Abstract
Salinity is an abiotic stress factor that reduces yield and threatens food security in the world’s arid and semi-arid regions. The development of salt-tolerant genotypes is critical for mitigating yield losses, and this journey begins with the identification of sensitive and tolerant plants. Numerous physiologic and molecular markers for detecting salt-tolerant wheat genotypes have been developed. One of them is proline, which has been used for a long time but has received little information about proline-related genes in wheat genotypes. In this study, proline content and the expression levels of proline-related genes (TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB) were examined in sensitive, moderate, and tolerant genotypes under salt stress (0, 50, 150, and 250 mM NaCl) for 0, 12, and 24 h. Our results show that salt stress increased the proline content in all genotypes, but it was found higher in salt-tolerant genotypes than in moderate and sensitive genotypes. The salinity stress increased gene expression levels in salt-tolerant and moderate genotypes. While salt-stress exposure for 12 and 24 h had a substantial effect on gene expression in wheat, TaPTF1, TaPIMP1, TaMIP, TaHKT1;4, and TaMYB genes were considerably upregulated in 24 h. The salt-tolerant genotypes showed a higher positive interaction than a negative interaction. The TaPTF1, TaP5CS, TaGSK1, and TaSRG genes were found to be more selective than the other analyzed genes under salt-stress conditions. Despite each gene’s specific function, increasing proline biosynthesis functioned as a common mechanism for separating salt tolerance from sensitivity.
1. Introduction
Salinity is a major abiotic stressor that has a negative impact on seed germination, plant growth, and productivity in arid and semi-arid regions [1]. Saline soils are defined as having electrical conductivity (EC) of the saturation soil-paste extract greater than 4 dS/m at 25 °C, which corresponds to approximately 40 mM NaCl and generates an osmotic pressure of approximately 0.2 MPa [2]. It is estimated that salt stress affects approximately 800 million hectares or 6.5% of the world’s total land area, and salt has already damaged 45 million ha (19.5%) of the current 230 million ha of irrigated land [3]. High salinity problems are expected to affect approximately 50% of total arable agricultural land by 2050 [4]. Soil salinity is significant abiotic stress that has a devastating impact on crop production [5]. Salinity-related yield reduction in crops threatens food security with rapid growth in the world’s population, which is estimated to reach 9 billion by the end of the next 30 years [6]. Currently, projections show that approximately 690 million (11% of the global population) are hungry, and food demand is expected to increase by 85% (approximately 2.7 billion people) by 2050 [7,8].
Wheat plays a critical role in ensuring food and nutritional security; however, rapidly rising soil and water salinity pose a serious threat to global wheat production [9]. Wheat, like other glycophytes, is severely impacted by high salinity levels in soils, with 28–60% losses in grain yield under saline conditions [10,11]. Improving salt tolerance is one of the most effective and feasible strategies for reducing the negative effects of salinity on wheat production [12]. Several morphological, physiological, and molecular markers have been developed to assort salt-tolerant genotypes. The assessment of genetic variability using morphological and physiological markers is useful for early field-based characterization and selection, but these markers are heavily influenced by environmental fluxes [13]. Studies need to be supplemented with more robust genetically linked molecular and biochemical markers for selection precision in order to validate the morphological and physiological marker-based data [14]. Many tolerant genotypes have now been determined thanks to advances in molecular markers and modern genomic techniques [15].
Many studies have been conducted to investigate salt-tolerant genotypes in Arabidopsis and rice and their application in breeding programs, with the goal of identifying salt-tolerance genes [16]. Although most salinity-tolerance-related genes in Arabidopsis and rice have been identified, not enough genes have been identified in wheat due to the genome size and an insufficient gene map [17]. The genes identified in wheat were also investigated in Arabidopsis plants by transgenic studies [18,19,20,21]. Proline is one of the most significant amino acids synthesized and stored in the plant in response to salt stress, and proline accretion is a sign of abiotic stress tolerance in plants using different stress signaling pathways (phytohormones, calcium signaling, and mitogen-activated protein (MAP) kinase pathways) [22,23,24]. Proline has been shown to promote plant growth, physiology, biochemistry, anatomical features, and antioxidant system defense when exposed to salinity stress [25]. Proline is made from glutamate in the glutamate pathway by enzymes called 1-pyrroline-5-carboxylate synthetases (P5CS) and Δ1-pyrroline-5-carboxylate reductase (P5CR) genes [26,27]. Although the TaP5CS gene is a crucial gene for proline production, many other main genes such as pyrroline-5-carboxylate (P5C), pyrroline-5-carboxylate reductase (P5CR), and pyrroline-5-carboxylate dehydrogenase (P5CDH) have been found to also contribute to the ability to produce proline in plants [24]. Proline accumulation-related genes were previously identified utilizing the gene expression approach, which is widely utilized in this field [28,29,30,31,32]. Among identified genes, transcription factors (TFs) are involved in a variety of biological processes, including the regulation of stress tolerance [33]. MYB proteins are one of the most important TFs families in plants, and it is a key regulator gene involved in salt-stress adaptation in wheat [34,35]. ABA regulates the TaMYB and putative integral membrane protein 1 (TaPIMP1) genes, and TaPIMP1 overexpression boosted proline synthesis, resulting in increased drought tolerance [36]. The pi starvation-induced TF 1 (TaPTF1) gene is also a member of the basic helix–loop–helix TFs (bHLH) family, which improves plant salinity tolerance by increasing proline production [37]. In response to salt stress, the high-affinity K+ transporter (TaHKT1;4) gene aids in the exclusion of Na+ from leaf blades [38], and overexpression of the glycogen synthase kinase (TaGSK1) and salt response gene (TaSRG) increases salt tolerance by the reduction in the amount of Na+ in cells and proline accumulation [20,39]. An aquaporin gene NOD26-like membrane integral protein (TaMIP) accumulates higher K+ and proline content and lower Na+ concentration in salt-exposed plants when TaMIP is overexpressed [19]. The dehydrin (TaDHN) and salt-tolerant correlate (TaSC) genes, which regulate the rate-limiting stage of glutamate-derived proline biosynthesis, rapidly increase in mRNA levels in reaction to salt [21,40].
In this study, we aimed to test proline content and the activity of TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK1, TaP5CS, and TaMYB genes in two salt-sensitive, two moderate, and two salt-tolerant wheat genotypes under 0, 50, 150, and 250 mM NaCl stress conditions for 0, 12, and 24 h.
2. Results
The three-way analysis of variance (ANOVA) test was used to distinguish differences in proline content and the gene expression of TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB genes with varying Time (T; 0, 12, and 24 h), Salt levels (S; 0, 50, 150, and 250 mM), Genotypes (G; two salt-sensitive, moderate, and salt-tolerant), and their interactions (T × S, T × G, S × G, and T × S × G) (Table 1 and Table S2). The three-way ANOVA results revealed that T × S, T × G, S × G, and T × S × G interactions significantly (p < 0.001) affected proline content and all measured gene expressions.

Table 1.
Analysis of variance (ANOVA) table.
2.1. Proline Content and Phylogenetic Tree of Genes
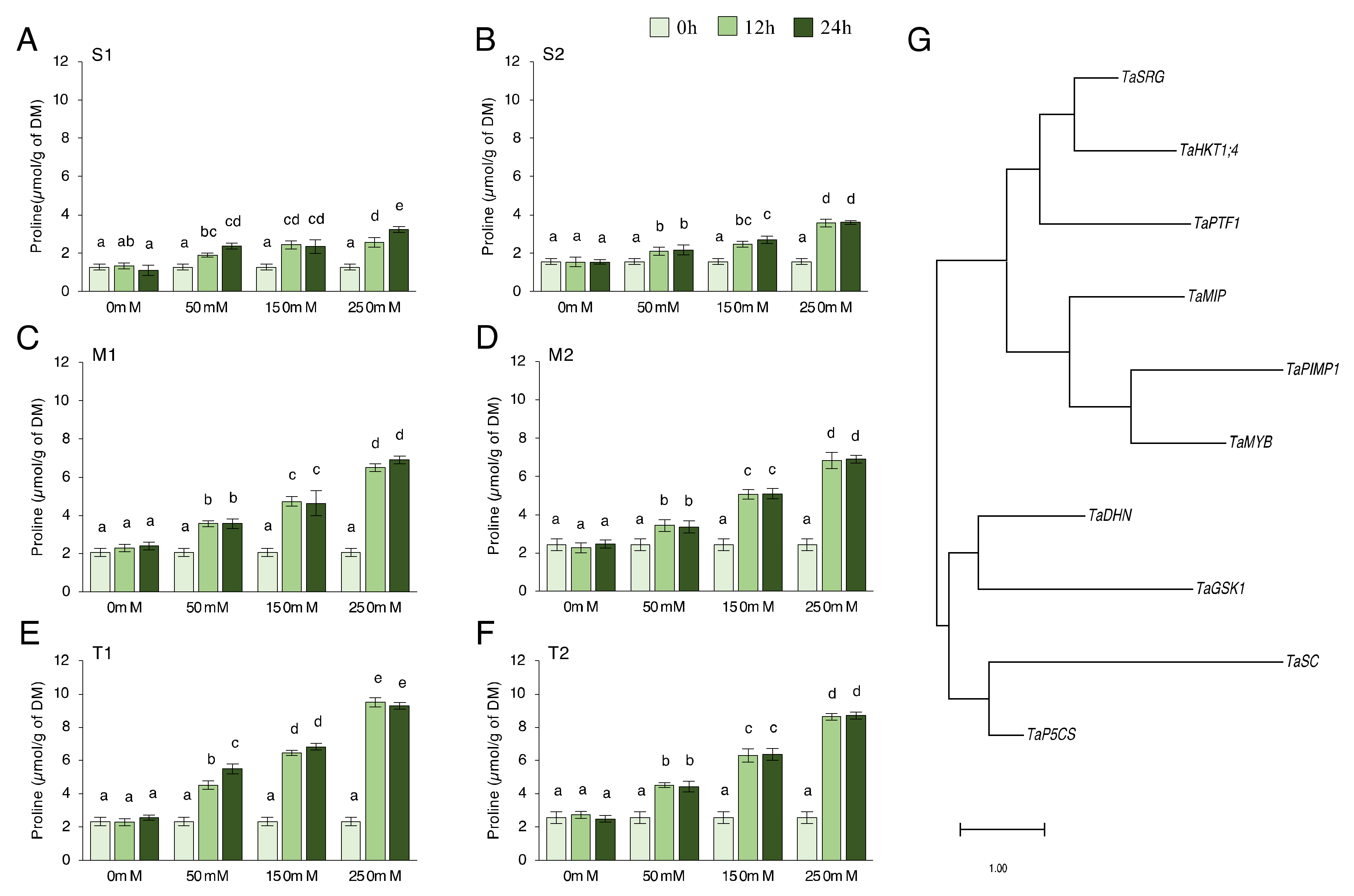
We checked proline content in salt-sensitive, moderate, and salt-tolerant genotypes under 0, 50, 150, and 250 mM NaCl for 0, 12, and 24 h. Proline content significantly increased under salt-stress conditions. The proline production after 12 and 24 h in almost all genotypes was found to not be statistically different. The moderate and tolerant genotypes generated more proline than the sensitive genotypes, but the proline concentration of the tolerant genotype was found to be higher (Figure 1A–F). In the S1 genotype, the proline content increased by a minimum of 50% after 12 h of 50 mM NaCl stress and a maximum of 155% after 24 h of 250 mM NaCl stress (Figure 1A). It increased by a minimum of 34% after 12 h of 50 mM NaCl stress and a maximum of 129% after 24 h of 250 mM NaCl stress in the S2 genotype (Figure 1B). In the M1 genotype, proline content increased by a minimum of 72% after 12 h of 50 mM NaCl stress and a maximum of 233% after 24 h of 250 mM NaCl stress (Figure 1C). It increased by a minimum of 41% after 12 h of 50 mM NaCl stress and a maximum of 183% after 24 h of 250 mM NaCl stress in the M2 genotype (Figure 1D). The proline content increased by a minimum of 94 % at 12 h of 50 mM NaCl stress and a maximum of 298 % at 24 h of 250 mM NaCl stress in the T1 genotype (Figure 1E). It was increased by a minimum of 76 % at 12 h of 50 mM NaCl stress and a maximum of 238 % at 24 h of 250 mM NaCl stress in the T2 genotype (Figure 1F). The higher proline content was measured in the T1 genotype under 250 mM NaCl stress conditions (Figure 1E). The proline content of salt-sensitive genotypes was almost doubled, and a triple increment was seen in the moderate and tolerant genotypes. To explore the phylogenetic relationship among proline-related genes in Triticum aestivum L., a rooted maximum-likelihood phylogenetic tree with 10 (TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB) genes (Figure 1G) was inferred from the amino acid sequences (Table S2, Figure A1) using the MEGA 11.0.13 program [41]. The genes can be subdivided into two main clusters and four well-conserved subclusters with the highest log likelihood (−2352.72). The evolutionary phylogenetic tree showed that TaP5CS and TaSC, TaGSK1, and TaDHN genes were found in the same cluster. TaPIMP1 and TaMYB, TaSRG, and TaHKT1;4 genes were found close to each other (Figure 1G).
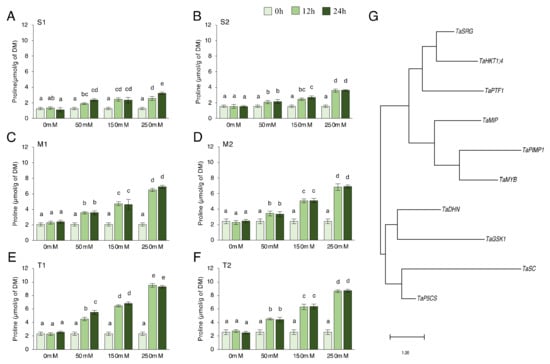
Figure 1.
Proline content and phylogenetic tree of genes. (A) The proline content of the salt-sensitive-1 (S1), (B) sensitive-2 (S2), (C) moderate-1 (M1), (D) moderate-2 (M2), (E) tolerant-1 (T1), (F) tolerant-2 (T2), and (G) evolutionary phylogenetic tree of TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB genes by using amino acid sequences in MEGA 11.0.13 software. The proline content in S1, S2, M1, M2, T1, and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, and 24 h. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
2.2. Expression Profiles of Proline-Related Genes
After the detection of the proline accumulation profile, we examined changes in the gene expression profile of proline-related genes (TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB) in all tested sensitive, moderate, and tolerant plants.
