In Vitro and In Vivo Nephroprotective Effects of Nelumbo nucifera Seedpod Extract against Cisplatin-Induced Renal Injury
Abstract
1. Introduction
2. Results
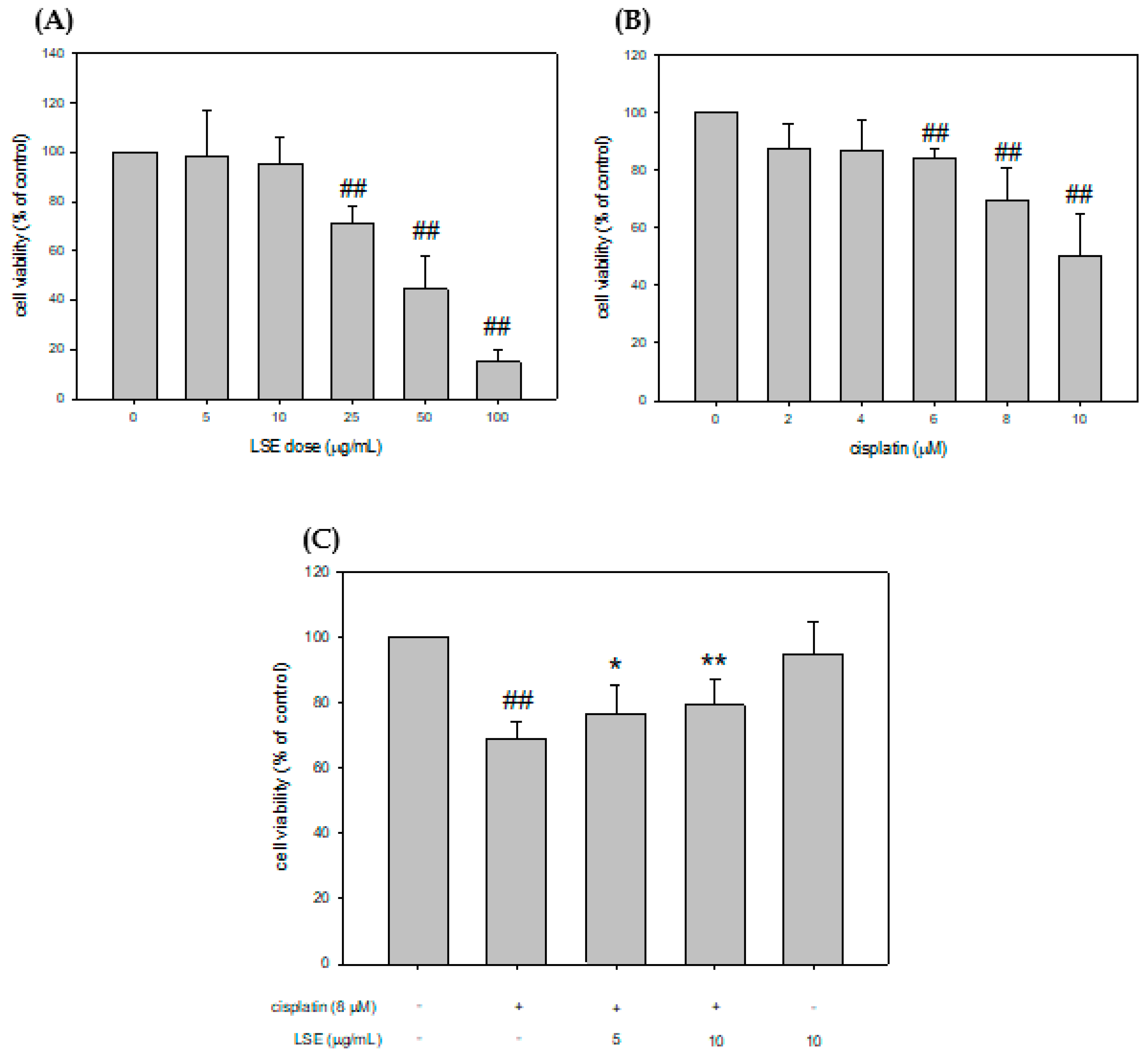
2.1. Effect of LSE on Cell Viability in Cisplatin-Induced NRK-52E Cells
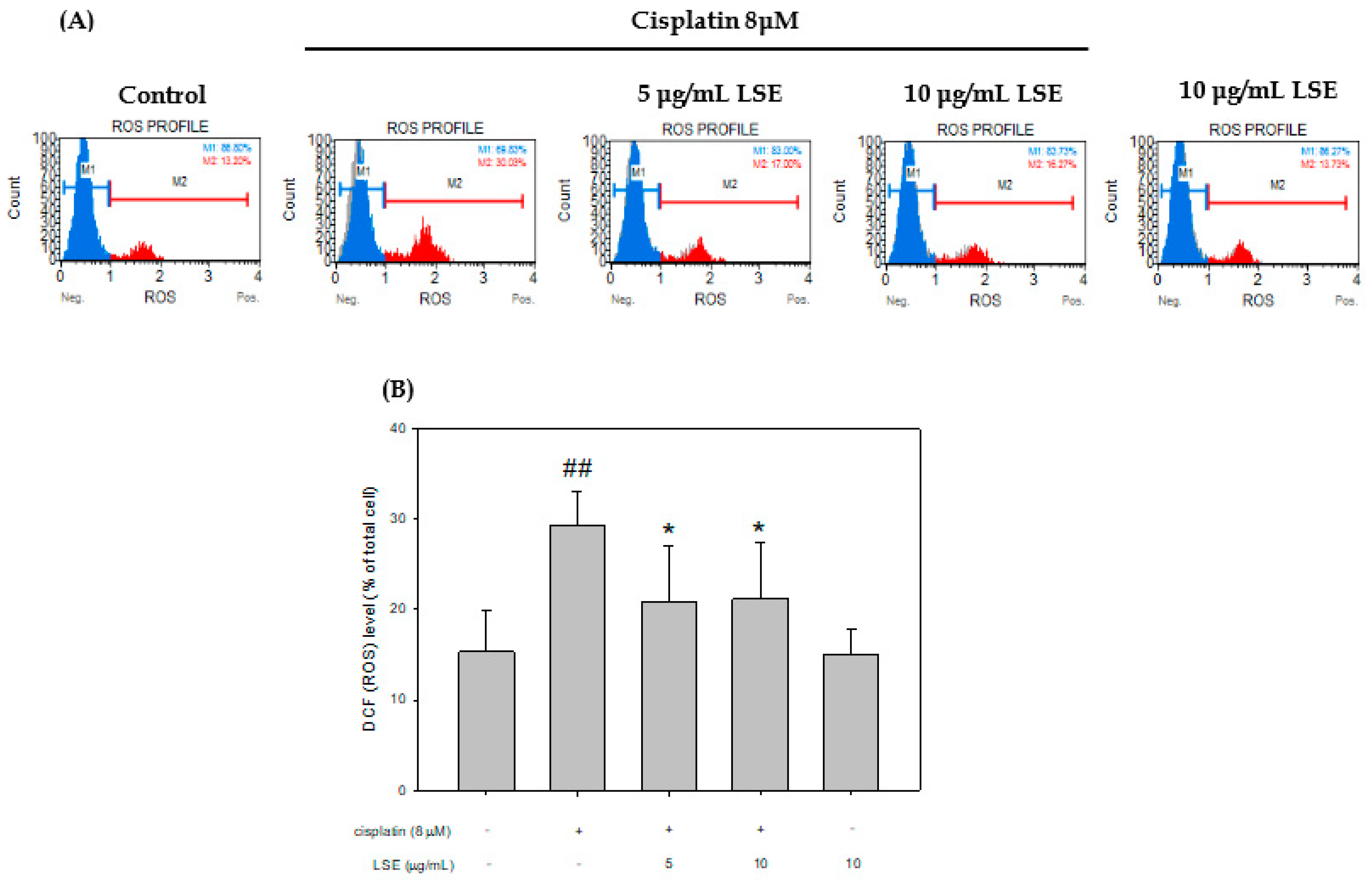
2.2. Effect of LSE on Cisplatin-Induced ROS Production in NRK−52E Cells
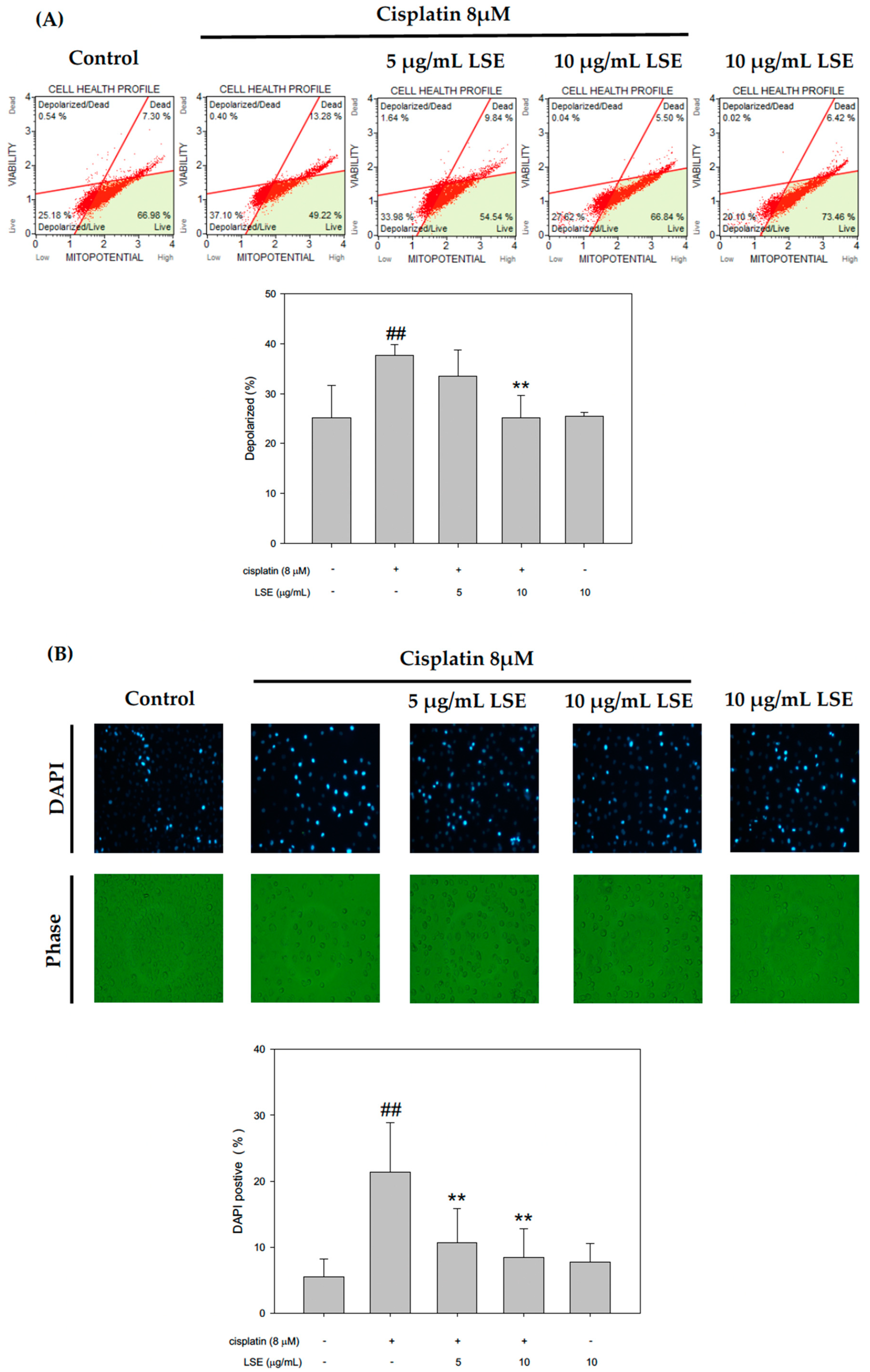
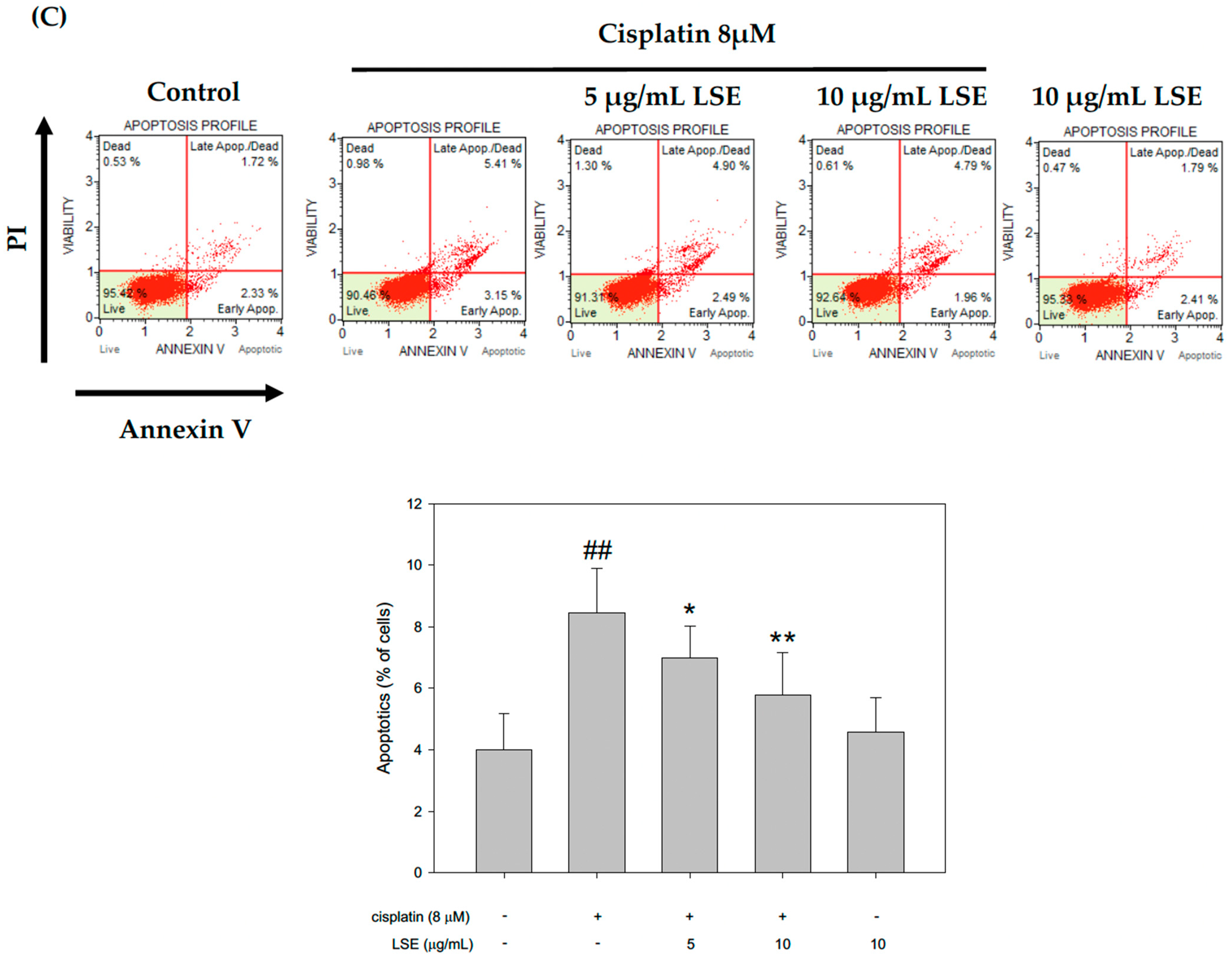
2.3. Effect of LSE on Cisplatin-Induced Apoptosis in NRK−52E Cells
2.4. Effect of LSE on Cisplatin-Induced Apoptotic Pathway in NRK-52E Cells
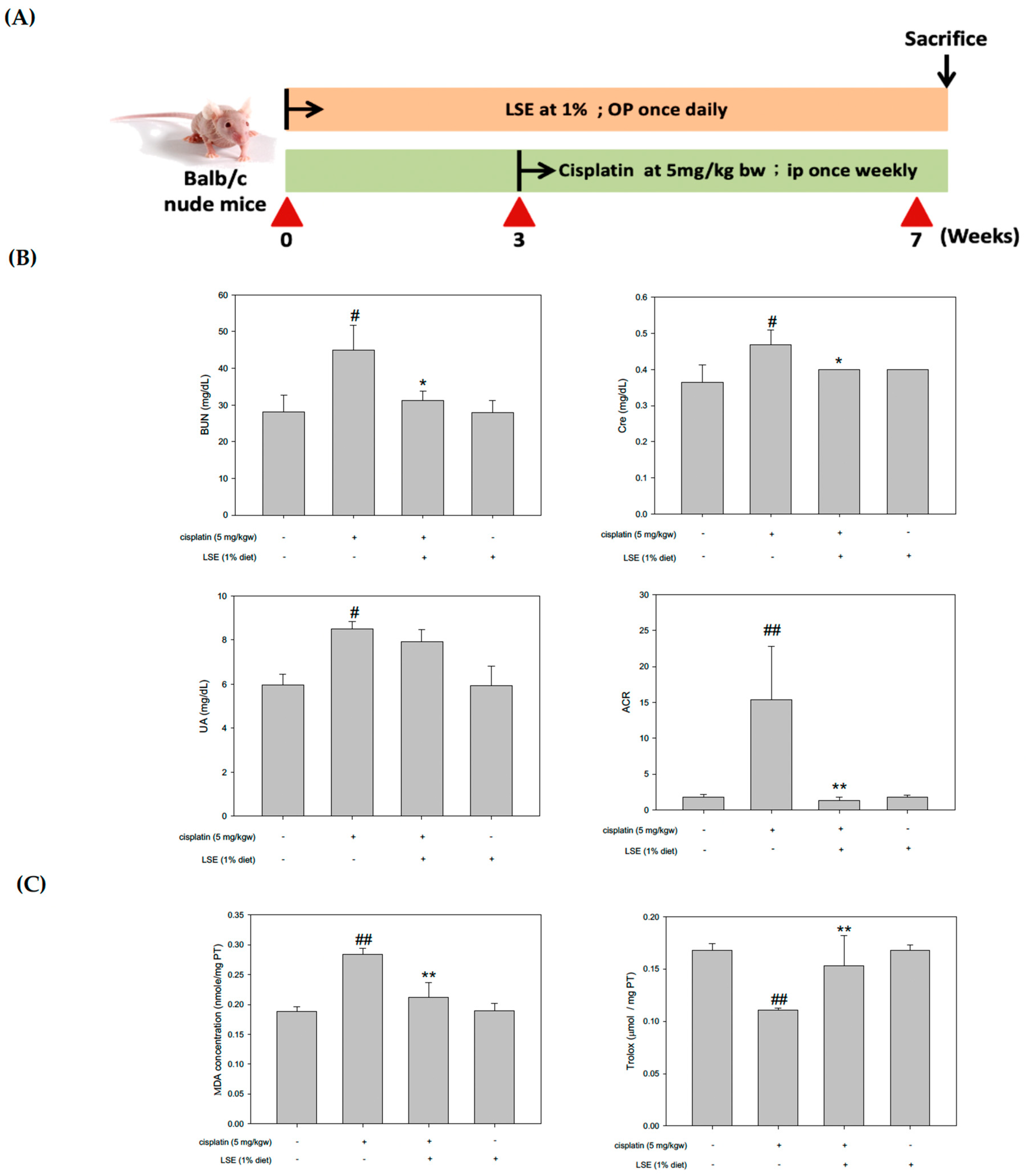
2.5. Effects of LSE on the Kidney Biochemical Parameters, Lipid Peroxidation, and Antioxidant Capacity in Nude Mice Induced by Cisplatin Treatment
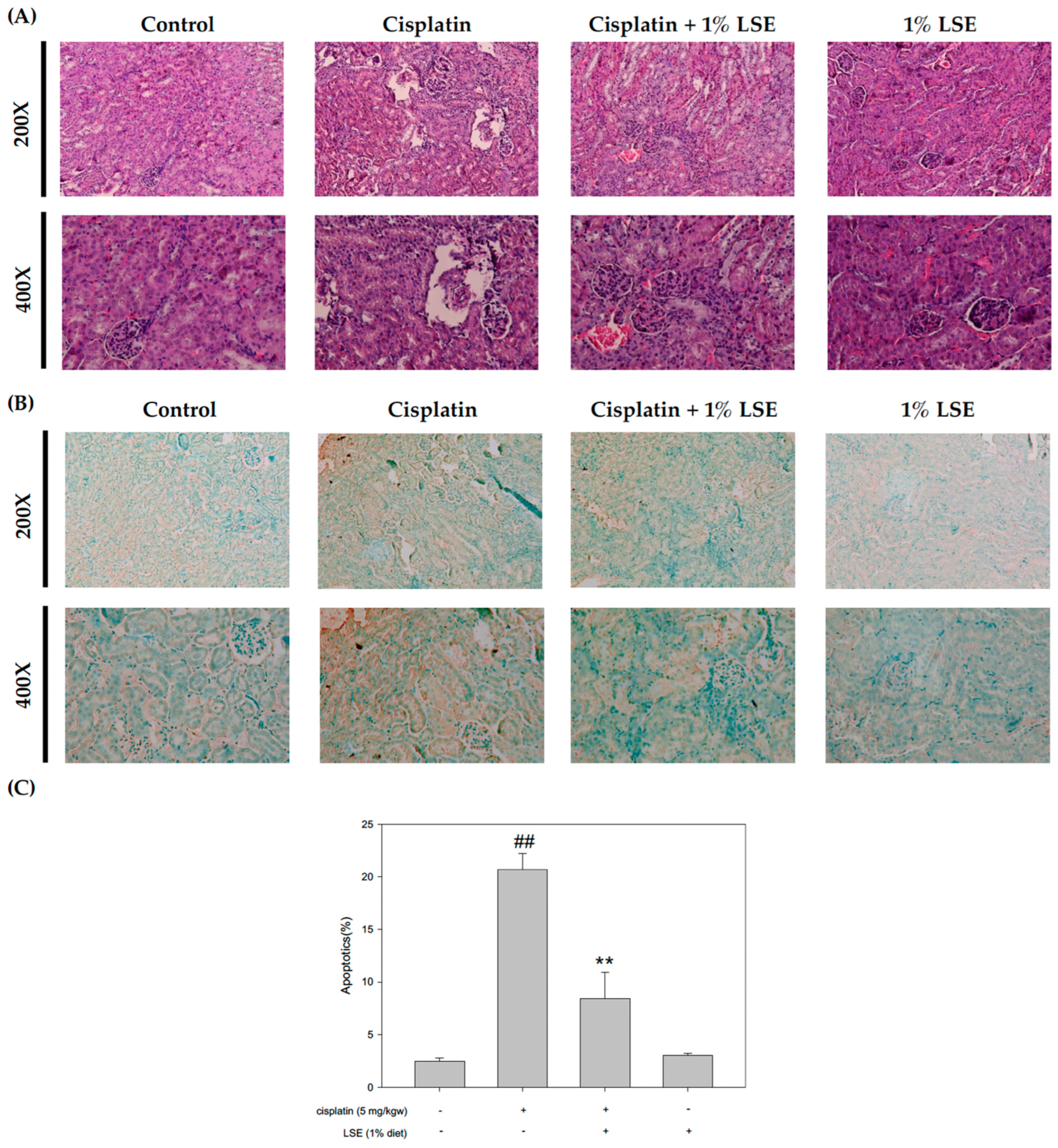
2.6. Effects of LSE on Kidney Histopathology and Apoptosis in Nude Mice Induced by Cisplatin Treatment
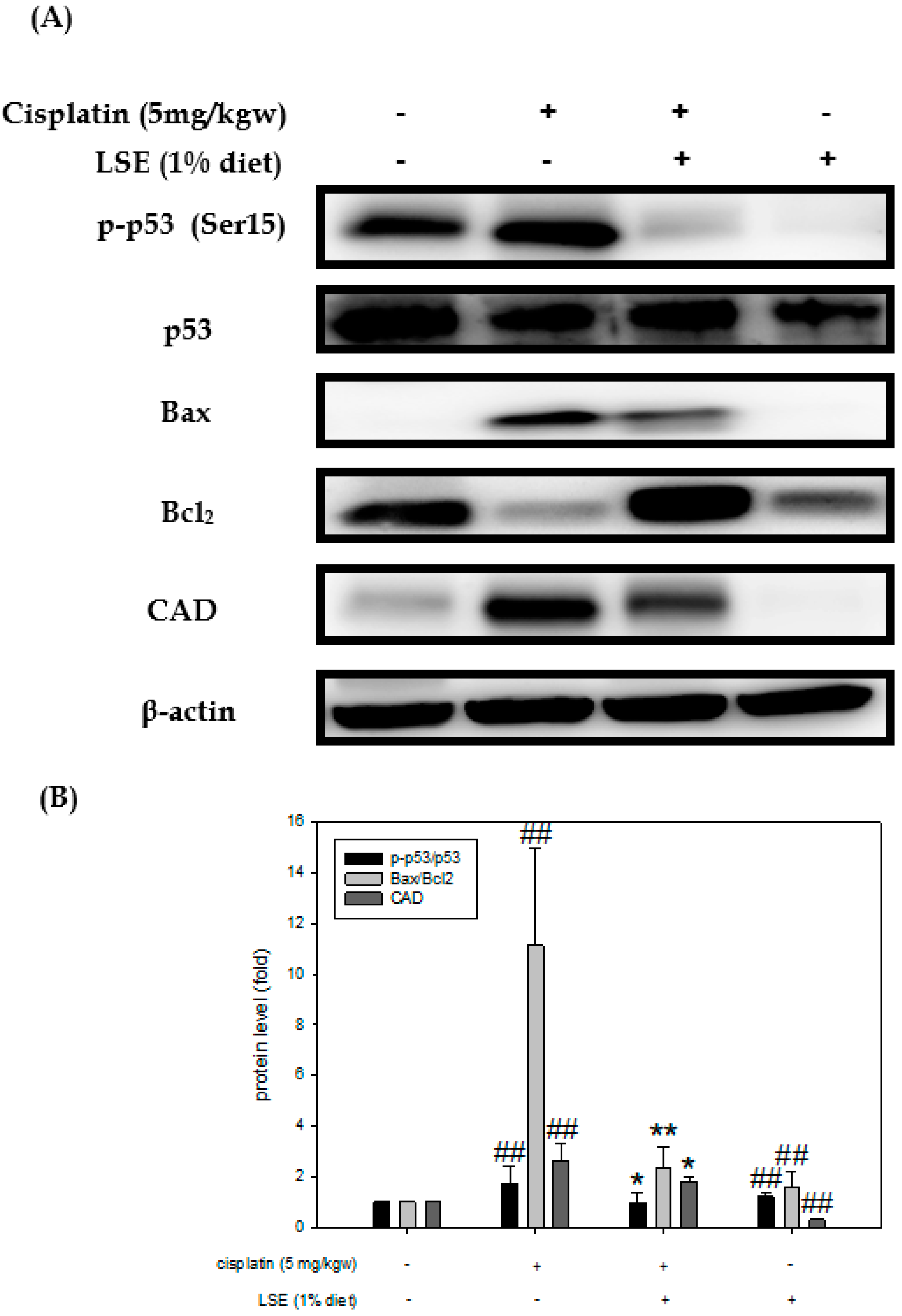
2.7. Effect of LSE on Kidney Expression of Apoptosis-Related Proteins in Nude Mice Induced by Cisplatin Treatment
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Lotus Seedpod Extract (LSE) Preparation
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. DAPI Stain Assay
4.6. Annexin V/PI Stain Assay
4.7. JC-1 Stain Assay
