Availability of Metribuzin-Loaded Polymeric Nanoparticles in Different Soil Systems: An Important Study on the Development of Safe Nanoherbicides
Abstract
1. Introduction
2. Results
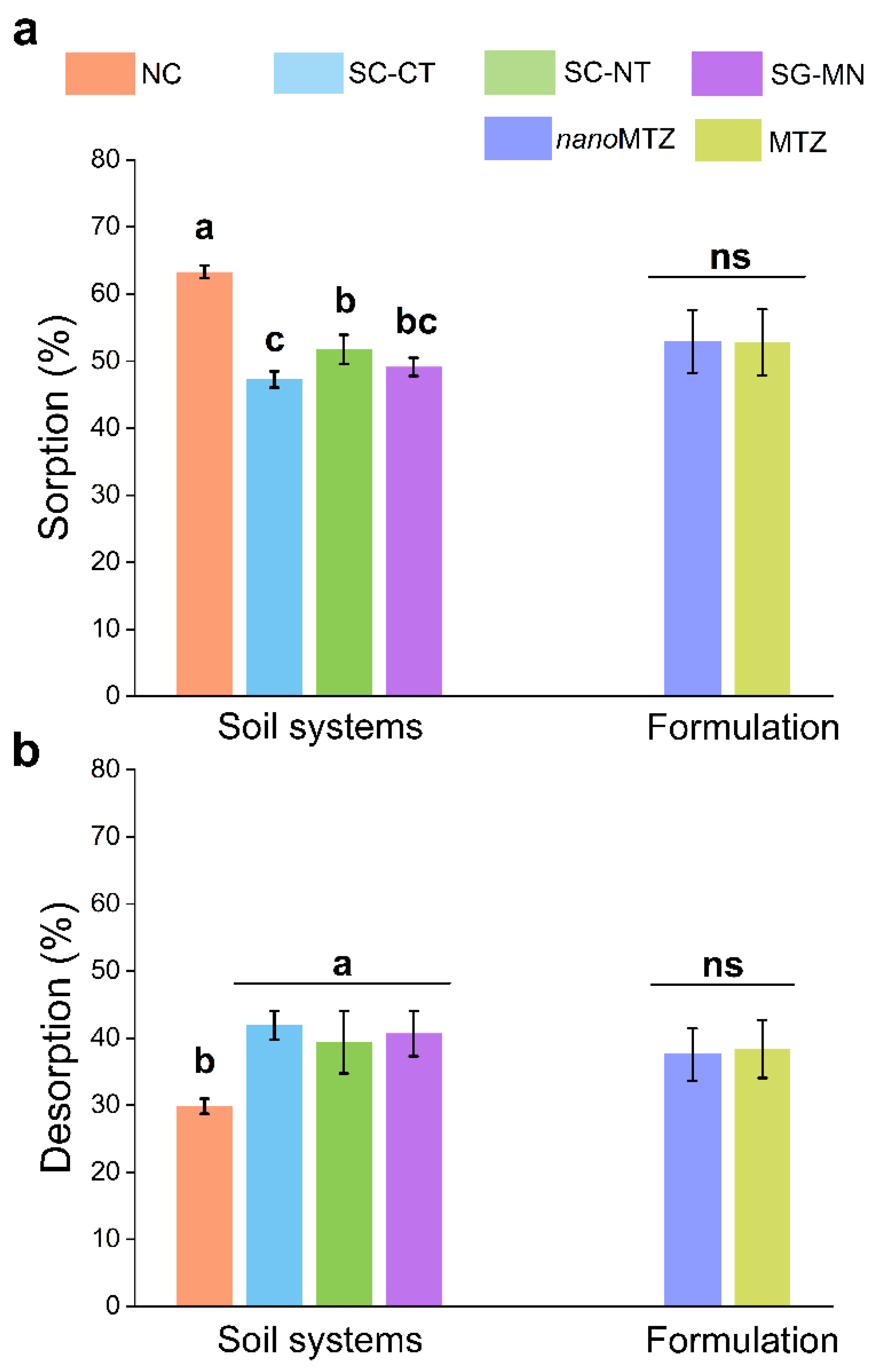
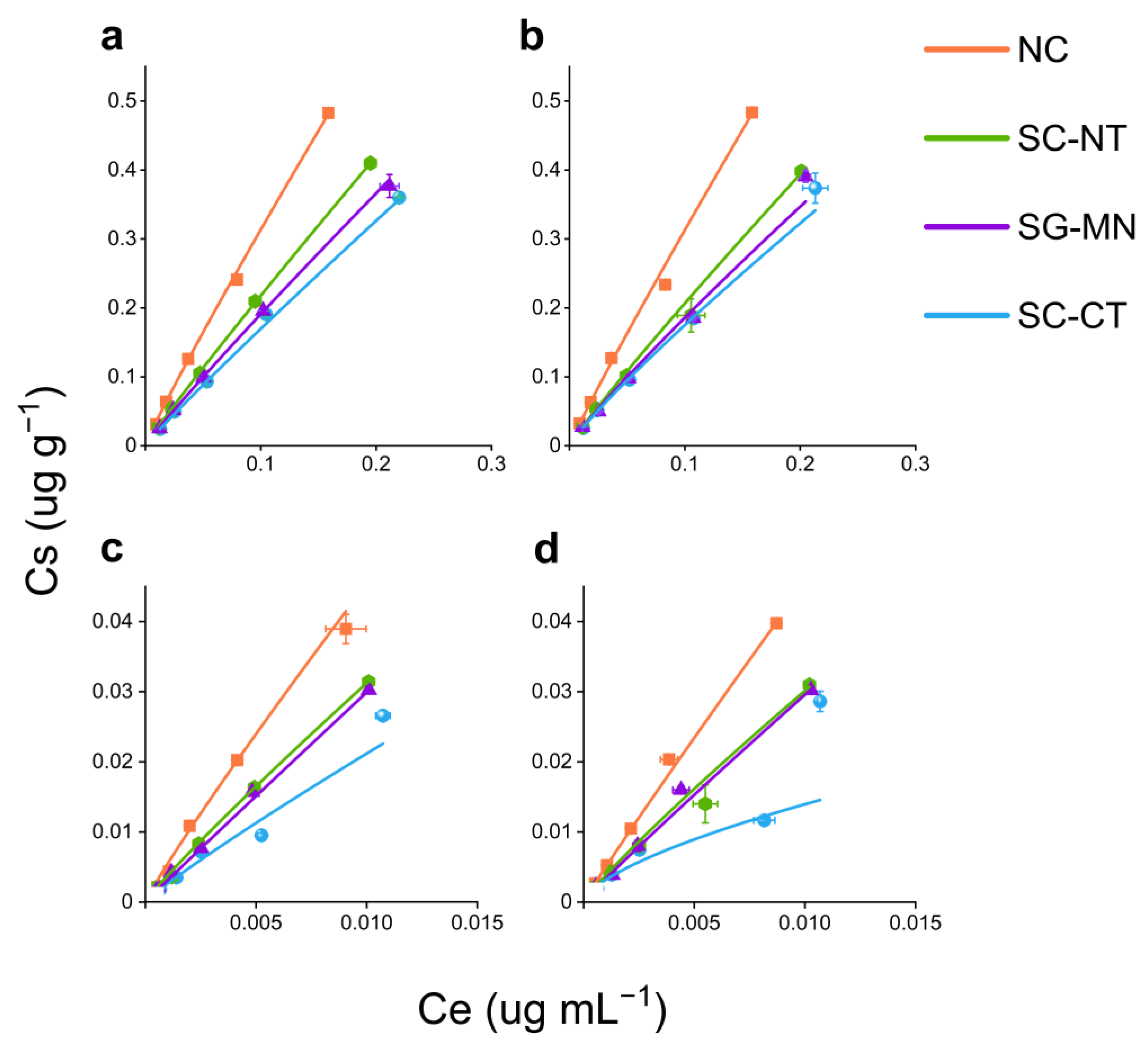
2.1. Metribuzin Nanoformulation and Commercial Formulation Sorption–Desorption in Different Soil Systems
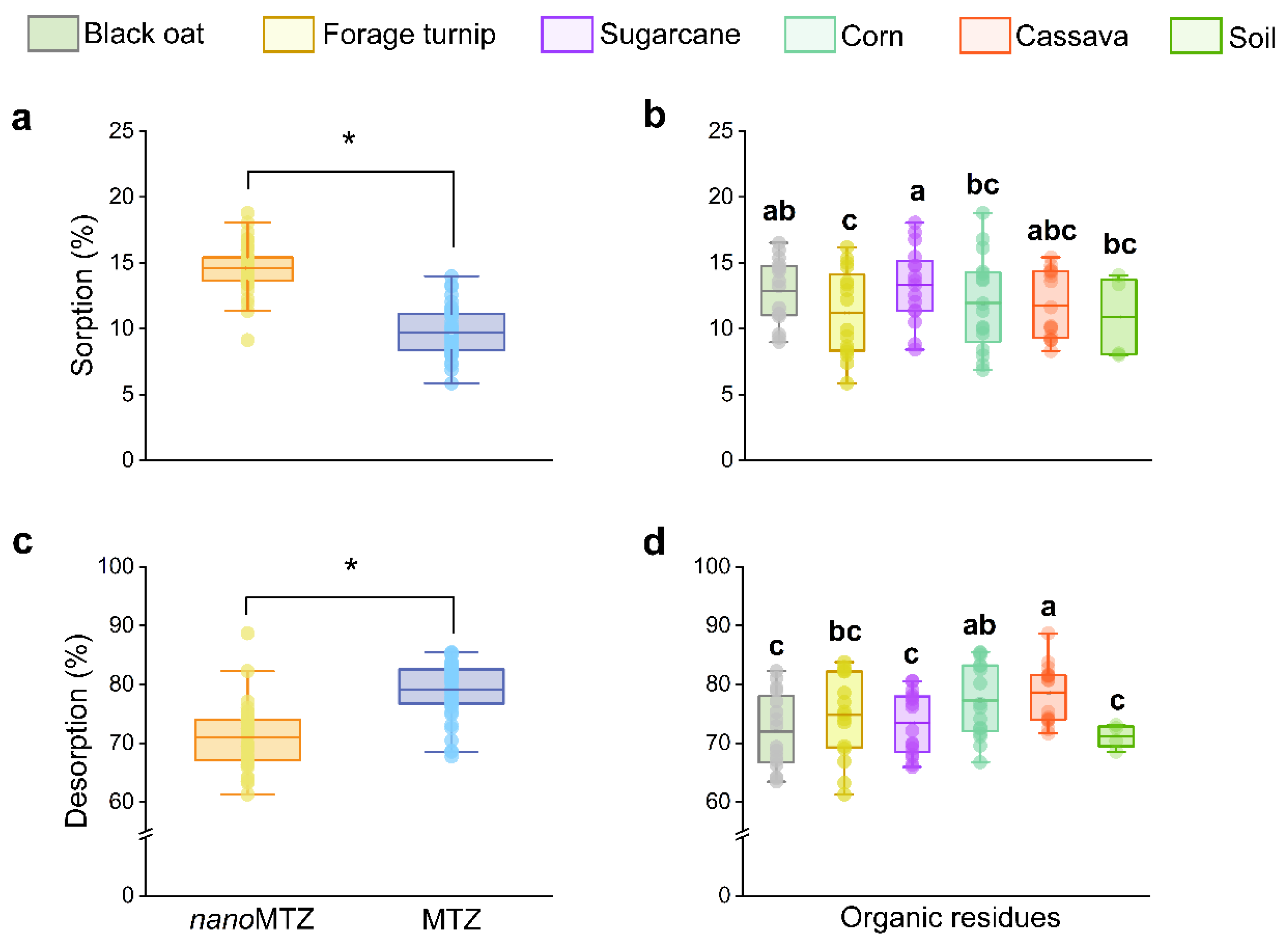
2.2. Organic Residue Effects on Sorption–Desorption of Metribuzin Nano and Conventional Formulations
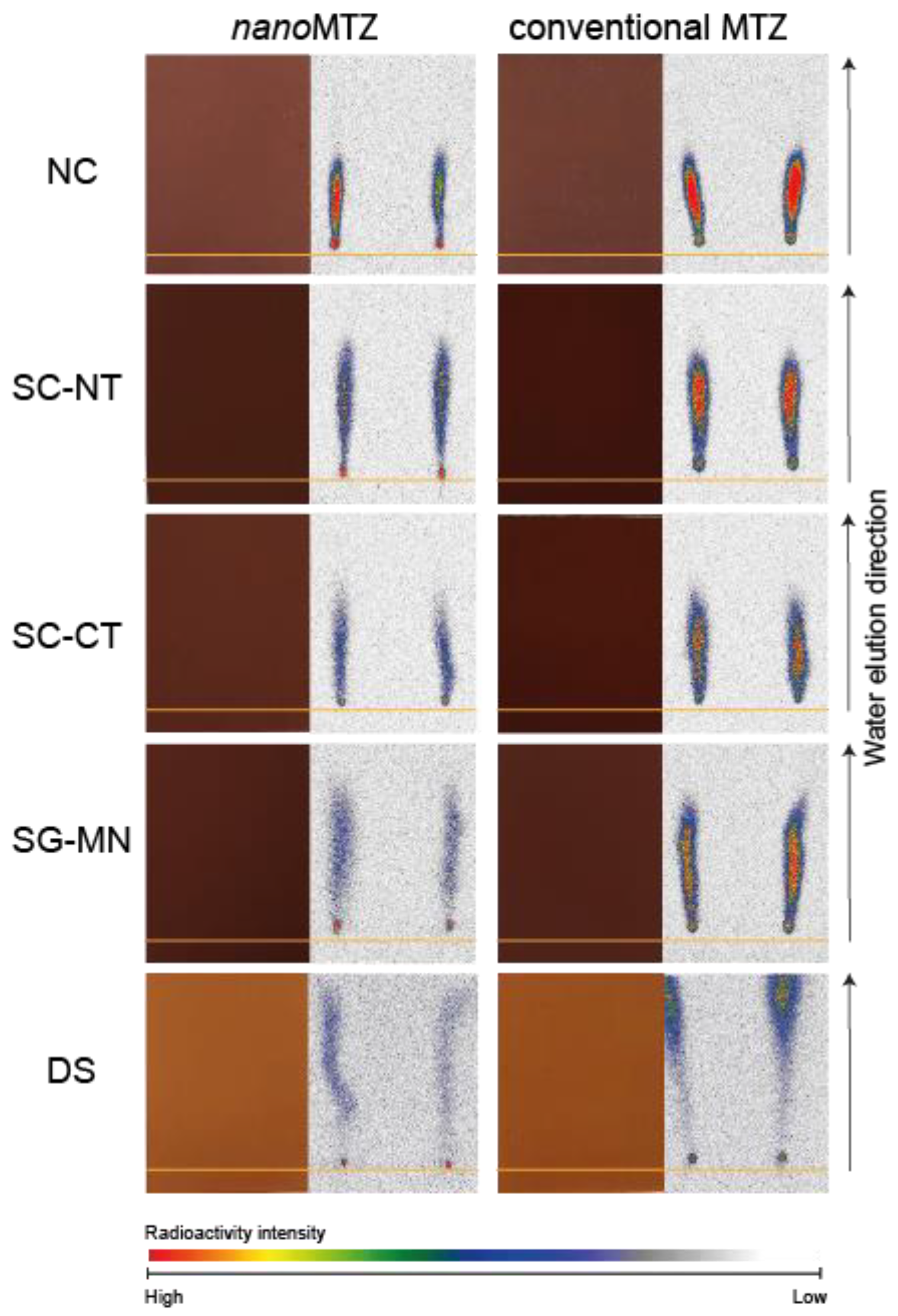
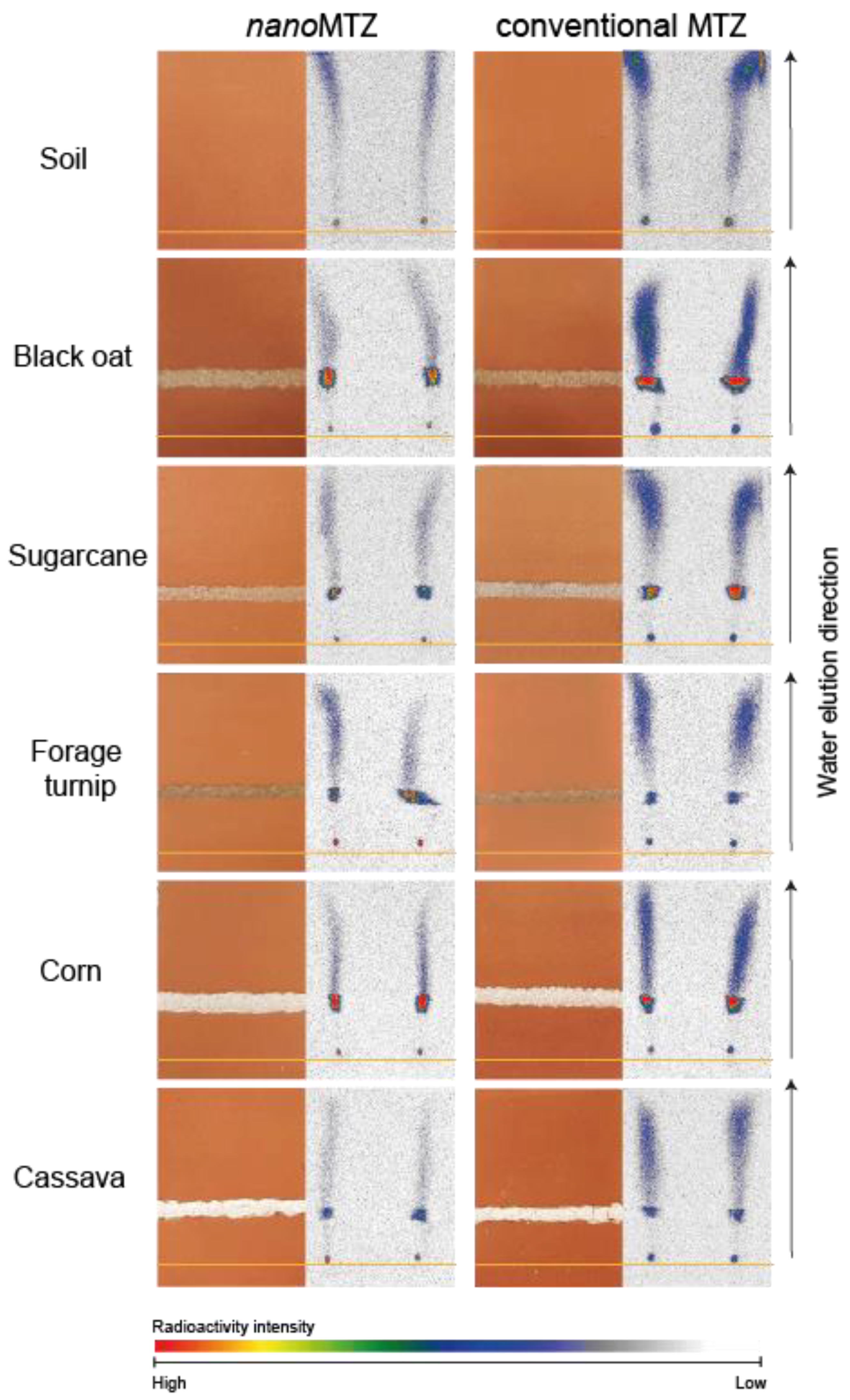
2.3. NanoMTZ and Conventional MTZ Soil Mobility
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of the Nanoformulation
4.3. Soil Collection and Preparation
4.4. Sorption–Desorption Assay in Different Soil Systems
4.5. Sorption–Desorption Assay as a Function of the Type and Amount of Fresh Organic Material in the Deep Soil
4.6. Soil Thin Layer Chromatography Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in targeted pesticides with environmentally responsive controlled release by nanotechnology. J. Nanomat. 2018, 8, 102. [Google Scholar] [CrossRef]
- Ahmed, B.; Rizvi, A.; Ali, K.; Lee, J.; Zaidi, A.; Khan, M.S.; Musarrat, J. Nanoparticles in the soil–plant system: A review. Environ. Chem. Lett. 2021, 19, 1545–1609. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech Revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsel-la, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agri-culture. Nat. Food 2020, 7, 416–425. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Stolf-Moreira, R.; Martinez, C.B.R.; Grillo, R.; de Jesus, M.B.; Fraceto, L.F. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PLoS ONE 2015, 7, e0132971. [Google Scholar] [CrossRef]