2.2.1. TaPTF1 and TaDHN Genes
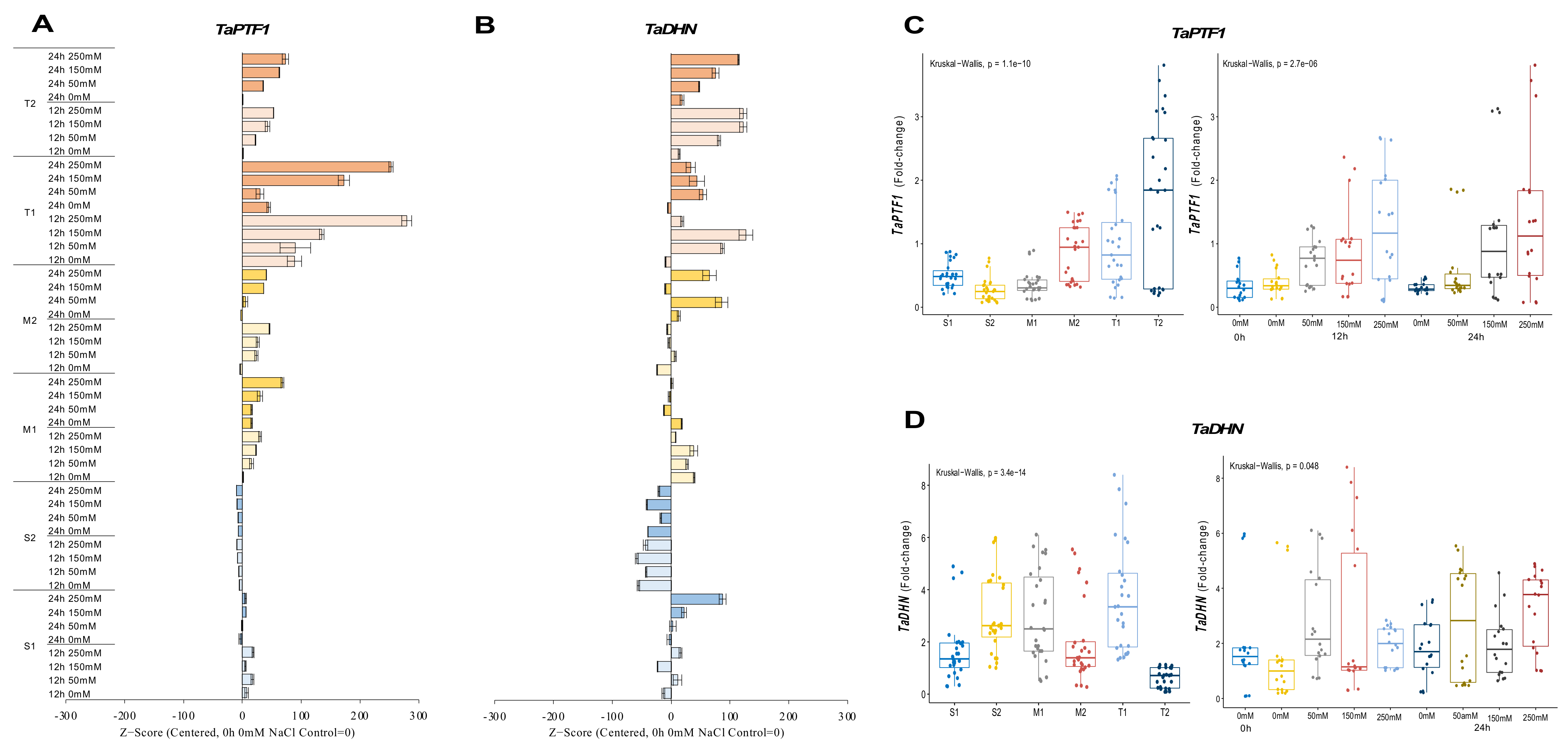
The expression of TaPTF1 was decreased from 0.7-fold (0 h of 0 mM NaCl—control) up to 0.1-fold (12 h of 250 mM NaCl) in the salt-sensitive-2 (S2) genotype under salt-stress conditions. Increasing salt doses (0 to 250 mM NaCl) also negatively affected mRNA transcript levels in the S2 genotype, but the transcript level of the S1 genotype slightly increased from 0.3-fold at control (0 h of 0 mM NaCl) to 0.8-fold at 12 h of salt stress under the applications of 50 and 250 mM NaCl. The moderate (M1 and M2) and tolerant (T1 and T2) genotypes showed higher increments in transcript level under salt-stress conditions. The M1 genotypes increased mRNA transcript levels from 0.1-fold at control to 0.8-fold at 24 h of 250 mM NaCl stress and from 0.4-fold at control to 1.4-fold at 12 h of 250 mM NaCl stress in the M2 genotype. The TaPTF1 transcript increased from 0.1-fold (control) to 2-fold at 12 h of 250 mM NaCl stress in the M1 genotype and from 0.2-fold (control) to 3.5-fold under 250 mM for 24 h in M2 genotype (Figure 2A and Figure A2A). The T2 genotype had a higher total fold-change in TaPTF1 gene expression. The TaPTF1 gene transcript fold-change was found to be higher under stress conditions of 12 h of 150 and 250 mM NaCl stresses (Figure 2C).
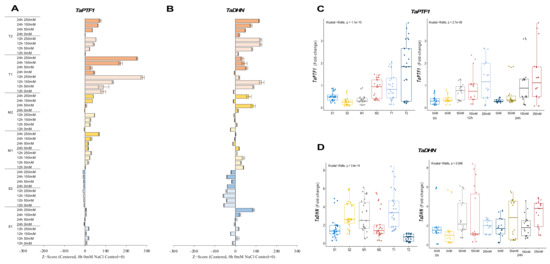
Figure 2.
The expression of TaPTF1 and TaDHN genes. (A) The gene expression pattern of TaPTF1 and (B) TaDHN genes; (C) the total gene expression profile of TaPTF1 for genotypes, salt stress, and time course; (D) the total gene expression profile of TaDHN for genotypes, salt stress, and time course. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Z-score centered to 0 h 0 mM treatment (Control = 0). Values represent the means ± SD (n = 3).
The transcript level of the TaDHN gene was increased from 0.1-fold (control) to 4.6-fold and 1.8-fold at 24 h of 150 and 250 mM NaCl in the S1 genotype, respectively. However, the mRNA transcripts were decreased in the S2 genotype from 5.8-fold at control to 7.4 and 1.9-fold under 150 mM NaCl stress for 12 and 24 h, respectively. The M1 genotype showed a significant increment from 1.7-fold (control) to 5.4-fold at 12 h of 150 mM salt stress, and TaDHN expression was increased from 1.3-fold (control) to 5.1- and 4.2-fold in M2 genotypes under 50 and 250 mM NaCl for 24 h. T1 genotypes showed a significant increment in TaDHN expression from 1.8-fold at control to 5.9- and 7.8-fold at 12 h of 50 and 150 mM NaCl stress conditions, respectively. Similarly, TaDHN gene transcripts were increased from 0.09-fold (control) to 1.7-fold at 12 h of 150 and 250 mM NaCl stress in the T2 genotype (Figure 2B and Figure A2B). The T1 genotype had a higher total expression fold-change, and 12 h of 150 mM NaCl had the highest expression fold-change compared to all other treatments (Figure 2D).
2.2.2. TaSRG and TaSC Genes
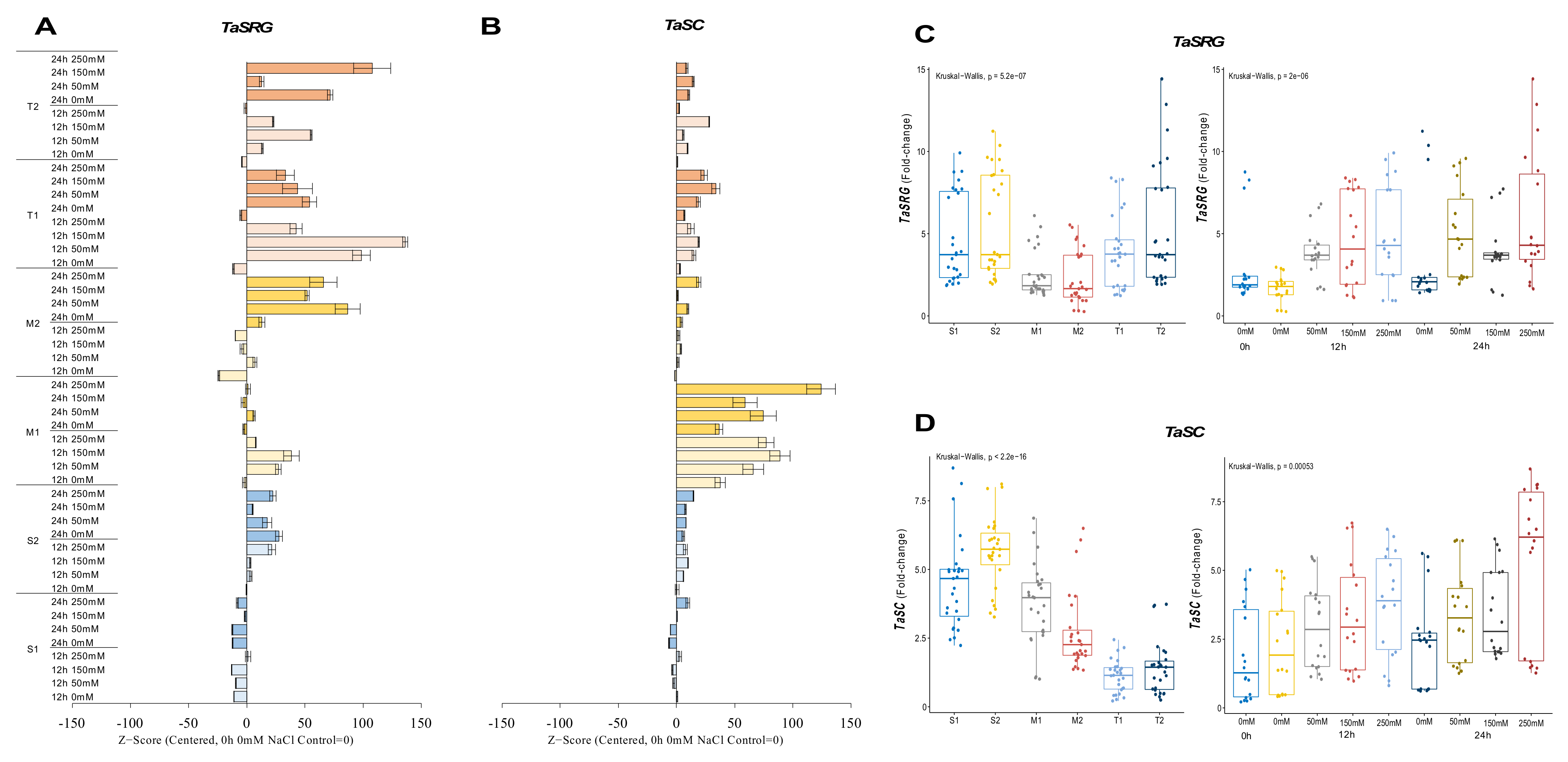
The TaSRG transcripts were decreased from 8.2-fold at control up to 1.9-fold under salt-stress conditions, except for 12 h of 250 mM, which was slightly increased from 8.2-fold (control) to 8.7-fold in the S1 genotype. Although a high fold change in the mRNA transcript level was recorded at 24 h under control and salt-stress conditions in the S2 genotype, under salt-stress conditions, it was decreased from 10.3-fold (control) to 9.3-, 7.7-, and 7.3-fold under 50, 150, and 250 mM NaCl stress conditions, respectively. In addition, only a significant increment (ca. 6.3-fold and up) was recorded in 12 h of 250 mM NaCl stress. The M1 genotype showed the highest increment from 1.7-fold (control) to 4.3- and 5.4-fold in the TaSRG gene transcript at 12 h of 50 and 150 mM NaCl stress conditions, respectively. The expression of TaSRG was increased from 1.3-fold (control) to 5.1- and 4.2-fold at 24 h of 50 and 250 mM NaCl stress in the M2 genotype, respectively. The expression of TaSRG was increased in all salt-stress applications and time courses in the T1 and T2 genotypes. The highest increments were recorded as 8.2-fold at 12 h of 150 mM NaCl stress in the T1 genotype and 12.8-fold at 24 h of 250 mM NaCl stress in the T2 genotype (Figure 3A and Figure A3A). However, the T2 genotype displayed an even greater TaSRG expression profile. The overall TaSRG gene expression fold-change was shown to be lower in moderate genotypes and higher in sensitive genotypes. Under salt-stress conditions, the TaSRG transcript fold-change significantly increased, especially after 24 h of 250 mM NaCl stress (Figure 3C).
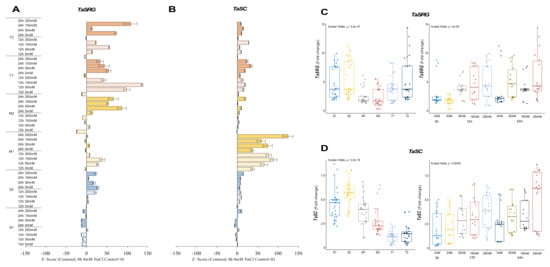
Figure 3.
The expression of TaSRG and TaSC genes. (A) The gene expression pattern of TaSRG and (B) TaSC genes; (C) the total gene expression profile of TaSRG for genotypes, salt stress, and time course; (D) the total gene expression profile of TaSC for genotypes, salt stress, and time course. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Z-score centered to 0 h 0 mM treatment (Control = 0). Values represent the means ± SD (n = 3).
The transcript level of TaSC was decreased from 4.6-fold (control) to 2.8-fold under 50 mM NaCl for 24 h, but it was increased by 1.1- and 3.5-fold under 250 mM NaCl for 12 and 24 h, respectively, in the S1 genotype. The S2 genotype showed an increment in the transcript level of TaSC under all salt-stress conditions from 3.6-fold (control) up to 6.6-fold. The highest gene expression was observed to be a 6.6-fold increase at 12 h of 150 mM in the S2 genotype. Similarly, the gene expression of TaSC was increased in the M1 genotype under all salt-stress treatments and time courses. The highest gene expression was recorded as 4.3-fold and 6.3-fold under 250 mM NaCl stress at 12 and 24 h, respectively. The TaSC expression of the M2 genotype was increased in all stress applications, and the highest gene expression was found to be 6-fold in 24 h of 250 mM NaCl stress application. A similar pattern was observed in the T1 and T2 genotypes, in which TaSC gene expression was increased from 0.2-fold (control) to 2.2-fold (24 h of 250 mM NaCl) and 0.3-fold (control) to 3.6-fold (12 h of 150 mM NaCl) in the T1 and T2 genotypes, respectively (Figure 3B and Figure A3B). The total transcript level of the TaSC gene was found to be higher in the S2 genotype, and 12 and 24 h of 250 mM NaCl stress increased the gene expression fold-change of the TaSC gene (Figure 3D).
