Morphoanatomical, Physiological, and Biochemical Indicators in Lactuca sativa L. Germination and Growth in Response to Fluoride
Abstract
:1. Introduction
2. Results
2.1. Germination and Initial Growth of L. sativa Seedlings
2.2. Greenhouse Experiment
2.2.1. Visible Symptoms
2.2.2. Fluoride Content
2.2.3. Physiological Traits
2.2.4. Biochemical Traits
2.2.5. Phenolic Compounds in L. sativa Roots and Leaves
2.2.6. Leaf Nutrients
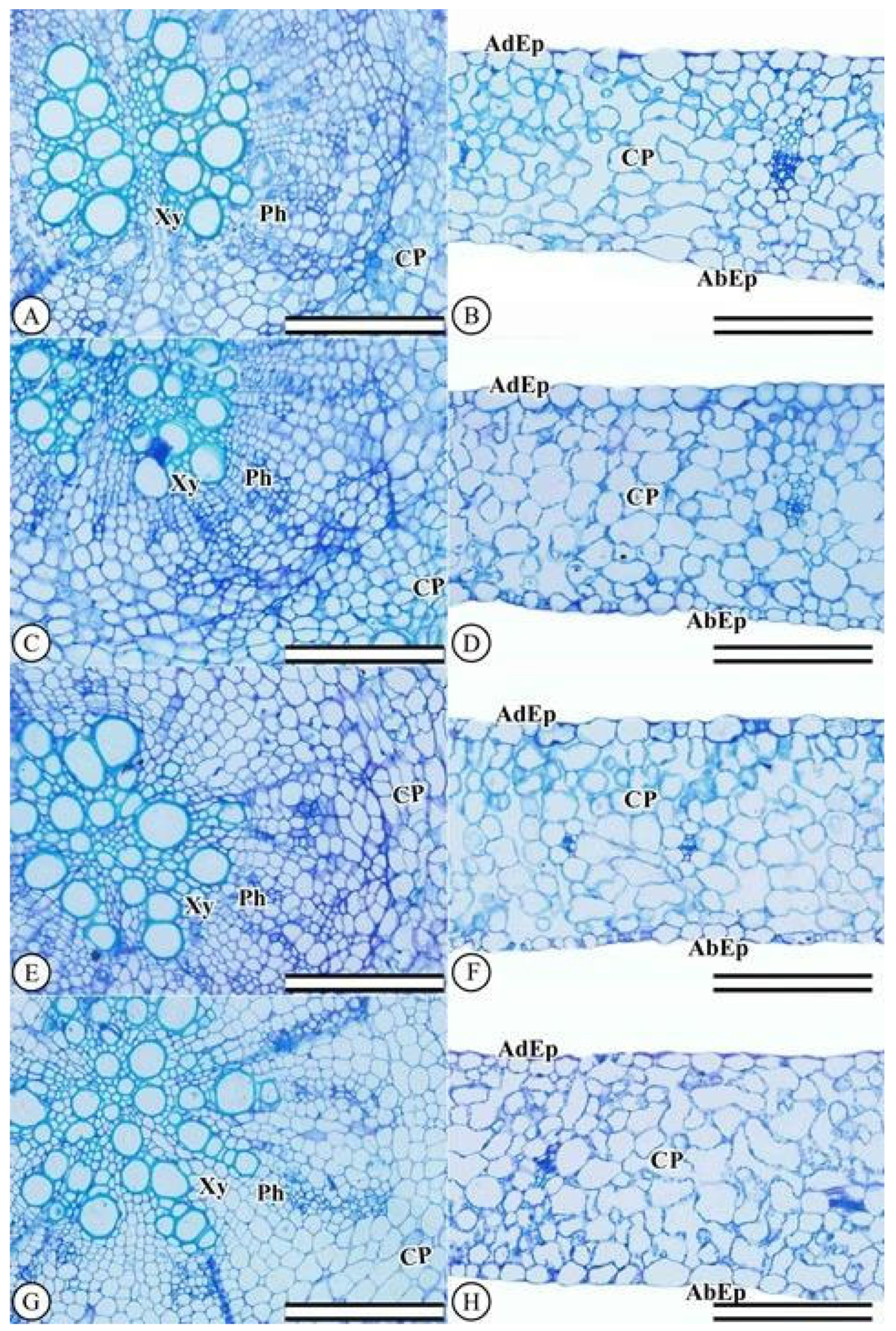
2.2.7. Anatomical Leaf and Root Characterizations
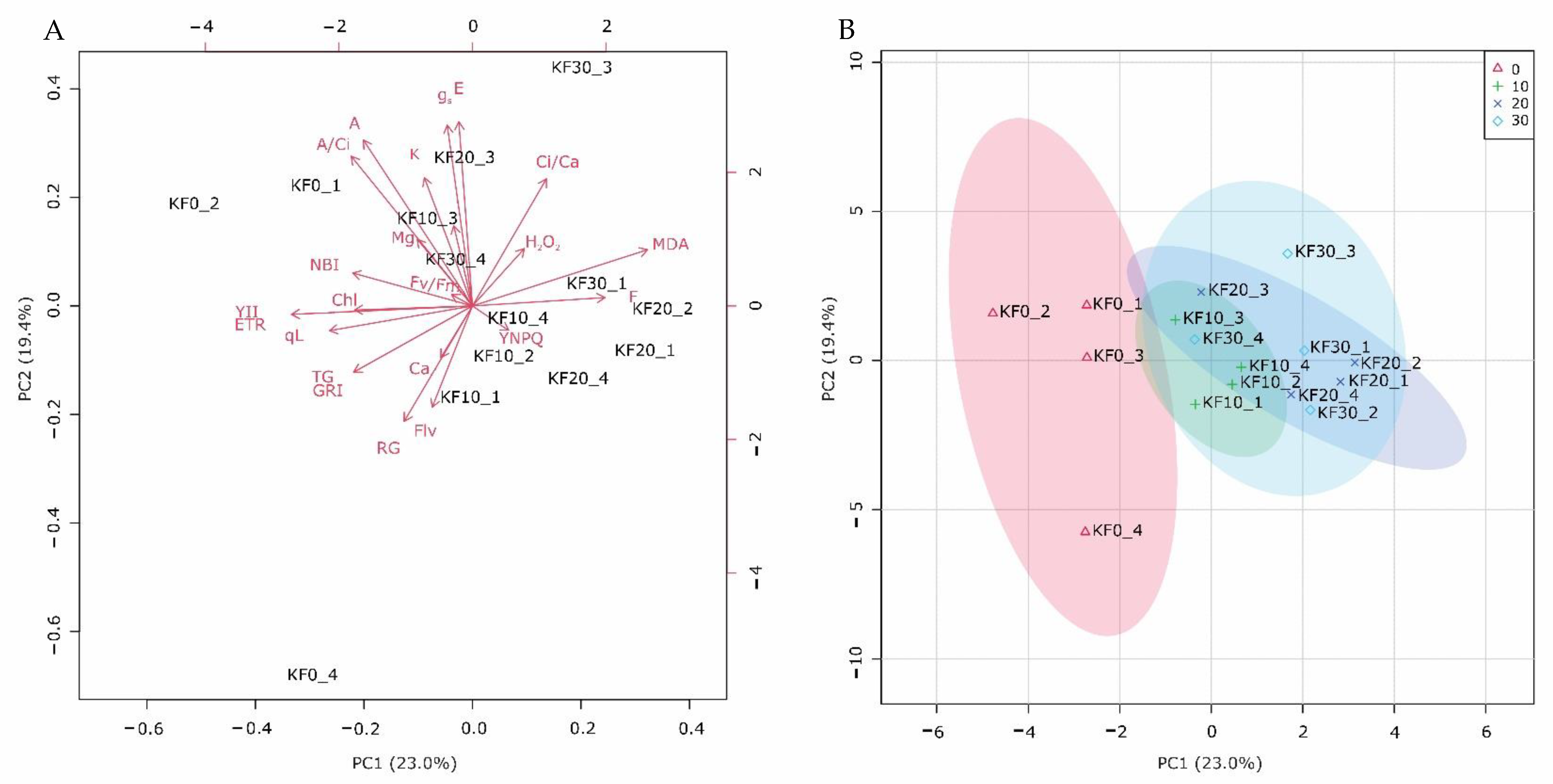
2.2.8. Principal Component Analysis
3. Discussion
4. Material and Methods
4.1. Germination Bioassay: Experimental Design and Evaluations
4.2. Greenhouse Experiment
4.2.1. Experimental Design and Evaluations
4.2.2. Fluoride Determinations in Seeds and Plants
4.2.3. Visible Symptoms
4.2.4. Physiological Traits
4.2.5. Biochemical Traits
4.2.6. Pigments, Flavonols, Nitrogen Balance Index, and Leaf Nutrient Contents
4.2.7. Morphoanatomical and Histochemical Seed Characterization
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mondal, N.K. Effect of fluoride on photosynthesis, growth and accumulation of four widely cultivated rice (Oryza sativa L.) varieties in India. Ecotoxicol. Environ. Saf. 2017, 144, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, L.H.; Davison, A.W. Fluorides in the Environment; CABI Publishing: Oxford, UK, 2004; 287p. [Google Scholar]