4.8. Reactive Oxygen Species (ROS) Analysis
4.9. Mitochondria Isolation
4.10. Animal and Experimental Design
4.11. Urine and Serum Biochemical Parameters
4.12. Thiobarbituric Acid Reactive Substances (TBARS) Assay
4.13. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
4.14. Hematoxylin-Eosin Staining
4.15. TUNEL Assay
4.16. Protein Extraction and Western Blotting
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.Q.; Li, M. Research Advancement on Cisplatin-Induced Nephrotoxicity. World J. Cancer Res. 2012, 2, 21–26. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 11, 7–15. [Google Scholar] [CrossRef]
- Harder, H.C.; Smith, R.G.; Leroy, A.F. Template primer inactivation by cis- and trans-dichlorodiammine platinum for human DNA polymerase alpha, beta; Rauscher murine leukemia virus reverse transcriptase, as a mechanism of cytotoxicity. Cancer Res. 1976, 36, 3821–3829. [Google Scholar] [PubMed]
- Harder, H.C.; Rosenberg, B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int. J. Cancer 1970, 6, 207–216. [Google Scholar] [CrossRef]
- Zhu, S.; Pabla, N.; Tang, C.; He, L.; Dong, Z. DNA damage response in cisplatin-induced nephrotoxicity. Arch. Toxicol. 2015, 89, 2197–2205. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Kaushal, V.; Hong, X.; Shah, S.V. Role and regulation of activation of caspases in cisplatin-induced injury to renal tubular epithelial cells. Kidney Int. 2001, 60, 1726–1736. [Google Scholar] [CrossRef]