2.2.3. TaPIMP1 and TaMIP Genes
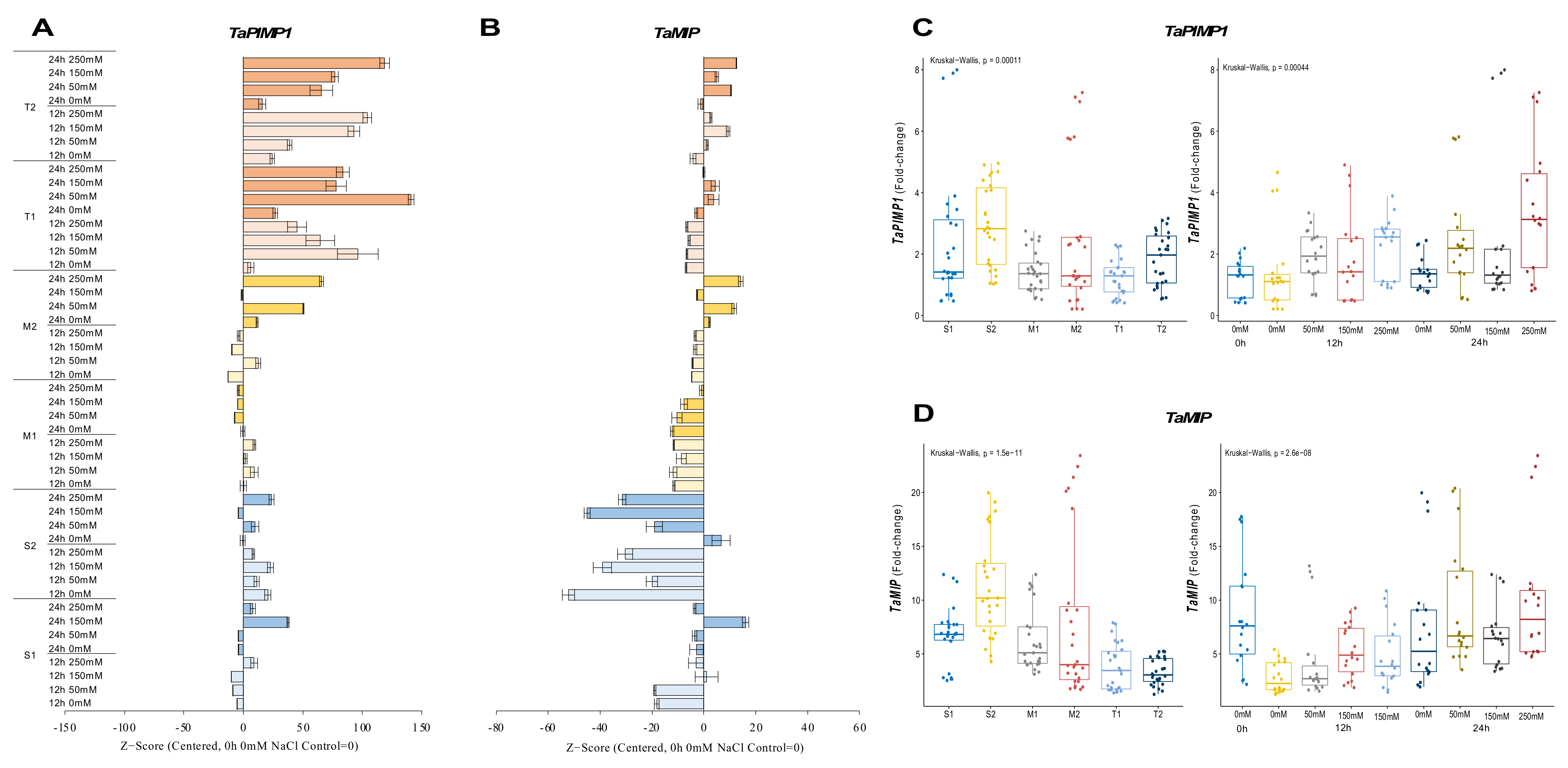
The expression of TaPIMP1 significantly decreased in the S1 genotype under salt, except under 12 h of 250 mM NaCl and 24 h of 150 and 250 mM NaCl stress, resulting in 3.4-, 7.8-, and 3.2-fold increases, respectively. Although gene expression of TaPIMP1 was increased in all salt treatments, it was decreased under 150 mM NaCl for 124 h. The TaPIMP1 transcript level of the M1 genotype was found to be higher (2.4-fold) in 12 h of 50 and 250 mM NaCl stress conditions. The tolerant genotypes showed a significant increment in TaPIMP1 expression under salt-stress conditions and time courses. The highest expression was recorded as 2.2-fold under 150 mM NaCl for 24 h in the T1 genotype and 3.6-fold at 12 h of 250 mM NaCl stress in the T2 genotype (Figure 4A and Figure A4A). The S2 genotype had overall fold-change increase in gene expression of TaPIMP1. Salt stress (250 mM NaCl) showed an increasing influence on the transcript fold-change of TaPIMP1 gene after 12 and 24 hours of exposure. Higher salt doses and long-term exposure raised the TaPIMP1 transcript fold-change in salt-sensitive and salt-tolerant genotypes (Figure 4C).
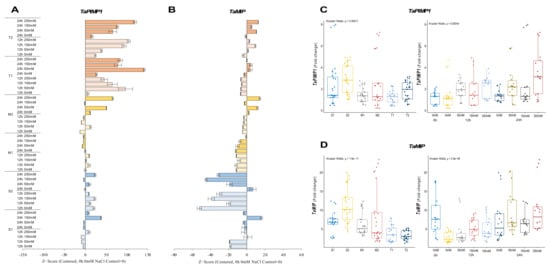
Figure 4.
The expression of TaPIMP1 and TaMIP genes. (A) The expression pattern of TaPIMP1 and (B) TaMIP genes; (C) the total gene expression profile of TaPIMP1 for genotypes, salt stress, and time course; (D) the total gene expression profile of TaMIP for genotypes, salt stress, and time course. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Z-score centered to 0 h 0 mM treatment (Control = 0). Values represent the means ± SD (n = 3).
The transcript level of TaMIP was decreased in all salt applications and time courses in the S1 genotype, but only 12 h of 150 mM NaCl increased gene expression from 7.7-fold (control) to 12-fold. A similar pattern was observed in the S2 and M1 genotypes, where gene expression only increased from 17.2-fold (control) to 19.1-fold under 0 mM NaCl for 24 hours in the S2 genotype. The M2 genotype showed higher expression of 16.6-fold and 22.4-fold after 24 h of 50 and 250 mM NaCl stress application, respectively. TaMIP gene expression was decreased is the rest of the treatments compared to the control in the M2 genotype. The gene expression was increased from 4.8-fold (control) to 6.7- and 7-fold at 24 h of 50 and 250 mM NaCl in the T1 genotype, respectively. However, the T2 genotype showed a significant increment in TaMIP expression in almost all salt treatments and time courses, except 0 mM NaCl for 12 and 24 h, which are time control treatments (Figure 4B and Figure A4B). The total fold-change in gene expression was found to be higher in the S2 genotype. The 0 h of 0 mM and 24 h 250 mM NaCl treatments showed increments in the expression fold-change of TaMIP genes (Figure 4D).
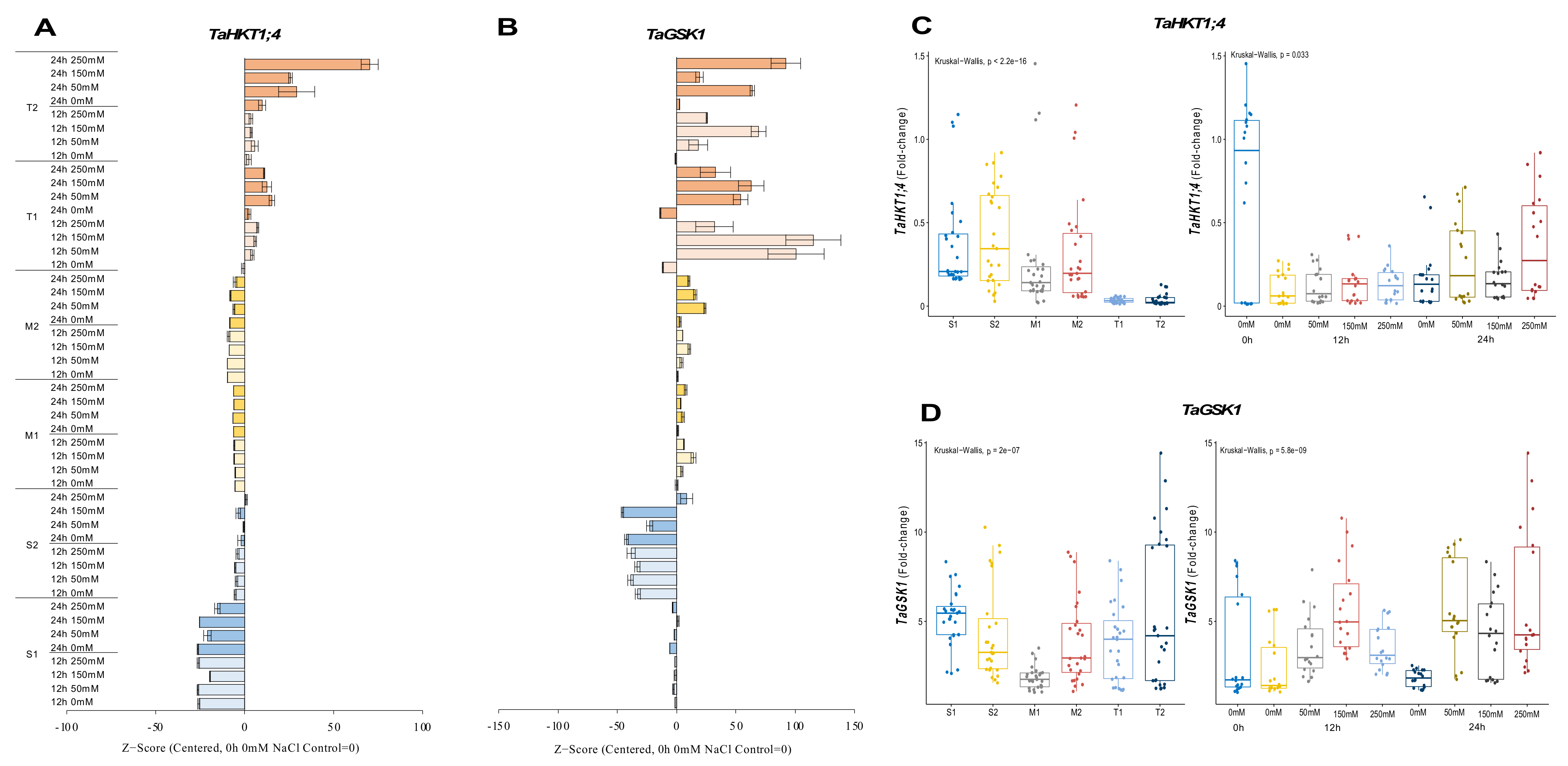
2.2.4. TaHKT1;4 and TaGSK1 Genes
TaHKT1;4 expression was significantly reduced for 12 and 24 h under all salt stress (50, 150, and 250 mM NaCl) applications in salt-sensitive and moderate genotypes. The expression was measured at a maximum of 0.5-fold under 250 mM NaCl for 24 h, but it was decreased by ca. 50% compared to 0 h of 0 mM NaCl conditions in the S1 genotype. The S2 genotype also showed a significant reduction in expression level from 0.7-fold (control) to 0.6-fold under 250 mM NaCl for 24 h. The transcript level of TaHKT1;4 was decreased from 1.2-fold (control) to 0.2-fold (12 h of 50 mM NaCl) and from 1-fold (control) to 0.5-fold (24 h of 250 mM NaCl) in the M1 and M2 genotypes, respectively. The mRNA transcripts of TaHKT1;4 were significantly increased in tolerant (T1 and T2) genotypes. The highest expressions were recorded as 0.057-fold and 0.050-fold at 24 h of 50 and 150 mM NaCl stress in the T1 genotype, respectively. Gene expression was increased at 24 h for all salt doses in T2 genotype. Higher TaHKT1;4 expression was detected under 50 mM (0.057-fold) and 250 mM (0.12-fold) NaCl for 24 h in the T2 genotype (Figure 5A and Figure A5A). The total gene expression fold change of TaHKT1;4 was found to be higher in the S2 genotype than in the other tested genotypes, and 0 h of 0 mM traits was the highest fold change in gene expression compared to other treatments (Figure 5C).
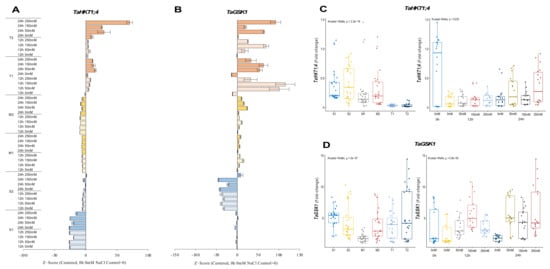
Figure 5.
The expression of TaHKT1;4 and TaGSK1 genes. (A) The gene expression pattern of TaHKT1;4 and (B) TaGSK1 genes; (C) the total gene expression profile of TaP5CS for genotypes, salt stress, and time course; (D) the total gene expression profile of TaGSK1 for genotypes, salt stress, and time course. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Z-score centered to 0 h 0 mM treatment (Control = 0). Values represent the means ± SD (n = 3).
The expression of TaGSK1 was decreased in the S1 genotype under all salt stress (50, 150, and 250 mM NaCl) treatments and time courses, but it was increased from 6.6-fold (control) to 7.6-fold under 24 h of 150 mM NaCl treatment in the S1 genotype. All salt treatments and time courses reduced the expression of TaGSK1 in S2 genotype. The highest gene expression was recorded as 5-fold when the control (0 h of 0 mM NaCl) was 8.2-fold in the S2 genotype. The TaGSK1 transcript level was increased in moderate (M1 and M2) genotypes under salt-stress conditions. The highest expression was observed as 3.2-fold under 150 mM NaCl stress for 12 h and 8.6-fold under 50 mM for 24 h in M1 and M2 genotypes, respectively. A similar pattern was observed in tolerant (T1 and T2) genotypes. The expression was increased from 1.8-fold (control) to 7.2-fold at 1 h of 150 mM NaCl stress in the T1 genotype, and it was increased from 1.3-fold (control) to 12.8-fold under 250 mM NaCl stress for 24 h in the T2 genotype (Figure 5B and Figure A5B). The total transcript level of TaGSK1 was found to be higher in tolerant genotypes after 12 and 24 h (Figure 5D).