- Kah, M.; Walch, H.; Hofmann, T. Environmental fate of nanopesticides: Durability, sorption and photodegradation of nanoformulated clothianidin. Environ. Sci. Nano 2018, 5, 882–889. [Google Scholar] [CrossRef]
- Preisler, A.C.; Pereira, A.E.S.; Campos, E.V.R.; Dalazen, G.; Fraceto, L.F.; Oliveira, H.C. Atrazine nanoencapsulation improves pre-emergence herbicidal activity against Bidens pilosa without enhancing long-term residual effect on Glycine max. Pest Manag. Sci. 2019, 76, 141–149. [Google Scholar] [CrossRef]
- Sopeña, F.; Cabrera, A.; Maqueda, C.; Morillo, E. Controlled release of the herbicide norflurazon into water from ethylcellulose formulation. J. Agric. Food Chem. 2005, 535, 3540–3547. [Google Scholar] [CrossRef]
- Garrido-Herrera, F.J.; González-Pradas, E.; Fernandez-Pérez, M. Controlled release of isoproturon, imidacloprid, and cyromazine from alginae-bentonite-activated carbon formulations. J. Agric. Food Chem. 2006, 54, 10053–10060. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Grillo, R.; de Medeiros, G.A.; Scognamiglio, V.; Rea, G.; Bartolucci, C. Nanotechnology in agriculture: Which innovation potential does it have? Front. Environ. Sci. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Silva, M.S.; Cocenza, D.S.; Grillo, R.; Melo, N.F.S.; Tonello, O.S.; Oliveira, L.C.; Cassimiro, D.L.; Rosa, A.H.; Fraceto, L.F. Paraquat-loaded alginate/chitosan nanoparticles: Preparation, characterization and soil sorption studies. J. Hazard. Mater. 