop plants and its recent advances: An overview of its analytical perspectives. Genet. Res. Inter. 2015, 2015, 431487. [Google Scholar] [CrossRef]
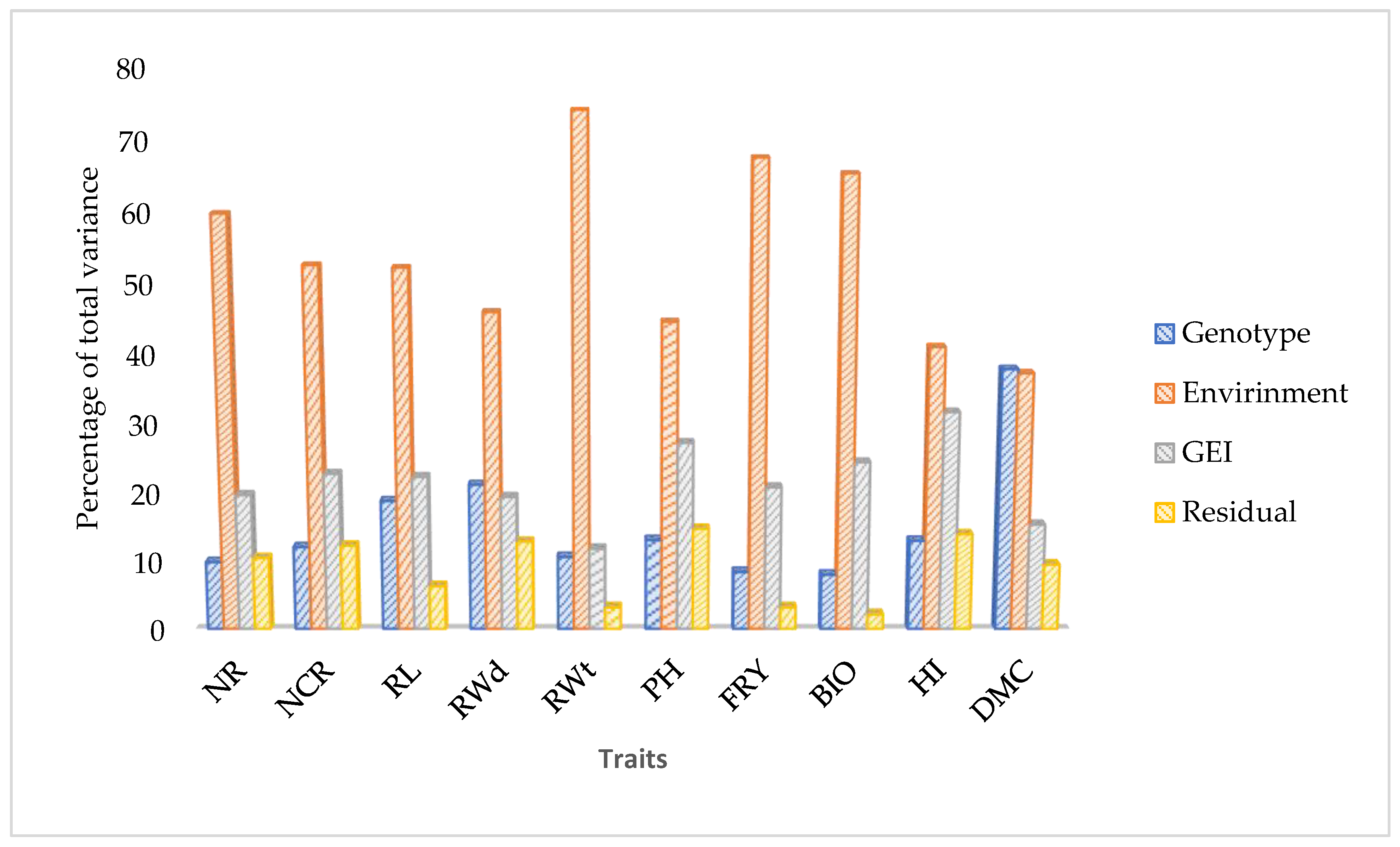
| Source | DF | NR | NCR | RL | RWd | RWt | PH | BIO | FRY | DMC | HI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Env | 5 | 153.3 ** | 58.97 ** | 1083.60 ** | 35.06 ** | 16.66 ** | 1.80 ** | 157823 ** | 83075 ** | 877.6 ** | 0.33 ** |
| Gen | 10 | 13.4 ** | 6.90 ** | 183.18 ** | 6.08 ** | 0.62 ** | 0.28 ** | 7455 ** | 2026 ** | 476.3 ** | 0.04 ** |
| Rep(E) | 12 | 1.37 | 0.60 | 7.20 | 1.31 | 0.08 | 0.06 | 962 | 650 | 15.0 | 0.01 |
| GEI | 50 | 8.0 ** | 3.93 ** | 75.53 ** | 2.01 ** | 0.27 ** | 0.17 ** | 7015 ** | 2208 ** | 61.3 ** | 0.04 ** |
| Error | 120 | 1.47 | 0.76 | 7.60 | 0.44 | 0.03 | 0.03 | 193 | 118 | 12.7 | 0.01 |
| H2 | 0.69 | 0.74 | 0.51 | 0.81 | 0.19 | 0.77 | 0.56 | 0.41 | 0.72 | 0.93 |
| Environment | KZN | Mpumalanga | Limpopo | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| Nseleni | Mabuyeni | Masibekela | Shatale | Mandlakazi | Mutale | Mean | CV (%) | |
| NR | 4.33 ± 0.8 | 8.00 ± 1.1 | 9.93 ± 0.9 | 7.19 ± 0.9 | 10.19 ± 1.3 | 7.09 ± 1.1 | 7.79 ± 1.0 | 15.6 |
| NCR | 2.86 ± 0.6 | 5.91 ± 0.9 | 4.53 ± 0.6 | 5.14 ± 0.7 | 6.61 ± 0.8 | 5.92 ± 1.0 | 5.16 ± 0.7 | 16.9 |
| RL | 23.5 ± 2.2 | 37.13 ± 2.6 | 30.05 ± 1.4 | 37.91 ± 2.3 | 33.28 ± 2.9 | 37.95 ± 2.4 | 33.3 ± 2.2 | 8.3 |
| RWd | 4.88 ± 0.6 | 5.87 ± 0.5 | 4.90 ± 0.6 | 6.14 ± 0.5 | 6.79 ± 0.5 | 7.48 ± 0.6 | 6.01 ± 0.6 | 11 |
| RWt | 0.48 ± 0.1 | 0.83 ± 0.1 | 0.72 ± 0.1 | 1.23 ± 0.2 | 1.69 ± 0.1 | 2.39 ± 0.1 | 1.22 ± 0.1 | 13.6 |
| PH | 1.38 ± 0.2 | 1.98 ± 0.2 | 1.76 ± 0.1 | 1.88 ± 0.2 | 2.04 ± 0.1 | 1.89 ± 0.2 | 1.82 ± 0.1 | 9.3 |
| BIO | 48.65 ± 8.2 | 177.32 ± 13.9 | 112.80 ± 13.4 | 108.31 ± 7.4 | 219.56 ± 10.4 | 198.14 ± 14.5 | 146.98 ± 12.1 | 9.5 |
| FRY | 31.14 ± 4.5 | 76.36 ± 5.1 | 55.49 ± 8.4 | 71.41 ± 10.3 | 117.06 ± 6.5 | 171.93 ± 22.3 | 87.23 ± 9.5 | 12.5 |
| DMC | 40.14 ± 1.6 | 38.61 ± 5.1 | 39.52 ± 2.0 | 51.59 ± 2.5 | 47.55 ± 2.0 | 43.65 ± 3.3 | 43.51 ± 2.9 | 8.2 |
| HI | 0.58 ± 0.1 | 0.47 ± 0.04 | 0.48 ± 0.05 | 0.68 ± 0.1 | 0.61 ± 0.04 | 0.72 ± 0.1 | 0.59 ± 0.1 | 12.6 |