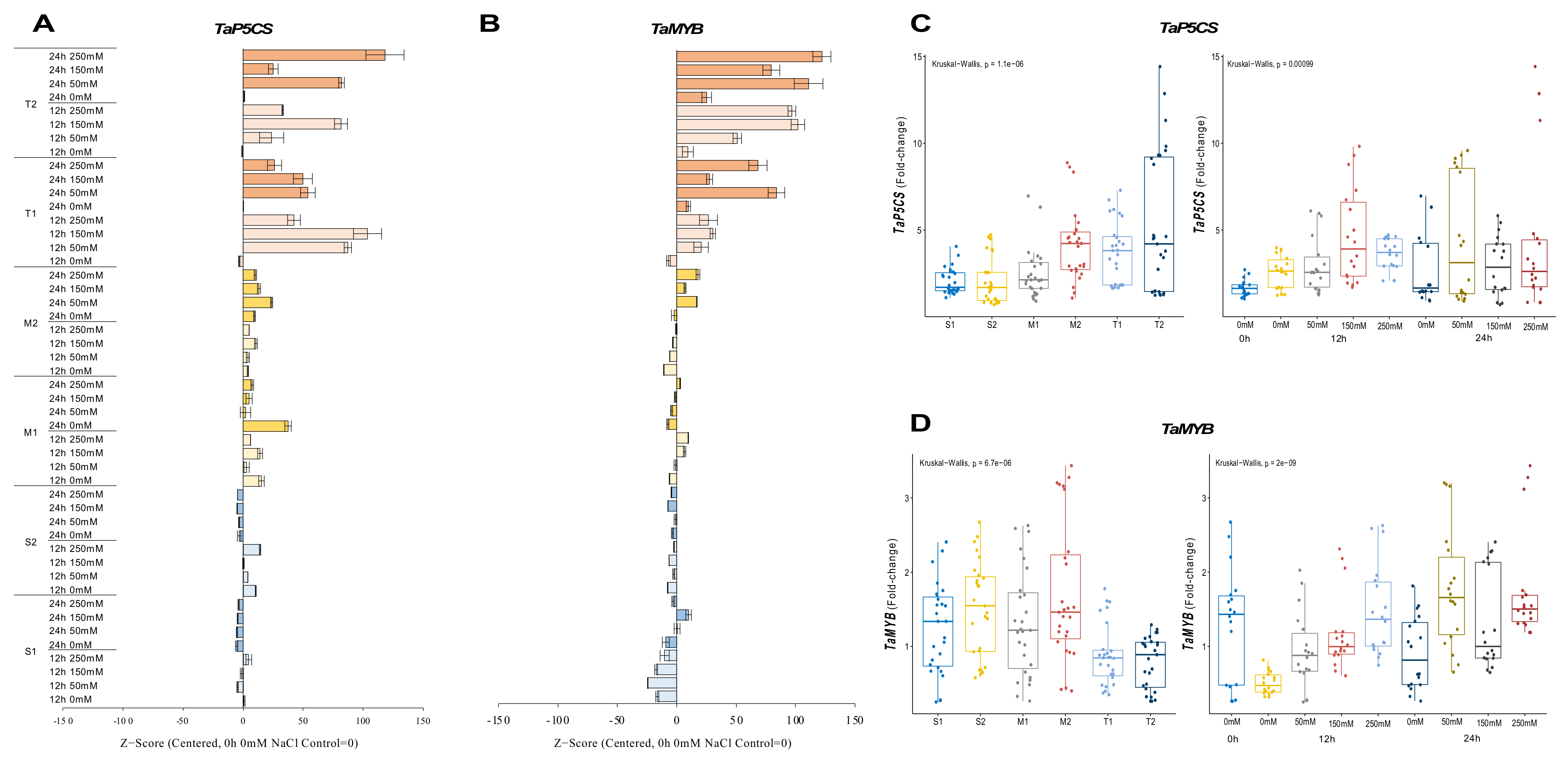
2.2.5. TaP5CS and TaMYB Genes
The expression of TaP5CS was reduced in sensitive (S1 and S2) genotypes under salt-stress conditions, but it was increased from 2.4-fold (control) to 3.5-fold at 12 h of 250 mM NaCl stress in the S1 genotype and from 1.7-fold (control) to 4.6-fold under 250 mM for 12 h in the S2 genotype. The transcription of the TaP5CS gene was found to be more abundant in moderate (M1 and M2) genotypes under salt-stress applications. The transcript level of TaP5CS was increased from 1.7-fold (control) to 3.2-fold in the M1 genotype and from 1.3-fold (control) to 8.6-fold in the M2 genotype under 50 mM (12 h) and 150 mM (24 h) NaCl salt-stress conditions, respectively. A similar gene expression pattern was observed in tolerant (T1 and T2) genotypes. The highest expression was recorded as 6.7-fold and 12.8-fold in T1 (12 h of 150 mM NaCl) and T2 (24 h of 250 mM NaCl) genotypes (Figure 6A and Figure A6A). The total gene expression fold-change was found higher in the T2 genotype, and 12 and 24 h of salt exposure increased the expression fold-change of the TaP5CS gene under salt-stress conditions (Figure 6C).
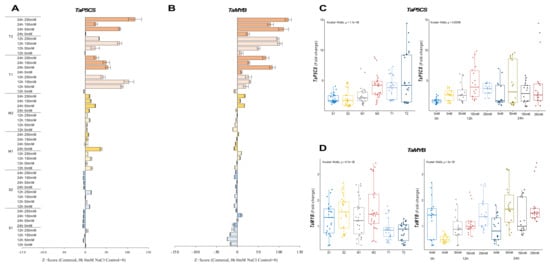
Figure 6.
The expression of TaP5CS and TaMYB genes. (A) The expression pattern of TaP5CS and (B) TaMYB genes; (C) the total gene expression profile of TaP5CS for genotypes, salt stress, and time course; (D) the total gene expression profile of TaMYB for genotypes, salt stress, and time course. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Z-score centered to 0 h 0 mM treatment (Control = 0). Values represent the means ± SD (n = 3).
The expression of TaMYB was significantly decreased in sensitive (S1 and S2) genotypes, with some exceptions. The expression was only increased from 2.4-fold (control) to 3.5-fold under 12 h of the 250 mM NaCl stress condition in the S1 genotype. TaMYB expression was found to be 2.1-fold and 2.5-fold higher at 12 h of 150 and 250 mM NaCl stress in the M1 genotype, respectively. The mRNA transcript level of TaMYB was found to be higher after 24 h compared to the 0 and 12 h periods. The highest gene expression was recorded as 3.2-fold at 24 h of 250 mM NaCl stress in the M2 genotype. The expression of TaMYB was increased by salt treatments (50 and 150 mM NaCl) in the tolerant (T1 and T2) genotypes. The highest gene expressions were observed as 1.6-fold and 1.3-fold at 24 h of 50 mM NaCl stress in the T1 and T2 genotypes, respectively (Figure 6B and Figure A6B). The total gene expression fold-change was found to be higher in the M2 genotype, but the effective activity of TaMYB was recorded as being higher after 24 h of salt-stress exposure (Figure 6D).
2.3. Principle Component, Hierarchical Clustering, and Correlation Network Analyses
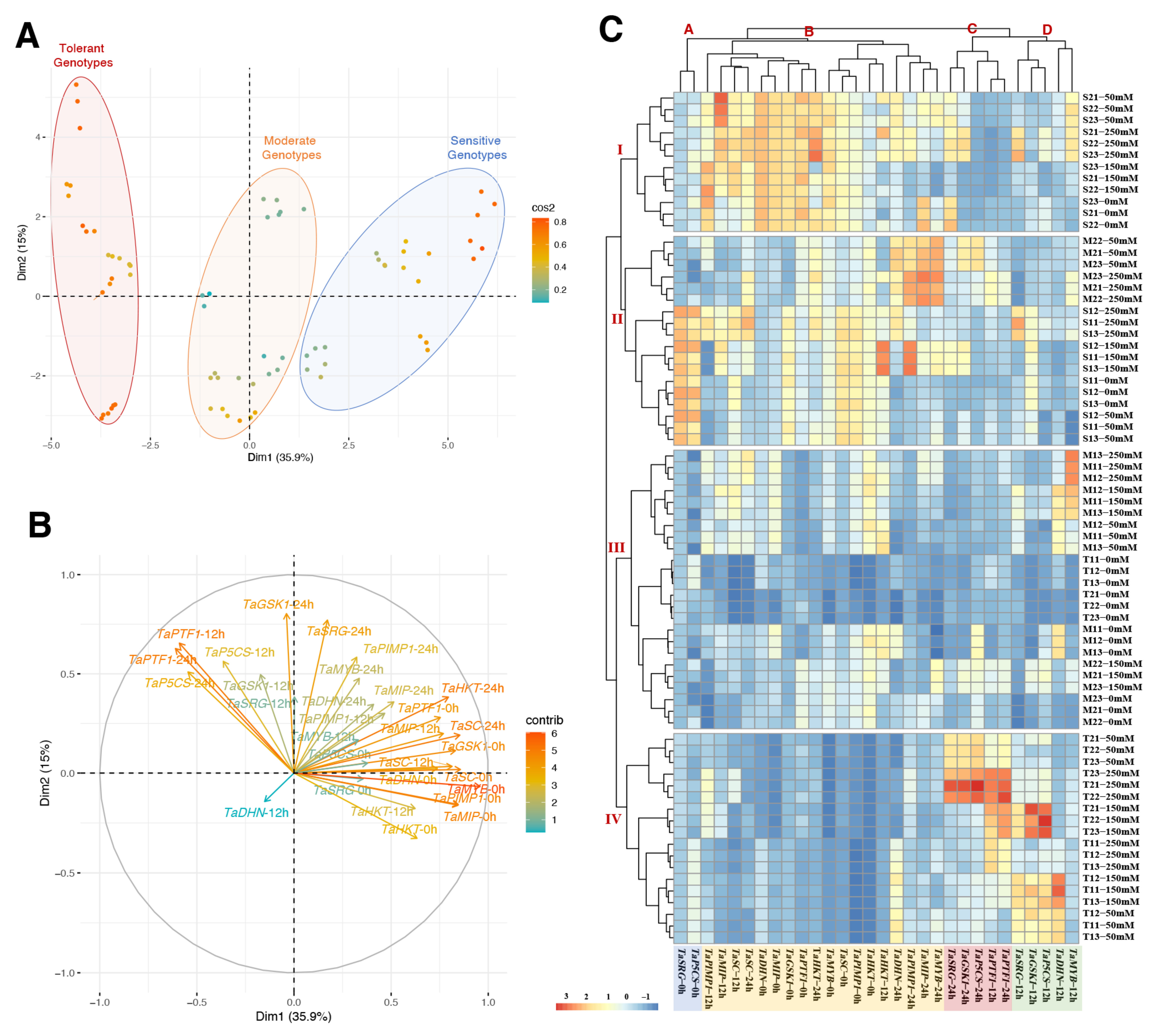
The PCA analysis was performed on gene expression data from two salt-sensitive, two moderate, and two salt-tolerant wheat genotypes to evaluate the genes’ variables and identify the factors with a predominant influence on usability for the detection of salt-tolerant genotypes. The PCA exhibits those genotypes under all saline conditions, time courses, and variables associated with Dimmention1 (35.9%) and Dim2 (15%), of which Dim1 was the major component (total 50.9%) (Figure 7A and Table S3). The colors of the individual variables represent their quality of representation of the principal component abbreviated as ‘Cos2′. Almost all variables were expressed as high quality in the analysis. The tolerant, moderate, and sensitive genotypes were clearly separate from each other in Figure 7A. The salt-tolerant genotypes result in a high gene expression of the TaPTF1 (12 and 24 h), TaP5CS (12 and 24 h), TaGSK1 (12 and 24 h), and TaSRG (12 and 24 h) genes on the left side of the first axis (Dm1) (Figure 7B).
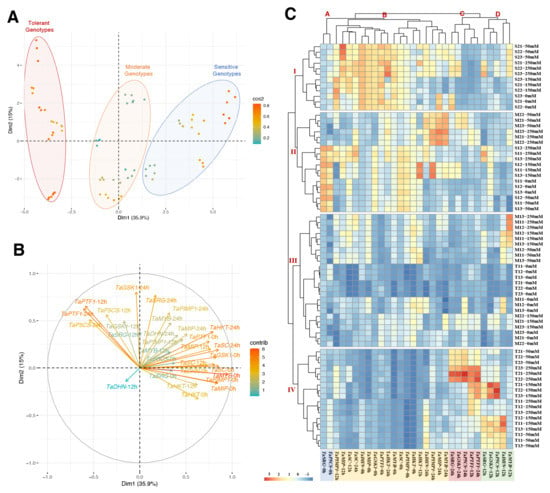
Figure 7.
Principle component and hierarchical clustering analyses of genotypes and genes under salt stress and time course. (A) Principal component analysis (PCA) of the spatialization of two salt-sensitive, two moderate, and two tolerant genotypes (Colors blue: salt-sensitive, orange: moderate, and red: salt-tolerant genotypes), (B) PCA of the studied traits, (C) hierarchical clustering analysis (HCA) of measured gene expression level in salt-sensitive (S1, S2), moderate (M1, M2), and salt-tolerant (T1, T2) genotypes under 0, 12, and 24 h of 0, 50, 150, and 250 mM NaCl treatments. Clusters represent genotypes (I to IV), and genes (A to D).
According to the heat map generated by the two-way hierarchical clustering analysis (HCA), all of the gene expression parameters under salt stress could be classified into four primary clusters (Figure 7C, groups A, B, C, D). A similar tendency has been observed for the genotypes after salt treatment. TaP5CS (0 h) and TaSRG (0 h) were separated into a distinct cluster (A) with high values in salt-sensitive genotypes. Cluster B showed the genes with higher values in sensitive and moderate genotypes under the control and salt-stress conditions. The TaPTF1 (12 and 24 h), TaP5CS (12 and 24 h), TaGSK1 (12 and 24 h), and TaSRG (12 and 24 h) genes were organized into two different clusters (C and D) with greater values under salinity conditions in tolerant genotypes, according to the HCA results. Under salinity stress, these characteristics are frequently reduced in salt-sensitive genotypes. The heatmap categorized the five germplasms into four separate clusters using data from salinity treatment (Figure 7C, groups I, II, III, IV). Under control and salt-stress conditions, Cluster I indicated the salt-sensitive genotype (S2). The M1 genotype under salt stress and the S1 genotype under control and salt-stress conditions were both assigned to cluster II. Cluster III represented the M1 genotype under both stress conditions, the M2 genotype under control conditions, and the tolerant genotypes under control conditions. Cluster IV was designated for only the salt-tolerant genotypes under salt-stress conditions.