2011, 190, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of poly(epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef]
- Diyanat, M.; Saeidian, H. The metribuzin herbicide in polycaprolactone nanocapsules shows less plant chromosome aberration than non-encapsulated metribuzin. Environ. Chem. Lett. 2019, 17, 1881–1888. [Google Scholar] [CrossRef]
- Sousa, B.T.; Pereira, A.E.S.; Fraceto, L.F.; Oliveira, H.C.; Dalazen, G. Effectiveness of nanoatrazine in post-emergent control of the tolerant weed Digitaria insularis. J. Plant Prot. Res. 2020, 60, 185–192. [Google Scholar] [CrossRef]
- Takeshita, V.; Sousa, B.T.; Preisler, A.C.; Carvalho, L.B.; Pereira, A.E.S.; Tornisielo, V.L.; Dalazen, G.; Oliveira, H.C.; Fraceto, L.F. Foliar absorption and field herbicidal studies of atrazine-loaded polymeric nanoparticles. J. Hazard. Mater. 2021, 418, 126350. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Rodríguez-Diéguez, A.; Horcajada, P. Metal–Organic Frameworks in Agriculture. ACS Appl. Mater. Interfaces 2022, 14, 16983–17007. [Google Scholar] [CrossRef]
- Iqbal, N.; Manalil, S.; Chauhan, B.S.; Adkins, S.W. Investigation of alternate herbicides for effective weed management in glyphosate-tolerant cotton. Arch. Agron. Soil Sci. 2019, 65, 1885–1899. [Google Scholar] [CrossRef]