- Casares, C.; Ramirez-Camacho, R.; Trinidad, A.; Roldan, A.; Jorge, E.; Garcia-Berrocal, J.R. Reactive oxygen species in apoptosis induced by cisplatin: Review of physiopathological mechanisms in animal models. Eur. Arch. Otorhinolaryngol. 2012, 269, 2455–2459. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar]
- Lemasters, J.J. Dying a thousand deaths: Redundant pathways from different organelles to apoptosis and necrosis. Gastroenterology 2005, 129, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, M.; Bai, Y.; Wang, X.; Dong, R.; Wu, C. Lotus Seedpod-Derived Hard Carbon with Hierarchical Porous Structure as Stable Anode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 12554–12561. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chyau, C.C.; Wang, C.P.; Wang, T.H.; Chen, J.H.; Lin, H.H. Flavonoids Identification and Pancreatic Beta-Cell Protective Effect of Lotus Seedpod. Antioxidants 2020, 9, 658. [Google Scholar] [PubMed]
- Wu, Q.; Li, S.; Li, X.; Fu, X.; Sui, Y.; Guo, T.; Xie, B.; Sun, Z. A significant inhibitory effect on advanced glycation end product formation by catechin as the major metabolite of lotus seedpod oligomeric procyanidins. Nutrients 2014, 6, 3230–3244. [Google Scholar] [CrossRef]