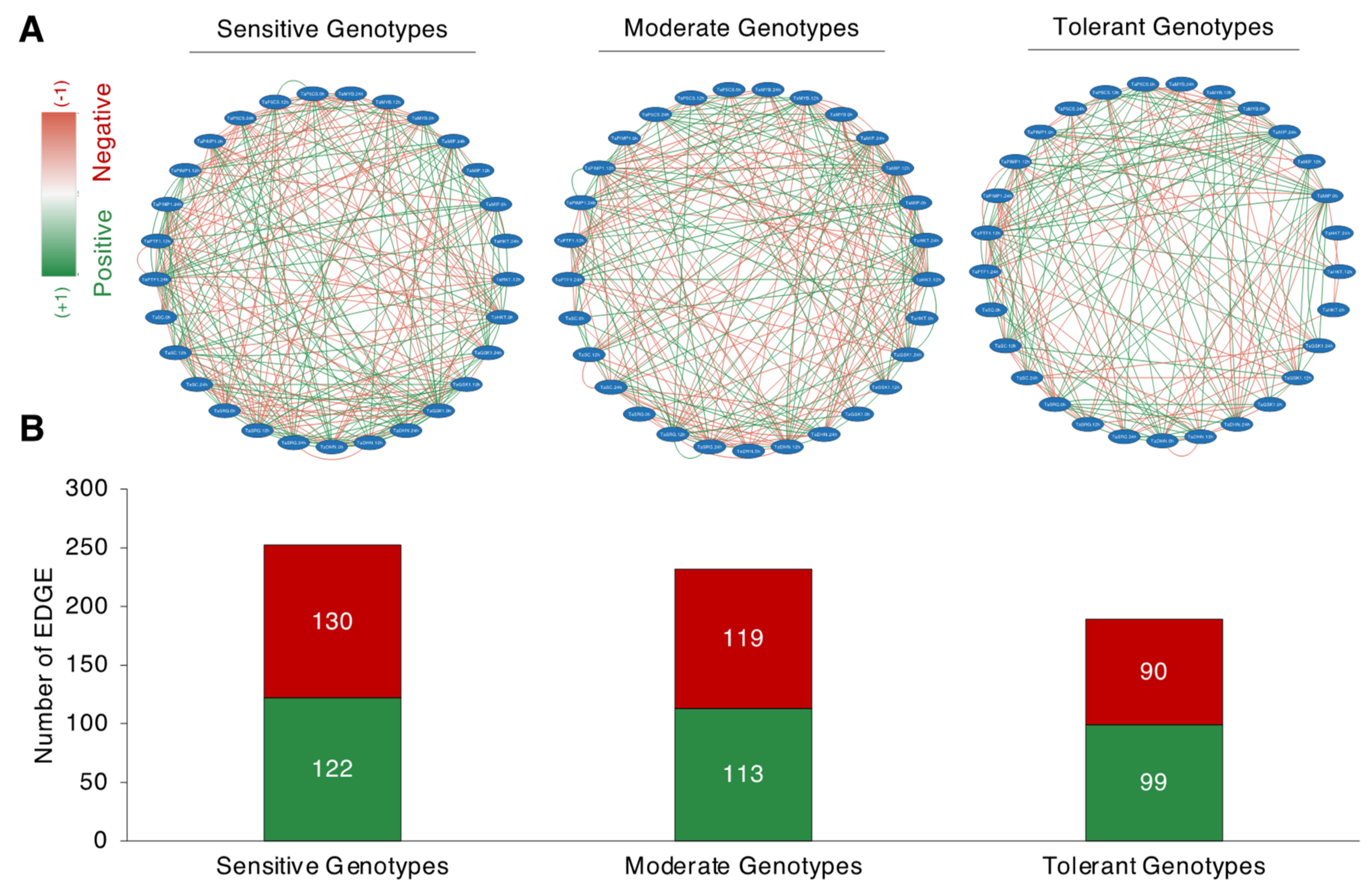
Following the separation derived from the PCA, correlation-based network analysis (CNA) was constructed for sensitive, moderate, and tolerant genotypes, under normal salt-stress conditions (Figure 8A). The total interaction number (number of edges, where an edge encodes an interaction or a tie between two traits) was shown to be larger in the salt-sensitive and moderate genotypes but lower in the salt-tolerant genotypes. The salt-sensitive genotypes had 252 edges, with 122 positive correlations resulting in a positive edge-to-negative edge (pe/ne) ratio of 0.93. The moderate genotypes had 232 edges, with 113 corresponding to positive correlations and 119 corresponding to negative correlations, yielding a pe/ne ratio of 0.94. The salt-tolerant genotypes had a total of 189 edges, with 99 corresponding to positive and 90 corresponding to negative correlations, yielding a pe/ne ratio of 1.1. The CNA discovered a similar pattern across salt-sensitive and moderate genotypes, with both having a higher negative edge than a positive edge. The salt-tolerant genotype, on the other hand, has a higher positive edge than a negative edge.
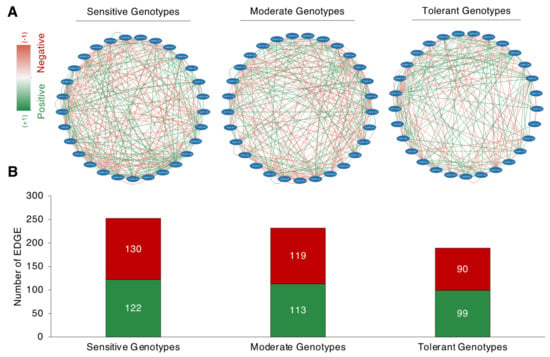
Figure 8.
Correlation network analysis (CNA) of studied traits. (A) Correlation network analysis of analyzed traits of salt-sensitive (left panel), moderate (middle panel), and salt-tolerant (right panel) genotypes; (B) Positive and negative EDGE (total interaction) numbers of salt-sensitive, moderate, and salt-tolerant genotypes. Red (−1) and green (+1) colors correspond to the negative and positive edge associations, respectively (p < 0.05). An edge encodes an interaction or a tie between two traits.
3. Discussion
Rising sea levels and wheat farming irrigation systems based on underground water have increased soil salinity and reduced wheat productivity. Wheat is seriously affected by salinity concentrations in soils. Approximately 34% of wheat cropland is currently irrigated, 80–126 million ha of modern rainfed wheat cropland do not have enough discharge to meet irrigation demand, and 30 to 47% of today’s rainfed wheat croplands need more irrigation. On a global scale, more than half of all wheat cropland (62%) exhibits evapotranspiration, which is a typical case of deficit irrigation [42]. Saline groundwater up to 6 dSm−1, from which wheat can still draw 40% of its needs, has been shown in certain trials to diminish yield by 30% [43]. According to a different study, under the salinity thresholds of 4 and 15 dSm−1, there are, correspondingly, 10–55% decreases in wheat grain yield [11]. When combined with environmental circumstances, genetics is thought to be one of the most important aspects defining a plant species’ ability to tolerate salt [44]. As a result, the determination of salt-tolerant genotypes is a critical and time-consuming undertaking for increasing agricultural output. Although various promising morphological, physiological, and molecular marker strategies for determining stress-tolerant genotypes are under development, the molecular marker strategy remains one of the fastest detection methods at present.
Although numerous genes have been identified as molecular marker genes for salinity tolerance in the wheat genome, ion accumulation, and proline synthesis are the key molecular pathways for salinity tolerance in plants [45,46,47]. Different growth stages of plants exhibit variable degrees of salt stress, with the seedling stage in wheat being the most vulnerable because this stage determines the formation of the tillers, which, in turn, determines the number of spikelets and, eventually, the yield [48]. The response of plant growth to salinity often occurs in two stages: a quick response to a rise in osmotic pressure, followed by a more gradual response following the accumulation of Na+ in mature tissues, which results in ion toxicity that affects plant growth and development [2]. Numerous physiological changes are brought on by osmotic stress, such as cell membrane distortion, protein aggregation, DNA damage, disorganized ROS generation, severe ion imbalance, and reduced photosynthetic activity [49].
Under environmental stressors, proline serves as an effective osmolyte, antioxidative defense, and signaling molecule [50]. When plants are stressed, accumulating proline helps to maintain cell osmotic balance, stabilizes membranes to prevent electrolyte leakage, and acts as an antioxidant to control the level of reactive oxygen species (ROS) [51]. Proline is synthesized from glutamate by 1-pyrroline-5-carboxylate synthase (P5CS) in the glutamate pathway [26]. In our experiment, salt-tolerant and moderate genotypes showed a significant increment in the transcript level of TaP5CS gene and proline content under salt-stress conditions (Figure 1 and Figure 5A). The salt-sensitive genotypes also increased proline content under control conditions, but the increment level was significantly lower in salt-sensitive genotypes (Figure 1A,B). The time course did not significantly affect proline content in almost all genotypes, but salt doses significantly increased proline content in all genotypes. Hien et al. [52] showed a relative rise in proline concentration in saline-tolerant rice cultivars 48 h after stress application, while no increases were observed in the sensitive cultivar, even after 72 h of treatment with 200 mM NaCl. These genotype-specific responses may be related to signaling cascades that regulate proline metabolism, which is controlled by diverse cellular mechanisms and should be explored further. Among the factors involved, transcription factors, ion accumulation or balance, and abscisic acid (ABA) involved in gene signaling and expression related to proline biosynthesis [53].
Transcription factors are the key genes that initiate and regulate the transcripts of the genes. The MYB proteins are one of the largest transcription factor families in plants [34]. TaMYB is a key regulator gene involved in wheat salt-stress adaptation [35]. In our experiment, salt-tolerant genotypes showed increasing expression levels of TaMYB gene under salt-stress conditions (Figure 6B,D). The overexpression of MYB2 genes increased proline accumulation in rice plants under salt-stress conditions [54]. Furthermore, a putative activation domain rich in acidic amino acid residues, glutamic acid, and aspartic acid significantly up-regulates TaMYB gene expression in drought and salt stress [35,55]. One other member of the R2R3-MYB transcription factor subfamily gene is TaPIMP1, which contains MYB DNA binding domains and enhances drought and salinity tolerance in plants [56]. The transcription level of the TaPIMP1 gene in salt-tolerant genotypes was increased under salt-stress conditions compared with salt-sensitive and moderate genotypes (Figure 4A,C). TaPIMP1 is controlled by ABA, and the overexpression of TaPIMP1 increased proline synthesis, resulting in greater drought tolerance [36]. The overexpression of TaPIMP1 improves salinity tolerance by enhancing chlorophyll content and superoxide dismutase (SOD) activity [56]. The most used genes in wheat are high-affinity potassium transporters (HKTs) that are responsible for Na+ and K+ ion homeostasis in wheat during salt stress [57]. The HKT family of proteins are expected to be important in plant salt-stress tolerance [58,59]. In our experiments, the expression level of TaHKT1;4 genes were increased in salt-tolerant genotypes, and it was reduced in salt-sensitive genotypes under salt-stress conditions. However, the total expression level was found higher in salt-sensitive genotypes under salt-stress conditions (Figure 5B,D). TaHKT1;4 has a higher functional variety among its members than the other HKT-type transporter groups and contributes to Na+ exclusion from leaf blades in response to salt stress. It was found to be expressed mostly in leaf sheaths and panicles [38,60,61].
Aquaporins (AQPs), which are water-selective channel proteins, mediate and control fast transmembrane water flow during activities such as seed germination, cell elongation, stomatal movement, phloem loading and unloading, reproductive growth, and stress responses [62]. According to protein subcellular location and amino acid sequence homology, the plant AQP family is split into three groups: small basic intrinsic protein (SIPs), nodulin 26-like intrinsic protein (NIPs), and plasma membrane intrinsic protein (PIPs) [63,64,65,66]. TaMIP, or TaNIP, is a novel aquaporin gene whose overexpression accumulates higher K+ and proline content and lower Na+ concentration in salt-exposed plants [19]. The salt-sensitive and moderate genotypes showed decreasing expression levels of TaMIP, but the expression level of TaMIP was increased in salt-tolerant genotypes under salt-stress conditions (Figure 4B,D). Additionally, the expression of several PIP-type genes was elevated in maize 2 h after 100 mM NaCl exposed maize for 2 h by collecting ABA [67]. Another study showed that a salt-tolerant rice genotype showed higher expression levels of aquaporin genes (OsTIPs and OsPIPs), OsP5CS, and proline accumulation under salt-stress conditions [68]. In response to salt, proline buildup is preceded by a rapid increase in the mRNA levels of TaP5CS and TaDHN genes, which regulate the rate-limiting stage of glutamate-derived proline biosynthesis [40]. The transcript level of the TaDHN gene was significantly increased in salt-tolerant genotypes under salt-stress conditions (Figure 3B,D), and the expression level of the TaP5CS gene was reduced in salt-sensitive genotypes but significantly increased in salt-tolerant genotypes under salt-stress conditions (Figure 6A,C). Transgenic plants demonstrate enhanced P5CS activity due to DHN-5 genes accumulating substantial amounts of proline [69].
TaP5CS transcript accumulation is tissue-specific and can be induced by salt and ABA. TaSRG, TaPTF1, TaSC, TaPIMP1, and TaGSK1 genes were discovered to regulate osmotic stress via ABA signaling [20,21,36,70]. The expression level of the TaSRG gene was increased after 24 h of NaCl exposure in salt-tolerant genotypes (Figure 3A,C). TaSRG might also control P5CS gene expression, which could have helped the transgenic plants’ tolerance to salt by maintaining a high K+/Na+ ratio [20]. The basic helix–loop–helix transcription factors (bHLH) improve salinity tolerance in plants [37]. TaPTF1 is a homolog of OsPTF1, a bHLH transcription factor, and OsPTF1 has been demonstrated to provide tolerance to Pi deficiency in rice [71]. Previous studies showed that the transcript number of TaPTF1 increased with the increase in TaP5CS and proline accumulation in salt-tolerant wheat genotypes [72]. In our experiment, the expression level of TaPTF1 was increased in salt-tolerant and moderate genotypes, and it was decreased in salt-sensitive genotypes under salt-stress conditions (Figure 2A,C). TaSC overexpression in transgenic plants boosted free proline levels as well as P5CS gene expression. Under stress, ABA and NaCl increase TaSC gene expression [21]. The transcription number of TaSC was increased in salt-tolerant and moderate genotypes under salt-stress conditions, and the expression level of TaSC was found to be higher in salt-sensitive genotypes compared with salt-tolerant and moderate genotypes (Figure 3B,D). When salt stress was applied to the cells, the TaSC gene promoter perceived the ABA accumulation signal, which up-regulated its expression and increased the concentration of the second messenger Ca2+, finally activating the CDPK pathway. As a result of the expression of downstream genes in the pathway, proline accumulated, the intracellular K+/Na+ ratio increased, and chloroplast activity was improved [21]. A salt-inducible gene called TaGSK1 exhibits a high degree of similarity with mammalian GSK3, a highly conserved serine/threonine protein kinase that controls the formation of glycogen [39]. In our experiment, the transcription level of TaGSK1 was decreased in the salt-sensitive genotypes but significantly increased in salt-tolerant and moderate genotypes under salt-stress conditions (Figure 5B,D). Exogenously applied ABA and NaCl both stimulated AtGSK1 expression, and AtGSK1 overexpression improved NaCl tolerance in Arabidopsis [73]. Transgenic plants with overexpressed TaGSK1 have reduced cellular osmotic turgor and Na+ concentration, as well as increased salt tolerance [39].
Previously, we found the wheat genotypes ‘Maycan’ (T1) and ‘Yildiz’ (T2) as salt-tolerant; ‘Kinaci-98’ (M1) and ‘Dogu-88’ (M2) as moderate; and ‘Esperia’ and ‘Sonmez-01’ as salt-sensitive [72]. Under salt conditions, the tolerant genotypes had higher osmoregulator proline content and antioxidant enzyme activity than the moderate and sensitive genotypes. All gene expression results demonstrated that salt-tolerant, salt-moderate, and salt-sensitive genotypes were categorized individually in the PCA analysis (Figure 7A), which is highly supported by our prior findings [72]. Under salt-stress conditions, salt stress raised the expression level of all studied genes in salt-tolerant genotypes. The salt-exposed time course revealed that 24 h of salt stress had a greater increasing effect on the gene expression levels of TaPTF1, TaPIMP1, TaMIP, TaHKT1;4, and TaMYB genes in salt-tolerant genotypes than 12 h did. Despite the fact that all genes related to proline accumulation and ion accumulation were studied, we found that the traits of TaPTF1 (12 and 24 h), TaP5CS (12 and 24 h), TaGSK1 (12 and 24 h), and TaSRG (12 and 24 h) were highly associated with tolerant genotypes (Figure 7C). Positive and negative correlations between the observed parameters indicate whether or not the investigated plant is stress-resistant. If negative correlations occur less often in the same plant than positive correlations, this suggests that the plant is salt-tolerant [74]. In our experiment, the correlation-network analysis revealed that salt-tolerant genotypes have a higher pe/ne ratio than salt-sensitive and salt-moderate genotypes (Figure 8B).