- Beckie, H.J.; Flower, K.C.; Ashworth, M.B. Farming without glyphosate? Plants 2020, 9, 96. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Hill, R.R. Impact of soil amendments on metribuzin and DCPA Half-lives and Mobility into agricultural runoff water. J. Environ. Sci. Health B 2014, 48, 313–323. [Google Scholar] [CrossRef]
- Stenrod, M.; Almvik, M.; Eklo, O.M.; Gimsing, A.L.; Holten, R.; Kunnis-Beres, K.; Larsbo, M.; Putelis, L.; Siimes, K.; Turka, I.; et al. Pesticide regulatory risk assessment, monitoring, and fate studies in the Northern zone: Recommendations from a Nordic-Baltic workshop. Environ. Sci. Pollut. Res. 2016, 23, 15779–15788. [Google Scholar] [CrossRef]
- Kolupaeva, V.; Kokoreva, A.; Bondareva, T. The study of metribuzin migration in Lysimeters. In E3S Web of Conferences, Proceedings of XIII International Scientific and Practical Conference “State and Prospects for the Development of Agribusiness—INTERAGROMASH 2020”, Rostovon-Don, Russia, 26–28 February 2020; Rudoy, E., Ed.; EDP Sciences: Les Ulis, France, 2020; Volume 175, p. 07008. [Google Scholar] [CrossRef]
- Takeshita, V.; Carvalho, L.B.; Galhardi, J.A.; Munhoz-Garcia, G.V.; Pimpinato, R.F.; Oliveira, H.C.; Tornisielo, V.L.; Fraceto, L.F. Development of a Preemergent Nanoherbicide: From Efficiency Evaluation to the Assessment of Environmental Fate and Risks to Soil Microorganisms. ACS Nanosci. Au 2022, 2, 307–323. [Google Scholar] [CrossRef]
- Kumar, J.; Nisak, K.; Shakil, N.A.; Walia, S.; Parsad, R. Controlled release formulations of metribuzin: Release kinetics in water and soil. J. Environ. Sci. Health B 2010, 45, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Philips, T.; Boopathy, R. Biotransformation of metribuzin under various electron acceptor conditions. Environ. Qual. Manag. 2022, 32, 223–231. [Google Scholar] [CrossRef]