| Geno | NR | NCR | RL | RWd | RWt | PH | BIO | FRY | HI | DMC |
|---|---|---|---|---|---|---|---|---|---|---|
| 98/0002 | 6.50 f | 4.59 ef | 32.15 bc | 7.32 a | 1.43 a | 1.74 def | 149.1 cd | 86.77 cde | 0.61 b | 45.1 b |
| 98/0505 | 7.82 cd | 5.18 bcd | 31.83 bc | 6.21 c | 1.12 de | 1.73 def | 145.3 de | 85.59 de | 0.60 bc | 45.3 b |
| MSAF 2 | 8.78 ab | 5.59 bc | 32.25 bc | 5.57 def | 1.19 cd | 1.89 bc | 160.0 b | 100.85 a | 0.58 bc | 41.4 d |
| P1/19 | 7.97 cd | 4.19 f | 29.45 d | 5.33 f | 0.86 d | 1.72 ef | 102.8 g | 60.96 f | 0.56 c | 48.5 a |
| P4/10 | 7.55 cde | 5.04 a | 32.55 b | 5.97 cd | 1.24 bc | 1.95 b | 150.6 cd | 95.02 ab | 0.60 bc | 45.7 b |
| UKF3 | 7.35 de | 4.82 dc | 32.60 b | 6.67 b | 1.48 a | 1.72 ef | 135.4 f | 91.38 bcd | 0.68 a | 43.8 bc |
| UKF4 | 9.52 a | 6.50 a | 39.50 a | 6.19 c | 1.46 a | 1.85 bc | 156.6 bc | 96.11 ab | 0.63 b | 42.1 de |
| UKF5 | 7.36 de | 5.22 bcd | 30.55 cd | 5.57 def | 1.08 e | 1.84 bcd | 181.5 a | 81.77 e | 0.49 d | 30.6 e |
| UKF7 | 6.77 ef | 4.47 def | 33.10 b | 5.92 cd | 1.18 cde | 1.69 f | 133.6 f | 82.06 e | 0.60 bc | 45.5 b |
| UKF8 | 7.75 cd | 5.23 bcd | 32.11 b | 5.48 ef | 1.10 de | 2.10 a | 164.5 b | 80.07 cde | 0.55 c | 41.2 d |
| UKF9 | 8.31 bc | 5.68 b | 39.24 a | 5.88 cdef | 1.31 b | 1.82 cde | 137.3 ef | 92.96 bc | 0.61 b | 49.5 a |
| NR | - | |||||||||
| NCR | 0.69 *** | - | ||||||||
| RL | 0.41 *** | 0.73 *** | - | |||||||
| RWd | 0.12 | 0.52 *** | 0.52 *** | - | ||||||
| RWt | 0.28 *** | 0.62 *** | 0.61 *** | 0.85 *** | - | |||||
| PH | 0.40 *** | 0.54 *** | 0.47 *** | 0.19 | 0.28 *** | - | ||||
| BIO | 0.40 *** | 0.69 *** | 0.49 *** | 0.54 *** | 0.65 *** | 0.52 *** | - | |||
| HI | 0.02 | 0.21 * | 0.38 *** | 0.51 *** | 0.51 *** | 0.01 | −0.12 | - | ||
| DMC | 0.01 | 0.04 | 0.18 * | 0.20 * | 0.26 ** | 0.10 | −0.18 | 0.43 *** | - | |
| FRY | 0.39 *** | 0.72 *** | 0.61 *** | 0.72 *** | 0.89 *** | 0.42 *** | 0.84 *** | 0.36 *** | 0.09 | - |
| NR | NCR | RL | RWd | RWt | PH | BIOM | HI | DMC | FRY |
| Trait | PC1 | PC2 | PC3 |
|---|---|---|---|
| Above-ground biomass (BIOM) | 0.35 | −0.32 | −0.31 |
| Dry matter content (DMC) | 0.08 | 0.48 | 0.50 |
| Harvest index (HI) | 0.18 | 0.57 | 0.13 |
| No. commercial roots (NCR) | 0.39 | −0.19 | 0.18 |