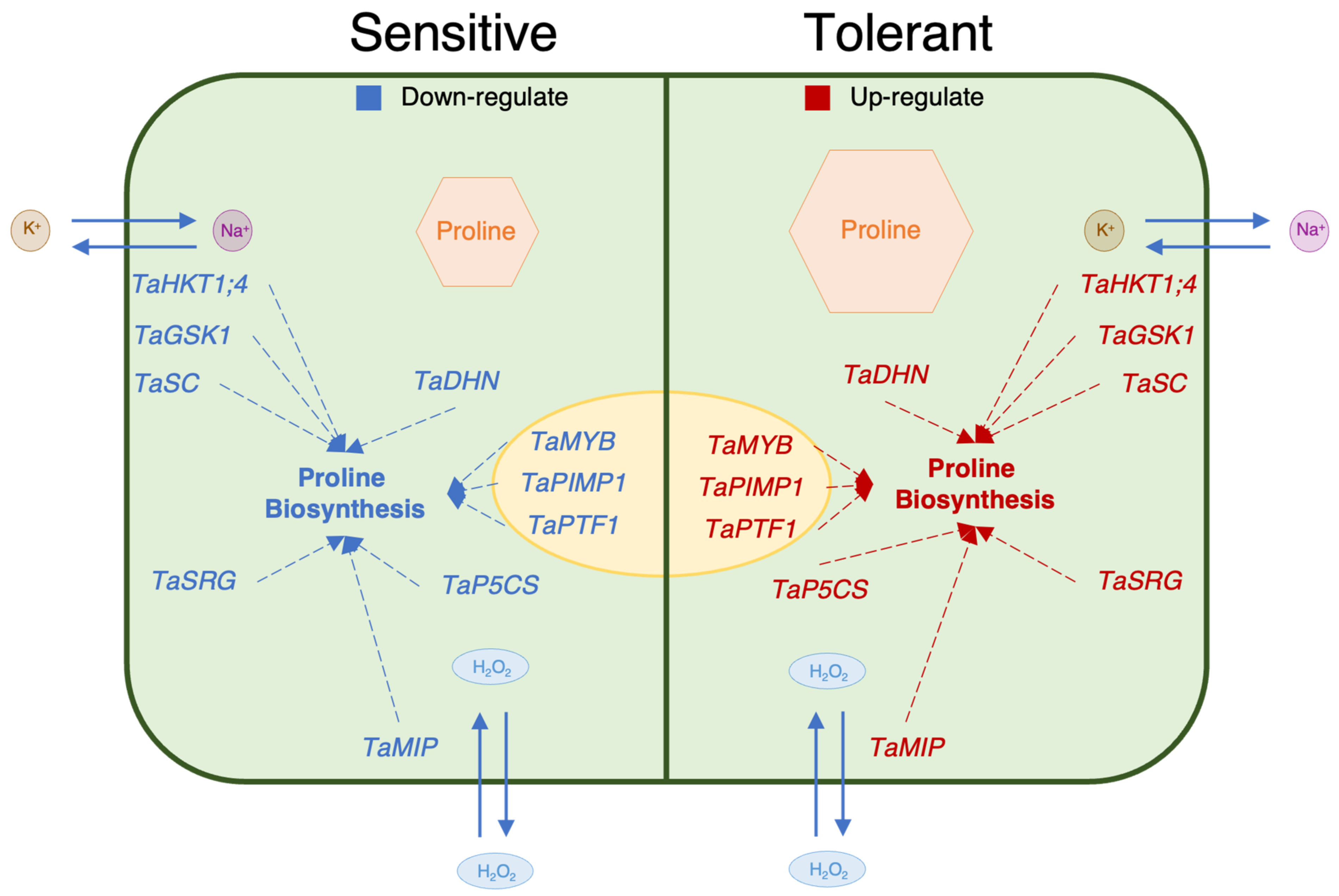
In the genotypes that are more tolerant to salt stress, the levels of transcript genes are more associated with the proline content [75]. The TaSRG may have influenced TaP5CS gene expression and increasing proline concentration in tolerant genotypes may be associated with increased TaP5CS gene transcript levels in tolerant genotypes as compared to sensitive genotypes [20,40]. The proline content of the genes encoding the transcription factors TaMYB, TaPIMP1, and TaPTF1 increased during salt stress in salt-tolerant genotypes in the past [35,36,72]. In this investigation, we discovered that these transcription factors had higher levels of gene expression and enhance the proline concentration in genotypes that are tolerant. Another important mechanism for plants to tolerate salinity is the ion balance, which is controlled by the TaHKT1;4, TaGSK1, and TaSC genes. These genes also enhance proline concentration in plants under salt-stress conditions [21,39,70,76]. Aquaporin genes such as TaMIP play a crucial role in cell defense against salt stress with dehydration (TaDHN) genes, which is the major mechanism for salinity tolerance in plants after ion balance [18,67]. Additionally, prior research indicates that this gene contributes to proline accumulation in plants under salt stress [69]. Our findings demonstrated that, despite each gene’s unique role, improving proline biosynthesis served as a shared mechanism for differentiating between salt tolerance and sensitivity (Figure 9).
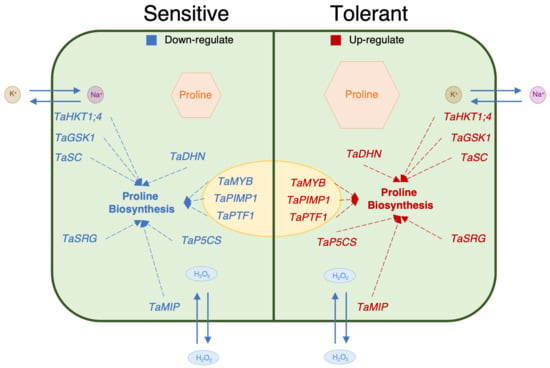
Figure 9.
Suggested schematic illustration of a working model depicting the relationship between proline content and proline-related genes.
4. Materials and Methods
4.1. Plant Material and Experimental Design
Two salt-sensitive (S1; ‘Esperia’ and S2; ‘Sönmez-01’), moderate (M1; ‘Doğu-88’ and M2; ‘Kınacı-97’), and tolerant (T1; ‘Maycan’ and T2; ‘Yildiz’) wheat (Triticum aestivum L.) genotypes were used for the experiment [72]. The seeds were obtained from the Department of Breeding and Genetics, Central Research Institute for Field Crops, Ankara, Türkiye.
The experiment was performed with three independent replicates (each a pool of 5 plants). Seeds were surface-sterilized, rinsed three times with sterile distilled water, and germinated on moist blotting paper in plastic Petri dishes. After 3 days of incubation germinated seeds were transferred to 0.5 L pots filled with peat-based growing media (propagation substrate SF1, SuliFlor, Radviliškis, Lithuania) with the following characteristics: pH of 6, electrical conductivity (EC) of 0.65, 80% organic matter, and N-P2O5-K2O ratio of 14:16:18. Pots were placed in individual trays in a controlled growth chamber under light intensity equal to 27 μmol/m2/s. The temperature was maintained at 25/23 °C Day/night and relative humidity of 70%. Plants were irrigated with sterile water, and the same water level was maintained for all plants. Two-week-old wheat seedlings were subjected to 0, 50, 150, and 250 mM NaCl for 12 and 24 h.
4.2. RNA Extraction and cDNA Library
The total RNA was extracted from wheat leaves by using the TRizol method [77] according to the manufacturer’s instructions (Invitrogen, Waltham, MA, USA), following treatment with RNAase-free DNAase I (ThermoFisher, Waltham, MA, USA). Total RNA quantity and quality were determined using instructions from NanoDrop ND-1000 spectrophotometer (ThermoFisher, Waltham, MA, USA). The First Strand cDNA Synthesis Kit was used to generate cDNA templates from total RNA samples via reverse transcription (ThermoFisher, Waltham, MA, USA).
4.3. Real-Time Quantitative PCR
Transcript levels were analyzed in a CFX Connect™ 96 Real-Time PCR Detection System (Bio-Rad Laboratories GmbH, CA, USA). RT-PCR amplifications were conducted in a 15 µL reaction volume mixture containing 1.5 µL of cDNA, 4 µL ddH2O, 1 µL of 10 pmol forward (sense) primer, 1 µL of 10 pmol reverse (antisense) primer, and 7.5 µL iTaq™ Universal SYBER® Green Supermix (Bio-Rad Laboratories GmbH, CA, USA). Each reaction for each gene was performed in triplicate following PCR protocol: 5 min 94 °C, 30 s 94 °C, 5 s 65 °C, 10 s 75 to 95 °C for melting curve (30 cycles). PCR amplification was performed with different cycles to ensure a linear response in the PCR. Primers used for RT-PCR are shown in Table 2, and their specificity was checked by separating the PCR products on 1.8% agarose gels. The TaACTIN (AB181991.1) gene was used as a reference gene, and the expression levels of genes were calculated by using the 2−ΔΔCT method [78].

Table 2.
Primer sequence of genes used in the experiment.
4.4. Proline Content
Free proline content was determined according to the method described by Bates et al. [79], with a slight modification. Fresh leaf samples were homogenized in 10 mL of 3% sulfosalicylic acid and incubated for 24 h at 4 °C. The homogenate was centrifuged at 10000 rpm at 25 °C for 5 min, and the supernatant (1 mL) was reacted with 1 mL ninhydrin reagent and 1 mL glacial acetic acid in a test tube at 100 °C for 1 h before stopping the reaction by immersing the tubes in an ice bath for 20 min. Proline was extracted with 2 mL of toluene and incubated for 30 min at room temperature. The toluene phase was separated, and absorbance at 520 nm was measured with a UVmini-1240 spectrophotometer (Shimadzu, Japan). Proline content was assessed from biological triplicated.
4.5. Evolutionary Analysis by Maximum Likelihood Method
The evolutionary history was interfered with by using the Maximum Likelihood method and Jones–Taylor–Thornton (JTT) matrix-based model [80]. The tree with the highest log likelihood (−2352.72) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor–Join and BioNJ algorithms to a matrix of pairwise distance estimated using the JTT model and then selecting the topology with superior log likelihood value. The tree is drawn to scale with branch lengths measured in the number of substitutions per site. This analysis involved 10 amino acid sequences. There was a total of 156 positions in the final dataset. Evolutionary analyses were conducted in the MEGA11.0.13 program [41,81].
4.6. Statistical, Principal Component, Hierarchical Clustering, and Network Analyses
The recorded data for each trait were initially standardized, obtaining the Z scores using means of the expression Z= (X − Y)/W, where Z is the value of the standardized variable corresponding to the respective trait and Y is the observation of 0 h and 0 mM NaCl. Y is the overall mean of the 0 mM 0 h trait in three replications, and W is the phenotypic standard deviation of the 0 mM 0 h trait. Collected data were subjected to a three-way analysis of variance (ANOVA) using R software (V4.2.1, https://www.r-project.org/, accessed on 28 November 2022) to assess differences among cultivars, salinity doses, and time course. Means separation was determined using Tukey’s honest significant difference (HSD) test at p < 0.05 with R software, including the ‘glht’ function in the ‘multcomp’ package [82]. Principal component analysis (PCA) was performed on the correlation matrix of 6 cultivars and transcript levels of genes under salt-stress conditions in a time course. Index values for each treatment were first calculated by assessing the salt-stress response vs. its control value. All the traits under each treatment were combined and used as index values for the PCA analysis. These index values were used to identify the correlation of response variable vectors and genotypes across the ordination space. A two-way heatmap clustering analysis (HCA) was performed on the same dataset as used in the PCA analysis. Pearson correlation was used as a correlation-based distance method, and ‘euclidean algorithm’ was used to compute the dissimilarity matrix. PCA and HCA were created using the R software, including the ‘prcomp’ function in the ‘factoextra’ package [83]. Data were hierarchically clustered using the heatmap function in the ‘pheatmap’ package with R software [84]. The correlation-based network analysis (CNA) was created according to Aycan et al. [74]. The CNA is displayed as a pairwise correlation comparison matrix. The genotypic correlation between traits (using salt-tolerant, moderate, and salt-sensitive groups) was estimated using Spearman’s correlation coefficient in R software. Topological properties of co-occurrence networks were analyzed using the Cytoscape software plugin NetworkAnalyzer (v3.9.0, Cytoscape Consortium, U.S.).
5. Conclusions
All genotypes had their proline content raised by salt stress; however, salt-tolerant genotypes had higher proline contents than the moderate and sensitive genotypes. Gene expression levels in salt-tolerant and moderate genotypes increased as a result of the salinity stress. TaPTF1, TaPIMP1, TaMIP, TaHKT1;4, and TaMYB genes were significantly upregulated after 24 h, whereas salt-stress exposure for 12 and 24 h had a major impact on gene expression in wheat. When compared to the salt-sensitive and salt-moderate genotypes, the salt-tolerant genotypes displayed a stronger positive than negative interaction. As a selective trait for salt-stress tolerance with proline synthesis, the TaPTF1 (12 and 24 h), TaP5CS (12 and 24 h), TaGSK1 (12 and 24 h), and TaSRG (12 and 24 h) genes can be employed. Our results showed that, despite each gene’s specific function, increasing proline biosynthesis functioned as a common mechanism for separating salt tolerance from sensitivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11233401/s1, Table S1: Three-way analysis of variance (ANOVA) tables, *** significant at p < 0.001; Table S2: Amino acid sequences of TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB genes; Table S3: Loading values and percentage contribution of variables on the axis identified by the principal component analysis (PCA) for all cultivars under control and saline conditions.
Author Contributions
M.A. and M.Y. designed the study; M.A. performed the experiment, data analysis, bioinformatics and statistical analyses; M.B., T.M., and M.Y. provided feedback and valuable suggestions, M.B. and T.M. contributed to the conception of the work; M.A. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the General Directorate of Agricultural Research and Policies, Ministry of Agriculture and Forestry, The Republic of Türkiye (Grant number TAGEM 15/AR-GE/56 to M.Y); the FOSC project (Sus-Agri-CC) from the European Union’s Horizon 2020 research and innovation program under grant agreement 220N247 to M.Y; Strategic International Collaborative Research Program by the Japan Science and Technology Agency (JPMJSC16C5 to T.M); and a grant for the Promotion of KAAB Projects (Niigata University) from the Ministry of Education, Culture, Science and Technology (Japan).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Department of Breeding and Genetics, Central Research Institute for Field Crops, Ankara, Türkiye, for wheat seeds. We would also like to thank Mustafa Kayan (Ankara University), Deniz Kom (Ankara University), and Burak Onol (Ankara University) for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Figure A1.
Amino acid alignment of TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB genes.
Figure A1.
Amino acid alignment of TaPTF1, TaDHN, TaSRG, TaSC, TaPIMP1, TaMIP, TaHKT1;4, TaGSK, TaP5CS, and TaMYB genes.

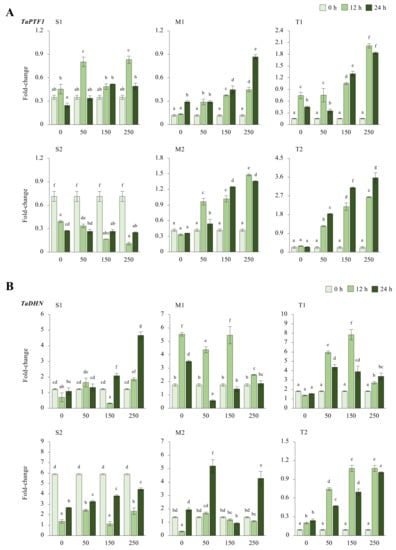
Figure A2.