- Govindasamy, P.; Liu, R.; Provin, T.; Rajan, N.; Hons, F.; Mowrer, J.; Bagavathiannan, M. Soil carbon improvement under long-term (36 years) no-till sorghum production in a sub-tropical environment. Soil Use Manag. 2020, 37, 37–48. [Google Scholar] [CrossRef]
- Bossolani, J.W.; Crusciol, C.A.; Leite, M.F.; Merloti, L.F.; Moretti, L.G.; Pascoaloto, I.M.; Kuramae, E.E. Modulation of the soil microbiome by long-term Ca-based soil amendments boosts soil organic carbon and physicochemical quality in a tropical no-till crop rotation system. Soil Biol. Biochem. 2021, 156, 108188. [Google Scholar] [CrossRef]
- Oorts, K.; Bossuyt, H.; Labreuche, J.; Merckx, R.; Nicolardot, B. Carbon and nitrogen stocks in relation to organic matter fractions, agregation and pore size distribution in no-tillage and conventional tillage in Northern France. Eur. J. Soil Sci. 2007, 58, 248–259. [Google Scholar] [CrossRef]
- Castañon, M.F.H.T.; Machado Filho, A.; Nemoto, P.R.L.; Oliveira Filho, J.; Cunha, M.S.C. Fitomassa de plantas de cobertura em diferentes densidades de plantio no cerrado de Mato Grosso. Agropecuária Científica No Semi-Árido 2014, 10, 14–18. [Google Scholar] [CrossRef]
- Tejada, M.; Benítez, C. Flazasulfuron behavior in a soil amended with different organic wastes. Appl. Soil Ecol. 2017, 117, 81–87. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in Adsorption. Part XI. A System of Classifcation of Solution Adsorption Isotherms, and its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Barriuso, E.; Laird, D.A.; Koskinen, W.C.; Dowdy, R.H. Atrazine Desorption from Smectites. Soil Sci. Soc. Am. 1994, 58, 1632–1638. [Google Scholar] [CrossRef]
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin, and quinclorac in soil. Geoderma 2019, 343, 40–49. [Google Scholar] [CrossRef]
- Sarkar, B.; Mukhopadhyay, R.; Mandal, A.; Mandal, S.; Vithanage, M.; Biswas, J.K. Sorption and desorption of agro-pesticides in soils. In Agrochemicals Detection, Treatment and Remediation, 1st ed.; Prassad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 189–205. [Google Scholar] [CrossRef]
- Sadegh-Zadeh, F.; Wahid, S.A.; Jalili, B. Sorption, degradation and leaching of pesticides in soils amended with organic matter: A review. Adv. Environ. Technol. 2017, 3, 119–132. [Google Scholar] [CrossRef]
- Report, E.S. Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Metribuzin. EFSA J. 2008, 6, 1–74. [Google Scholar] [CrossRef]
- FAO. APPENDIX 2 Parameters of Pesticides that Influence Processes in the Soil the Parameters Used to Characterize the Properties of Pesticides. Available online: https://www.fao.org/3/X2570E/X2570E06.htm (accessed on 3 November 2022).
- USEPA. Guidance for Reporting on the Environmental Fate and Transport of the Stressors of Concern in Problem Formulations. Available online: http://www.epa.gov/pesticides/science/efed/policy_guidance/team_authors/endangered_species_reregistration_workgroup/esa_reporting_fate.pdf (accessed on 3 November 2022).