- Gong, Y.S.; Guo, J.; Hu, K.; Gao, Y.Q.; Xie, B.J.; Sun, Z.D.; Yang, E.N.; Hou, F.L. Ameliorative effect of lotus seedpod proanthocyanidins on cognitive impairment and brain aging induced by D-galactose. Exp. Gerontol. 2016, 74, 21–28. [Google Scholar] [CrossRef]
- Xie, C.; Wang, S.; Cao, M.; Xiong, W.; Wu, L. (E)-9-Octadecenoic Acid Ethyl Ester Derived from Lotus Seedpod Ameliorates Inflammatory Responses by Regulating MAPKs and NF-kappaB Signalling Pathways in LPS-Induced RAW264.7 Macrophages. Evid. Based Complement. Alternat. Med. 2022, 2022, 6731360. [Google Scholar] [CrossRef]
- Cao, J.; Yu, X.; Deng, Z.; Pan, Y.; Zhang, B.; Tsao, R.; Li, H. Chemical Compositions, Antiobesity, Antioxidant Effects of Proanthocyanidins from Lotus Seed Epicarp and Lotus Seed Pot. J. Agric. Food Chem. 2018, 66, 13492–13502. [Google Scholar] [CrossRef]
- Heiskanen, K.M.; Bhat, M.B.; Wang, H.W.; Ma, J.; Nieminen, A.L. Mitochondrial depolarization accompanies cytochrome c release during apoptosis in PC6 cells. J. Biol. Chem. 1999, 274, 5654–5658. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Kruger, K.; Thomale, J.; Stojanovic, N.; Osmak, M.; Henninger, C.; Bormann, S.; Fritz, G. Platinum-induced kidney damage: Unraveling the DNA damage response (DDR) of renal tubular epithelial and glomerular endothelial cells following platinum injury. Biochim. Biophys. Acta 2015, 1853, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wong, Y.S.; Chen, T.; Zhang, H.; Liu, C.; Zheng, W. The reversal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-mercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials 2011, 32, 9068–9076. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.D.; Hou, J.G.; Yang, G.; Jiang, S.; Chen, C.; Wang, Z.; Liu, Y.Y.; Ren, S.; Li, W. Icariin ameliorates cisplatin-induced cytotoxicity in human embryonic kidney 293 cells by suppressing ROS-mediated PI3K/Akt pathway. Biomed. Pharmacother. 2019, 109, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sierra, T.; Eugenio-Perez, D.; Sanchez-Chinchillas, A.; Pedraza-Chaverri, J. Role of food-derived antioxidants against cisplatin induced-nephrotoxicity. Food Chem. Toxicol. 2018, 120, 230–242. [Google Scholar] [CrossRef]
- Higuchi, Y. Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem. Pharmacol. 2003, 66, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.N.; Liu, Z.; Wang, Z.; Ren, S.; Tang, S.; Wang, Y.P.; Xiao, S.Y.; Chen, C.; Li, W. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-kappaB signaling pathways. Food Chem. Toxicol. 