| No. roots (NR) | 0.24 | −0.32 | 0.45 |
| Plant height (PH) | 0.25 | −0.28 | 0.37 |
| Root length (RL) | 0.35 | 0.02 | 0.21 |
| Root width (RWd) | 0.34 | 0.27 | −0.34 |
| Root weight (RWt) | 0.39 | 0.21 | −0.23 |
| Root fresh yield (FRY) | 0.41 | 0.00 | −0.23 |
| Eigenvalue | 5.04 | 1.80 | 1.09 |
| Percentage variance (%) | 50.35 | 17.98 | 10.92 |
| Cumulative variance (%) | 50.35 | 68.33 | 79.25 |
| Cultivar | Species | Type | Application | Source | Remark |
|---|---|---|---|---|---|
| 98/0002 | M. esculenta | Clones | Food and industrial | IITA | |
| 98/0505 | M. esculenta | Clones | Food and industrial | IITA | |
| MSFA2 | M. esculenta | Clones | Food | ARC | |
| P1/19 | M. esculenta | Clones | Industrial | ARC | |
| P4/10 | M. esculenta | Clones | Industrial | ARC | |
| UKF3 | M. esculenta | Clones | Food and industrial | UKZN | |
| UKF4 | M. esculenta | Clones | Food and industrial | UKZN | |
| UKF5 | M. esculenta | Clones | Food and industrial | UKZN | |
| UKF7 | M. esculenta | Clones | Food and industrial | UKZN | |
| UKF8 | M. esculenta | Clones | Food and industrial | UKZN | |
| UKF9 | M. esculenta | Clones | Food and industrial | UKZN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amelework, A.B.; Bairu, M.W.; Marx, R.; Owoeye, L.; Laing, M.; Venter, S.L. On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) Cultivars in South Africa. Plants 2022, 11, 3339. https://doi.org/10.3390/plants11233339
Amelework AB, Bairu MW, Marx R, Owoeye L, Laing M, Venter SL. On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) Cultivars in South Africa. Plants. 2022; 11(23):3339. https://doi.org/10.3390/plants11233339
Chicago/Turabian StyleAmelework, Assefa B., Michael W. Bairu, Roelene Marx, Lawrence Owoeye, Mark Laing, and Sonja L. Venter. 2022. "On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) Cultivars in South Africa" Plants 11, no. 23: 3339. https://doi.org/10.3390/plants11233339
APA StyleAmelework, A. B., Bairu, M. W., Marx, R., Owoeye, L., Laing, M., & Venter, S. L. (2022). On-Farm Multi-Environment Evaluation of Selected Cassava (Manihot esculenta Crantz) Cultivars in South Africa. Plants, 11(23), 3339. https://doi.org/10.3390/plants11233339