- Guimarães, A.C.D.; Mendes, K.F.; dos Reis, F.C.; Campion, T.F.; Christoffoleti, P.J.; Tornisielo, V.L. Role of soil physicochemical properties in quantifying the fate of diuron, hexazinone, and metribuzin. Environ. Sci. Pollut. Res. 2018, 25, 12419–12433. [Google Scholar] [CrossRef]
- Rigi, M.R.; Farahbakhsh, M.; Rezaei, K. Adsorption and desorption behavior of herbicide metribuzin in different soils of Iran. J. Agric. Sci. Technol. 2015, 17, 777–787. [Google Scholar]
- Mielke, K.C.; Laube, A.F.S.; Guimarães, T.; Brochado, M.G.S.; Medeiros, B.A.P.; Mendes, K.F. Pyrolysis Temperature and Application Rate of Sugarcane Straw Biochar Influence Sorption and Desorption of Metribuzin and Soil Chemical Properties. Processes 2022, 10, 1924. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency—USEPA. Candidate Contaminant List Regulatory Determination Support Document for Metribuzin. Available online: https://www.epa.gov/sites/default/files/2014-09/documents/support_cc1_metribuzin_ccl_regdet.pdf (accessed on 3 November 2022).
- Pot, V.; Benoit, P.; Menn, M.; Eklo, O.M.; Sveistrup, T.; Kværner, J. Metribuzin transport in undisturbed soil cores under controlled water potential conditions: Experiments and modelling to evaluate the risk of leaching in a sandy loam soil profile. Pest Manag. Sci. 2011, 67, 397–407. [Google Scholar] [CrossRef]
- Montagner, C.C.; Vidal, C.; Acayaba, R.D. Contaminantes emergentes em matrizes aquáticas do Brasil: Cenário atual e aspectos analíticos, ecotoxicológicos e regulatórios. Química Nova 2017, 40, 1094–1110. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Bouchard, D.C.; Lavy, T.L.; Marx, D.B. Fate of Metribuzin, Metolachlor, and Fluometuron in Soil. Weed Sci. 1982, 30, 629–632. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ε-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Gahane, A.; Thakur, A.K. Deciphering the mechanism and structural features of polysorbate 80 during adsorption on PLGA nanoparticles by attenuated total reflectance-Fourier transform infrared spectroscopy. RSC Advances 2016, 6, 108545–108557. [Google Scholar] [CrossRef]
- Briceño, G.; Palma, G.; Durán, N. Influence of organic amendment on the biodegradation and movement of pesticides. Crit. Rev. Environ. Sci. Technol. 2007, 37, 233–271. [Google Scholar] [CrossRef]
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Barriuso, E.; Houot, S.; Serra-Wittling, C. Influence of Compost Addition to Soil on the Behaviour of Herbicides. Pestic. Sci. 1997, 49, 65–75. [Google Scholar] [CrossRef]
- Sluszny, C.; Graber, E.R.; Gerstl, Z. Sorption of s-triazine herbicides in organic matter amended soils: Fresh and incubated systems. Wat. Air Soil Pollut. 1999, 115, 395–410. [Google Scholar] [CrossRef]
- Gonçalves, S.P.C.; Delite, F.S.; Côa, F.; Neto, L.L.R.; da Silva, G.H.; de Sá Bortolozzo, L.; Ferreira, A.G.; de Medeiros, A.M.Z.; Strauss, M.; Martinez, D.S.T. Biotransformation of Nanomaterials in the Soil Environment: Nanoecotoxicology and Nanosafety Implications. In Nanomaterials Applications for Environmental Matrices: Water, Soil and Air, 1st ed.; Nascimento, R.F., Ferreira, O.P., De Paula, A.J., Sousa Neto, V.O., Eds.; Elsevier: Amsterdam, Holland, 2019; pp. 265–304. [Google Scholar] [CrossRef]
- Rasool, S.; Rasool, T.; Gani, K.M. A review of interactions of pesticides within various interfaces of intrinsic and organic residue amended soil environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- Zmora-Nahum, S.; Markovitch, O.; Tarchitzky, J.; Chen, Y. Dissolved organic carbon (DOC) as a parameter of compost maturity. Soil Biol. Biochem. 2005, 37, 2109–2116. [Google Scholar] [CrossRef]
- He, X.; Aker, W.G.; Hwang, H.M. Assessing the effect of different natural dissolved organic matters on the cytotoxicity of titanium dioxide nanoparticles with bacteria. J. Environ. Sci. 2016, 48, 230–236. [Google Scholar] [CrossRef]