2017, 110, 62–73. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Gamad, N.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Telmisartan ameliorates cisplatin-induced nephrotoxicity by inhibiting MAPK mediated inflammation and apoptosis. Eur. J. Pharmacol. 2015, 748, 54–60. [Google Scholar] [CrossRef]
- Khan, R.; Rehman, M.U.; Khan, A.Q.; Tahir, M.; Sultana, S. Glycyrrhizic acid suppresses 1,2-dimethylhydrazine-induced colon tumorigenesis in Wistar rats: Alleviation of inflammatory, proliferation, angiogenic; apoptotic markers. Environ. Toxicol. 2018, 33, 1272–1283. [Google Scholar] [CrossRef]
- Wang, S.W.; Xu, Y.; Weng, Y.Y.; Fan, X.Y.; Bai, Y.F.; Zheng, X.Y.; Lou, L.J.; Zhang, F. Astilbin ameliorates cisplatin-induced nephrotoxicity through reducing oxidative stress and inflammation. Food Chem. Toxicol. 2018, 114, 227–236. [Google Scholar] [CrossRef]
- Suhaili, S.H.; Karimian, H.; Stellato, M.; Lee, T.H.; Aguilar, M.I. Mitochondrial outer membrane permeabilization: A focus on the role of mitochondrial membrane structural organization. Biophys. Rev. 2017, 9, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Mercantepe, F.; Mercantepe, T.; Topcu, A.; Yilmaz, A.; Tumkaya, L. Protective effects of amifostine, curcumin; melatonin against cisplatin-induced acute kidney injury. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Bartz, S.K.; Caldas, M.C.; Tomsa, A.; Krishnamurthy, R.; Bacha, F. Urine Albumin-to-Creatinine Ratio: A Marker of Early Endothelial Dysfunction in Youth. J. Clin. Endocrinol. Metab. 2015, 100, 3393–3399. [Google Scholar] [CrossRef]
- Dobyan, D.C.; Levi, J.; Jacobs, C.; Kosek, J.; Weiner, M.W. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther. 1980, 213, 551–556. [Google Scholar] [PubMed]
- Perse, M.; Veceric-Haler, Z. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. Biomed. Res. Int. 2018, 2018, 1462802. [Google Scholar] [CrossRef]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Prevention. Semin. Hear. 2019, 40, 197–204. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Crespy, V.; Demigne, C.; Texier, O.; Regerat, F.; Remesy, C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998, 426, 331–336. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside; rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhang, D.Y.; Gao, X.J.; Parry, J.; Liu, K.; Liu, B.L.; Wang, M. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Lin, H.H.; Hsu, C.C.; Chen, B.C.; Chen, J.H. Aqueous Extract of Pepino (Solanum muriactum Ait) Leaves Ameliorate Lipid Accumulation and Oxidative Stress in Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 931. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Y.; Tsai, C.-L.; Tseng, C.-Y.; Yu, P.-R.; Chang, Y.-H.; Wong, Y.-C.; Lin, H.-H.; Chen, J.-H. In Vitro and In Vivo Nephroprotective Effects of Nelumbo nucifera Seedpod Extract against Cisplatin-Induced Renal Injury. Plants 2022, 11, 3357. https://doi.org/10.3390/plants11233357
Chen J-Y, Tsai C-L, Tseng C-Y, Yu P-R, Chang Y-H, Wong Y-C, Lin H-H, Chen J-H. In Vitro and In Vivo Nephroprotective Effects of Nelumbo nucifera Seedpod Extract against Cisplatin-Induced Renal Injury. Plants. 2022; 11(23):3357. https://doi.org/10.3390/plants11233357
Chicago/Turabian StyleChen, Jui-Yi, Chia-Lin Tsai, Chiao-Yun Tseng, Pei-Rong Yu, Yu-Hsuan Chang, Yue-Ching Wong, Hui-Hsuan Lin, and Jing-Hsien Chen. 2022. "In Vitro and In Vivo Nephroprotective Effects of Nelumbo nucifera Seedpod Extract against Cisplatin-Induced Renal Injury" Plants 11, no. 23: 3357. https://doi.org/10.3390/plants11233357
APA StyleChen, J.-Y., Tsai, C.-L., Tseng, C.-Y., Yu, P.-R., Chang, Y.-H., Wong, Y.-C., Lin, H.-H., & Chen, J.-H. (2022). In Vitro and In Vivo Nephroprotective Effects of Nelumbo nucifera Seedpod Extract against Cisplatin-Induced Renal Injury. Plants, 11(23), 3357. https://doi.org/10.3390/plants11233357