(A) The gene expression of the TaPTF1 and (B) TaDHN in the leaves of salt-sensitive (S1), S2, moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
Figure A2.
(A) The gene expression of the TaPTF1 and (B) TaDHN in the leaves of salt-sensitive (S1), S2, moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
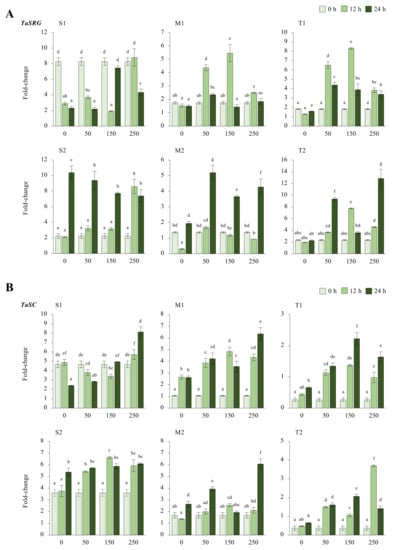
Figure A3.
(A) The gene expression of the TaSRG, and (B) TaSC in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
Figure A3.
(A) The gene expression of the TaSRG, and (B) TaSC in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
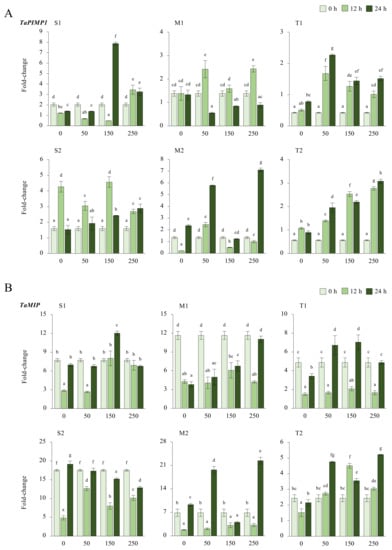
Figure A4.
(A) The gene expression of the TaPIMP1, and (B) TaMIP in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
Figure A4.
(A) The gene expression of the TaPIMP1, and (B) TaMIP in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
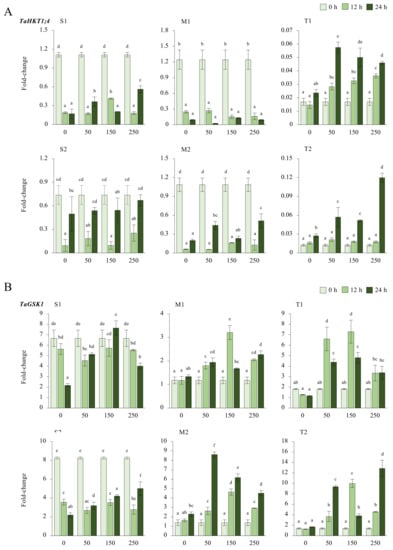
Figure A5.
(A) The gene expression of the TaHKT1;4 gene, and (B) TaGSK1 in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
Figure A5.
(A) The gene expression of the TaHKT1;4 gene, and (B) TaGSK1 in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
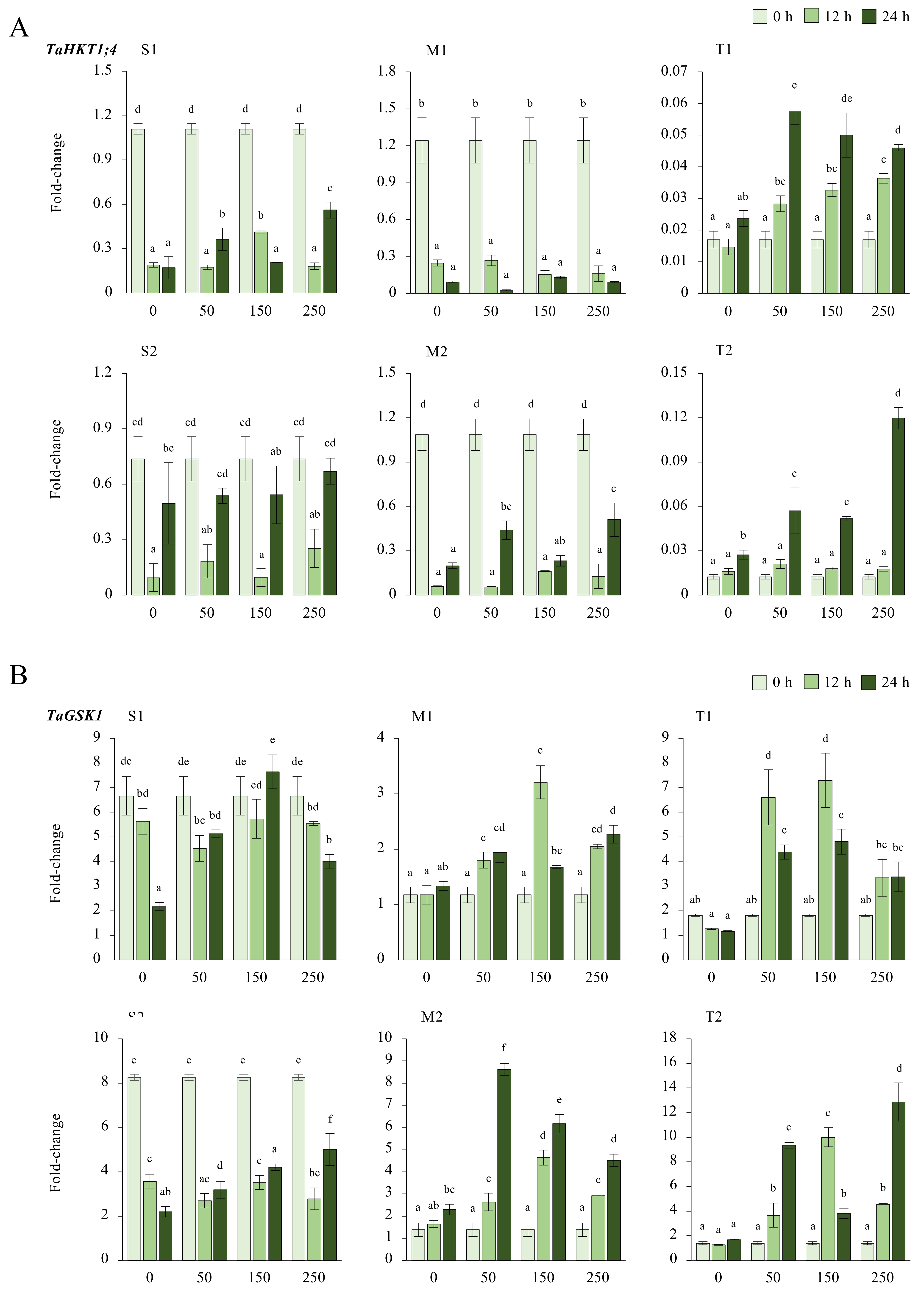
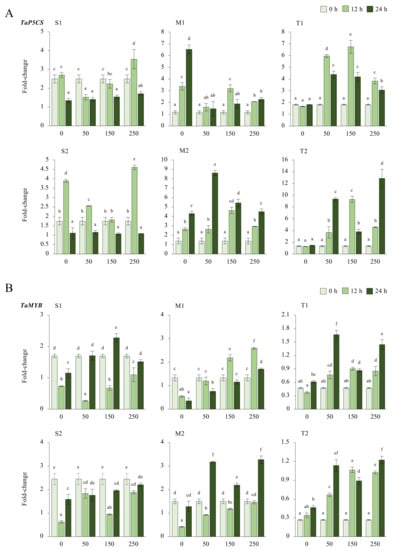
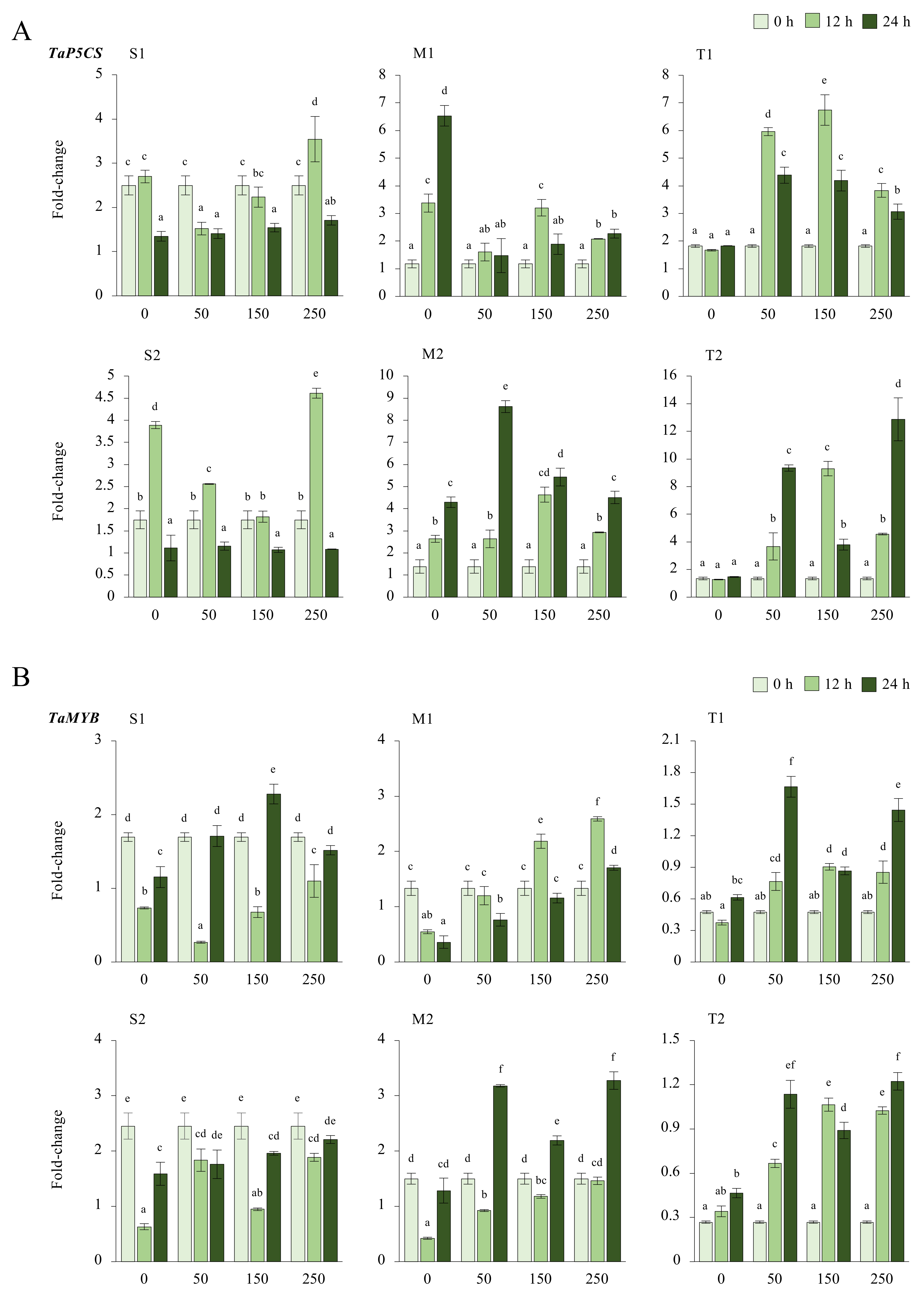
Figure A6.
(A) The gene expression of the TaP5CS, and (B) TaMYB in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
Figure A6.
(A) The gene expression of the TaP5CS, and (B) TaMYB in the leaves of salt-sensitive (S1), S2, -moderate (M1), M2, -tolerant (T1), and T2 genotypes exposed to 0, 50, 150, and 250 mM NaCl stress for 0, 12, 14 h. The Cq values formed the basis of qRT-PCR. The reference gene TaACTIN was used to standardize the Cq value for each sample. Means (±standard deviation) within the same graph followed by letters are significantly different at p < 0.05 according to the Tukey HSD test from independent biological triplicates (n = 3).
References
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Flowers, T. Ecology: Crops for a Salinized World. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Bannari, A.; Al-Ali, Z.M. Assessing Climate Change Impact on Soil Salinity Dynamics Between 1987–2017 in Arid Landscape Using Landsat TM, ETM+ and OLI Data. Remote Sens. 2020, 12, 2794. [Google Scholar] [CrossRef]
- Kumar, V.; Shriram, V.; Jawali, N.; Shitole, M.G. Differential Response of Indica Rice Genotypes to NaCl Stress in Relation to Physiological and Biochemical Parameters. Arch. Agron. Soil Sci. 2007, 53, 581–592. [Google Scholar] [CrossRef]
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards Food Security: Current State and Future Prospects of Agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- UN. United Nations Set out 17 Sustainable Development Goals (SDGs). Available online: https://www.un.org/sustainabledevelopment/hunger/ (accessed on 12 November 2022).
- FAO. The Future of Food and Agriculture: Trends and Challenges. Available online: http://www.fao.org/3/a-I6583e.Pdf (accessed on 30 September 2022).