- Hou, L.; Fortner, J.D.; Wang, X.; Zhang, C.; Wang, L.; Chen, W. Complex interplay between formation routes and natural organic matter modification controls capabilities of C-60nanoparticles (nC(60)) to accumulate organic contaminants. J. Environ. Sci. 2017, 51, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, J.; Yin, Y.; Shen, M. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects. J. Environ. Sci. 2018, 63, 198–217. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, K.B.; Lehmann, J.; Walter, M.T. Atrazine leaching from biochar-amended soils. Chemosphere 2014, 95, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Khorram, M.S.; Wang, Y.; Jin, X.; Fang, H.; Yu, Y. Reduced mobility of fomesafen through enhanced adsorption in biochar-amended soil. Environ. Toxicol. Chem. 2015, 34, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Kumar, A.; Singh, N. Sorption mechanisms of pesticides removal from effluent matrix using biochar: Conclusions from molecular modelling studies validated by single-, binary and ternary solute experiments. J. Environ. Manag. 2021, 295, 113104. [Google Scholar] [CrossRef]
- Helling, C.S.; Turner, B.C. Pesticide mobility: Determination by soil thin-layer chromatography. Science 1968, 162, 562–563. [Google Scholar] [CrossRef]
- Deng, H.; Feng, D.; He, J.X.; Li, F.Z.; Yu, H.M.; Ge, C.J. Influenceofbiocharamendmentstosoilonthemobilityofatrazineusingsorption-desorptionandsoilthin-layerchromatography. Ecol. Eng. 2017, 99, 381–390. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 106: Adsorption—Desorption Using a Batch Equilibrium Method; OECD Publishing: Paris, France, 2000. [Google Scholar]
- United States Environmental Protection Agency—USEPA. Fate, Transport and Transformation Test Guidelines OPPTS 835.3300 Soil Biodegradation; USEPA: Washington, DC, USA, 1998. [Google Scholar]

| nanoMTZ | MTZ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | NC | SC-CT | SC-NT | SG-MN | NC | SC-CT | SC-NT | SG-MN | |
| Sorption | Kd (mL g−1) | 3.4 ± 0.13 | 1.75 ± 0.01 | 2.23 ± 0.01 | 1.96 ± 0.05 | 3.5 ± 0.08 | 1.84 ± 0.08 | 2.07 ± 0.19 | 1.91 ± 0.11 |
| Kf (mL g−1) | 2.66 ± 0.17 | 1.49 ± 0.03 | 1.93 ± 0.01 | 1.66 ± 0.07 | 2.73 ± 0.08 | 1.33 ± 0.09 | 1.76 ± 0.06 | 1.48 ± 0.16 | |
| 1/n | 0.929 | 0.944 | 0.947 | 0.940 | 0.940 | 0.882 | 0.931 | 0.900 | |
| R² (adj) | 0.995 | 0.999 | 0.999 | 0.998 | 0.998 | 0.996 | 0.998 | 0.988 | |
| Hysteresis | - | - | - | - | |||||
| Desorption | Kd (mL g−1) | 5.46 ± 0.51 | 2.88 ±0.22 | 3.45 ± 0.19 | 3.05 ± 0.06 | 4.91 ± 0.27 | 2.95 ± 0.26 | 3.24 ± 0.64 | 3.34 ± 0.61 |
| Kf (mL g−1) | 3.17 ± 1.13 | 2.79 ± 0.56 | 1.39 ± 0.54 | 2.15 ±0.06 | 3.78 ± 0.22 | 0.269 ± 0.12 | 1.89 ± 0.15 | 2.35 ± 0.51 | |
| 1/n | 0.922 | 0.985 | 0.909 | 0.919 | 0.959 | 0.643 | 0.899 | 0.951 | |
| R² (adj) | 0.992 | 0.992 | 0.945 | 0.999 | 0.999 | 0.922 | 0.997 | 0.993 | |
| Hysteresis | 0.99 | 1.04 | 0.96 | 0.98 | 1.02 | 0.73 | 1.07 | 1.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshita, V.; Munhoz-Garcia, G.V.; Werk Pinácio, C.; Cardoso, B.C.; Nalin, D.; Tornisielo, V.L.; Fraceto, L.F. Availability of Metribuzin-Loaded Polymeric Nanoparticles in Different Soil Systems: An Important Study on the Development of Safe Nanoherbicides. Plants 2022, 11, 3366. https://doi.org/10.3390/plants11233366
Takeshita V, Munhoz-Garcia GV, Werk Pinácio C, Cardoso BC, Nalin D, Tornisielo VL, Fraceto LF. Availability of Metribuzin-Loaded Polymeric Nanoparticles in Different Soil Systems: An Important Study on the Development of Safe Nanoherbicides. Plants. 2022; 11(23):3366. https://doi.org/10.3390/plants11233366
Chicago/Turabian StyleTakeshita, Vanessa, Gustavo Vinicios Munhoz-Garcia, Camila Werk Pinácio, Brian Cintra Cardoso, Daniel Nalin, Valdemar Luiz Tornisielo, and Leonardo Fernandes Fraceto. 2022. "Availability of Metribuzin-Loaded Polymeric Nanoparticles in Different Soil Systems: An Important Study on the Development of Safe Nanoherbicides" Plants 11, no. 23: 3366. https://doi.org/10.3390/plants11233366
APA StyleTakeshita, V., Munhoz-Garcia, G. V., Werk Pinácio, C., Cardoso, B. C., Nalin, D., Tornisielo, V. L., & Fraceto, L. F. (2022). Availability of Metribuzin-Loaded Polymeric Nanoparticles in Different Soil Systems: An Important Study on the Development of Safe Nanoherbicides. Plants, 11(23), 3366. https://doi.org/10.3390/plants11233366