- El Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in The Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Khan, M.S.; Rizvi, A.; Saif, S.; Zaidi, A. Phosphate-solubilizing Microorganisms in Sustainable Production of Wheat: Current Perspective. In Probiotics in Agroecosystem; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 51–81. [Google Scholar]
- Satir, O.; Berberoglu, S. Crop Yield Prediction Under Soil Salinity Using Satellite Derived Vegetation Indices. Field Crop. Res. 2016, 192, 134–143. [Google Scholar] [CrossRef]
- Luo, Q.; Teng, W.; Fang, S.; Li, H.; Li, B.; Chu, J.; Li, Z.; Zheng, Q. Transcriptome Analysis of Salt-Stress Response in Three Seedling Tissues of Common Wheat. Crop J. 2019, 7, 378–392. [Google Scholar] [CrossRef]
- Srivastava, D.; Khan, N.A.; Shamim, M.; Yadav, P.; Pandey, P.; Singh, K.N. Assessment of The Genetic Diversity in Bottle Gourd (Lagenaria Siceraria [Molina] Standl.) Genotypes Using SDS-PAGE and RAPD Markers. Natl. Acad. Sci. Lett. 2014, 37, 155–161. [Google Scholar] [CrossRef]
- Talebi, R.; Nosrati, S.; Etminan, A.; Naji, A.M. Genetic Diversity and Population Structure Analysis of Landrace and Improved Safflower (Cartamus Tinctorious L.) Germplasm Using Arbitrary Functional Gene-Based Molecular Markers. Biotechnol. Biotechnol. Equip. 2018, 32, 1183–1194. [Google Scholar] [CrossRef]
- Raina, A.; Laskar, R.A.; Tantray, Y.R.; Khursheed, S.; Wani, M.R.; Khan, S. Characterization of Induced High Yielding Cowpea Mutant Lines Using Physiological, Biochemical and Molecular Markers. Sci. Rep. 2020, 10, 3687. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.v.; Mondal, T.K. Advances in Understanding Salt Tolerance in Rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple Wheat Genomes Reveal Global Variation in Modern Breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Brini, F.; Hanin, M.; Lumbreras, V.; Amara, I.; Khoudi, H.; Hassairi, A.; Pagès, M.; Masmoudi, K. Overexpression of Wheat Dehydrin DHN-5 Enhances Tolerance to Salt and Osmotic Stress in Arabidopsis thaliana. Plant Cell Rep. 2007, 26, 2017–2026. [Google Scholar] [CrossRef]
- Gao, Z.; He, X.; Zhao, B.; Zhou, C.; Liang, Y.; Ge, R.; Shen, Y.; Huang, Z. Overexpressing a Putative Aquaporin Gene from Wheat, TaNIP, Enhances Salt Tolerance in Transgenic Arabidopsis. Plant Cell Physiol. 2010, 51, 767–775. [Google Scholar] [CrossRef]
- He, X.; Hou, X.; Shen, Y.; Huang, Z. TaSRG, a Wheat Transcription Factor, Significantly Affects Salt Tolerance in Transgenic Rice and Arabidopsis. FEBS Lett. 2011, 585, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, Y.; Jiao, B.; Chen, G.; Huang, S.; Guo, F.; Shen, Y.; Huang, Z.; Zhao, B. Overexpression of the Wheat Salt Tolerance-Related Gene TaSC Enhances Salt Tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 5463–5473. [Google Scholar] [CrossRef]
- Marin, J.A.; Andreu, P.; Carrasco, A.; Arbeloa, A. Determination of Proline Concentration, an Abiotic Stress Marker, in Root Exudates of Excised Root Cultures of Fruit Tree Rootstocks under Salt Stress. In Proceedings of the 3ème Meeting International: Aridoculture et Cultures Oasisennes: Gestion et Valorisation des Ressources et Applications Biotechnologiques dans les Agrosystèmes Arides et Sahariens, Jerba, Tunisie, 15–17 December 2010; 2010. [Google Scholar]
- Huang, J.; Hirji, R.; Adam, L.; Rozwadowski, K.L.; Hammerlindl, J.K.; Keller, W.A.; Selvaraj, G. Genetic Engineering of Glycinebetaine Production toward Enhancing Stress Tolerance in Plants: Metabolic Limitations. Plant Physiol. 2000, 122, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The Changes Induced in the Physiological, Biochemical and Anatomical Characteristics of Vicia faba by the Exogenous Application of Proline under Seawater Stress. S. Afr. J. Bot. 2014, 93, 56–63. [Google Scholar] [CrossRef]
- Hu, C.A.A.; Delauney, A.J.; Verma, D.P.S. A Bifunctional Enzyme (Δ1-Pyrroline-5-Carboxylate Synthetase) Catalyzes the First Two Steps in Proline Biosynthesis in Plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ortega, C.O.; Ochoa-Alfaro, A.E.; Reyes-Agüero, J.A.; Aguado-Santacruz, G.A.; Jiménez-Bremont, J.F. Salt Stress Increases the Expression of P5cs Gene and Induces Proline Accumulation in Cactus Pear. Plant Physiol. Biochem. 2008, 46, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Rahali-osmane, S.; Boulahia, K.; Djebbar, R.; Abrous-belbachir, O. Assessment Of Oxidative Stress And Proline Metabolism Genes Expression Of Cowpea Plants (Vigna unguiculata L.) Under Saline Conditions. Analele Universităţii din Oradea Fascicula Biologi 2020, 27, 7–16. [Google Scholar]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline Metabolic Dynamics and Implications in Drought Tolerance of Peanut Plants. Plant Phsiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in Relative Water Content, Proline Accumulation and Stress Gene Expression in Two Cowpea Landraces under Drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef]
- Klsa, D. Responses of Phytochelatin and Proline-Related Genes Expression Associated with Heavy Metal Stress in Solanum lycopersicum. Acta Bot. Croat. 2019, 78, 9–16. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant Growth Promoting Rhizobacteria Dietzia natronolimnaea Modulates the Expression of Stress Responsive Genes Providing Protection of Wheat from Salinity Stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Fernando, V.C.D. Major Transcription Factor Families Involved in Salinity Stress Tolerance in Plants. In Transcription Factors for Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 99–109. [Google Scholar]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis Transcription Factors: Genome-Wide Comparative Analysis among Eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Rahaie, M.; Xue, G.P.; Naghavi, M.R.; Alizadeh, H.; Schenk, P.M. A MYB Gene from Wheat (Triticum aestivum L.) Is up-Regulated during Salt and Drought Stresses and Differentially Regulated between Salt-Tolerant and Sensitive Genotypes. Plant Cell Rep. 2010, 29, 835–844. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wang, X.; Zhou, M.; Zhou, X.; Ye, X.; Wei, X. An R2R3 MYB Transcription Factor in Wheat, TaPIMP1, Mediates Host Resistance to Bipolaris Sorokiniana and Drought Stresses through Regulation of Defense- and Stress-Related Genes. New Phytol. 2012, 196, 1155–1170. [Google Scholar] [CrossRef]
- Zhou, J.; Li, F.; Wang, J.l.; Ma, Y.; Chong, K.; Xu, Y. yuan Basic Helix-Loop-Helix Transcription Factor from Wild Rice (OrbHLH2) Improves Tolerance to Salt- and Osmotic Stress in Arabidopsis. J. Plant Physiol. 2009, 166, 1296–1306. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Zwart, A.B.; Hare, R.A.; Rathjen, A.J.; Munns, R. Impact of Ancestral Wheat Sodium Exclusion Genes Nax1 and Nax2 on Grain Yield of Durum Wheat on Saline Soils. Funct. Plant Biol. 2012, 39, 609–618. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Tian, J.; Yang, L.; Huang, Y.; Zhao, B.; Zhou, C.; Ge, R.; Shen, Y.; Huang, Z. Overexpressing a Glycogen Synthase Kinase Gene from Wheat, TaGSK1, Enhances Salt Tolerance in Transgenic Arabidopsis. Plant Mol. Biol. Rep. 2012, 30, 807–816. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS Expression Level and Proline Accumulation in the Sensitive and Tolerant Wheat Cultivars under Control and Drought Stress Conditions in the Presence/Absence of Silicon and Salicylic Acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Müller, C.; Elliot, J.; Mueller, N.D.; Ciais, P.; Jägermeyr, J.; Gerber, J.; Dumas, P.; Wang, C.; Yang, H.; et al. Global Irrigation Contribution to Wheat and Maize Yield. Nat. Commun. 2021, 12, 1235. [Google Scholar] [CrossRef]
- Gowing, J.W.; Rose, D.A.; Ghamarnia, H. The Effect of Salinity on Water Productivity of Wheat under Deficit Irrigation above Shallow Groundwater. Agric. Water Manag. 2009, 96, 517–524. [Google Scholar] [CrossRef]
- Klay, I.; Riahi, L.; Amara, H.S.; Daaloul, A. Genotypic Variability for Salt Stress Tolerance among Wild and Cultivated Wheat Germplasms at an Early Development Stage. Open Agric. 2019, 4, 375–380. [Google Scholar] [CrossRef]
- Chong, L.; Guo, P.; Zhu, Y. Mediator Complex: A Pivotal Regulator of Aba Signaling Pathway and Abiotic Stress Response in Plants. Int. J. Mol. Sci. 2020, 21, 7755. [Google Scholar] [CrossRef]
- El-Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Impact of Exogenously Applied ABA on Proline Metabolism Conferring Drought and Salinity Stress Tolerance in Wheat Genotypes. Cereal Res. Commun. 2020, 48, 309–315. [Google Scholar] [CrossRef]
- Maas, E.v.; Hoffman, G.J.; Chaba, G.D.; Poss, J.A.; Shannon, M.C. Salt Sensitivity of Corn at Various Growth Stages. Irrig. Sci. 1983, 4, 45–57. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline Biosynthesis and Osmoregulation in Plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Hien, D.T.; Jacobs, M.; Angenon, G.; Hermans, C.; Thu, T.T.; van Son, L.; Roosens, N.H. Proline Accumulation and Δ1-Pyrroline-5-Carboxylate Synthetase Gene Properties in Three Rice Cultivars Differing in Salinity and Drought Tolerance. Plant Sci. 2003, 165, 1059–1068. [Google Scholar] [CrossRef]
- Thiery, L.; Leprince, A.S.; Lefebvre, D.; Ali Ghars, M.; Debarbieux, E.; Savouré, A. Phospholipase D Is a Negative Regulator of Proline Biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 2004, 279, 14812–14818. [Google Scholar] [CrossRef]
- Yang, A.; Dai, X.; Zhang, W.H. A R2R3-Type MYB Gene, OsMYB2, Is Involved in Salt, Cold, and Dehydration Tolerance in Rice. J. Exp. Bot. 2012, 63, 2541–2556. [Google Scholar] [CrossRef]
- Xue, G.P. The DNA-Binding Activity of an AP2 Transcriptional Activator HvCBF2 Involved in Regulation of Low-Temperature Responsive Genes in Barley Is Modulated by Temperature. Plant J. 2003, 33, 373–383. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Dong, N.; Liu, X.; Zhang, H.; Zhang, Z. Expression of a Wheat MYB Gene in Transgenic Tobacco Enhances Resistance to Ralstonia solanacearum, and to Drought and Salt Stresses. Funct. Integr. Genom. 2011, 11, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Beena, A.S.; Awana, M.; Singh, A. Salt-Induced Tissue-Specific Cytosine Methylation Downregulates Expression of HKT Genes in Contrasting Wheat (Triticum aestivum L.) Genotypes. DNA Cell Biol. 2017, 36, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Maggio, A.; Bressan, R.A.; Yun, D.J. Role and Functional Differences of HKT1-Type Transporters in Plants under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1059. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Lu, M.; Naz, S.; Sehar, S.; Cao, F.; Wu, F. Resemblance and Difference of Seedling Metabolic and Transporter Gene Expression in High Tolerance Wheat and Barley Cultivars in Response to Salinity Stress. Plants 2020, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamaji, N.; Costa, A.; Okuma, E.; Kobayashi, N.I.; Kashiwagi, T.; Katsuhara, M.; Wang, C.; Tanoi, K.; Murata, Y.; et al. OsHKT1;4-Mediated Na+ transport in Stems Contributes to Na+ exclusion from Leaf Blades of Rice at the Reproductive Growth Stage upon Salt Stress. BMC Plant Biol. 2016, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Z.; Zhao, T.; Yang, Y.; Chen, T.; Yang, M.; Yu, W.; Zhang, B. Genome-Wide Analysis of BHLH Transcription Factor and Involvement in the Infection by Yellow Leaf Curl Virus in Tomato (Solanum lycopersicum). BMC Genom. 2015, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Eisenbarth, D.A.; Weig, A.R. Dynamics of Aquaporins and Water Relations during Hypocotyl Elongation in Ricinus communis L. Seedlings. J. Exp. Bot. 2005, 56, 1831–1842. [Google Scholar] [CrossRef]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The Complete Set of Genes Encoding Major Intrinsic Proteins in Arabidopsis Provides a Framework for a New Nomenclature for Major Intrinsic Proteins in Plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef]
- Chaumont, F.; Barrieu, F.; Wojcik, E.; Chrispeels, M.J.; Jung, R. Aquaporins Constitute a Large and Highly Divergent Protein Family in Maize. Plant Physiol. 2001, 125, 1206–1215. [Google Scholar] [CrossRef]
- Weaver, C.D.; Crombie, B.; Stacey, G.; Roberts, D.M. Calcium-Dependent Phosphorylation of Symbiosome Membrane Proteins from Nitrogen-Fixing Soybean Nodules: Evidence for Phosphorylation of Nodulin-26. Plant Physiol. 1991, 95, 222–227. [Google Scholar] [CrossRef]
- Karlsson, M.; Johansson, I.; Bush, M.; McCann, M.C.; Maurel, C.; Larsson, C.; Kjellbom, P. An Abundant TIP Expressed in Mature Highly Vacuolated Cells. Plant J. 2000, 21, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Schraut, D.; Hartung, W.; Schäffner, A.R. Differential Responses of Maize MIP Genes to Salt Stress and ABA. J. Exp. Bot. 2005, 56, 2971–2981. [Google Scholar] [CrossRef]
- Nahar, L.; Aycan, M.; Hanamata, S.; Baslam, M.; Mitsui, T. Impact of Single and Combined Salinity and High-Temperature Stresses on Agro-Physiological, Biochemical, and Transcriptional Responses in Rice and Stress-Release. Plants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Saibi, W.; Feki, K.; ben Mahmoud, R.; Brini, F. Durum Wheat Dehydrin (DHN-5) Confers Salinity Tolerance to Transgenic Arabidopsis Plants through the Regulation of Proline Metabolism and ROS Scavenging System. Planta 2015, 242, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.P.; Ma, W.S.; Huang, Z.J.; Xu, T.; Xue, Y.B.; Shen, Y.Z. Isolation and Characterization of TaGSK1 Involved in Wheat Salt Tolerance. Plant Sci. 2003, 165, 1369–1375. [Google Scholar] [CrossRef]
- Yi, K.; Wu, Z.; Zhou, J.; Du, L.; Guo, L.; Wu, Y.; Wu, P. OsPTF1, a Novel Transcription Factor Involved in Tolerance to Phosphate Starvation in Rice. Plant Physiol. 2005, 138, 2087–2096. [Google Scholar] [CrossRef]
- Aycan, M.; Baslam, M.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Development of New High-Salt Tolerant Bread Wheat (Triticum aestivum L.) Genotypes and Insight into the Tolerance Mechanisms. Plant Physiol. Biochem. 2021, 166, 314–327. [Google Scholar] [CrossRef]
- Piao, H.L.; Lim, J.H.; Kim, S.J.; Cheong, G.W.; Hwang, I. Constitutive Over-Expression of AtGSK1 Induces NaCl Stress Responses in the Absence of NaCl Stress and Results in Enhanced NaCl Tolerance in Arabidopsis. Plant J. 2001, 27, 305–314. [Google Scholar] [CrossRef]
- Aycan, M.; Baslam, M.; Ozdemir, B.; Asiloglu, R.; Mitsui, T.; Yildiz, M. Direct Contribution of the Maternal Genotype on the Transgenerational Salinity Tolerance in Wheat (Triticum aestivum L.). Environ. Exp. Bot. 2021, 192, 104648. [Google Scholar] [CrossRef]
- Benitez, L.C.; Vighi, I.L.; Auler, P.A.; do Amaral, M.N.; Moraes, G.P.; dos Santos Rodrigues, G.; da Maia, L.C.; de Magalhães Júnior, A.M.; Braga, E.J.B. Correlation of Proline Content and Gene Expression Involved in the Metabolism of This Amino Acid under Abiotic Stress. Acta Physiol. Plant. 2016, 38, 267. [Google Scholar] [CrossRef]
- Byrt, C.S.; Xu, B.; Krishnan, M.; Lightfoot, D.J.; Athman, A.; Jacobs, A.K.; Watson-Haigh, N.S.; Plett, D.; Munns, R.; Tester, M.; et al. The Na+ Transporter, TaHKT1;5-D, Limits Shoot Na+ Accumulation in Bread Wheat. Plant J. 2014, 80, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Simms, D.; Cizdziel, P.; Chomczynski, P. TRIzol: A New Reagent for Optimal Single-Step Isolation of RNA. Focus Madison 1993, 24, 99–102. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. J. Bioinform. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for MacOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 11 August 2022).
- Kolde, R. Pretty Heatmaps. R Package Version 1.0.10. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 20 September 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).