- Kabir, H.; Gupta, A.K.; Tripathy, S. Fluoride and human health: Systematic appraisal of sources, exposures, metabolism, and toxicity. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1116–1193. [Google Scholar] [CrossRef]
- Saini, P.; Khan, S.; Baunthiyal, M.; Sharma, V. Organ-wise accumulation of fluoride in Prosopis juliflora and its potential for phytoremediation of fluoride contaminated soil. Chemosphere 2012, 89, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Panda, D. Fluoride toxicity stress: Physiological and biochemical consequences on plants. Int. J. Agric. Amb. Bio-Res. 2015, 1, 70–84. [Google Scholar]
- Shitumbanuma, V.; Tembo, F.; Tembo, J.M.; Chilala, S.; Van Rans, E. Dental fluorosis associated with drinking water from hot springs in Choma district in Southern province, Zambia. Environ. Geochem. Health 2007, 29, 51–58. [Google Scholar] [CrossRef]
- Yadav, K.K.; Kumar, S.; Pham, Q.B.; Gupta, N.; Rezania, S.; Kamyab, H.; Cho, J. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review. Ecotoxicol Environ. Saf. 2019, 182, 109362. [Google Scholar] [CrossRef]
- Singh, G.; Kumari, B.; Sinam, G.; Kumar, K.N.; Mallick, S. Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspectivee a review. Environ. Pollut. 2018, 239, 95–108. [Google Scholar] [CrossRef]
- WHO. Fluorine and Fluoride. In Environmental Health Criteria 36; International Programme on Chemical Safety, World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- CETESB. Valores Orientadores Para Solo E Água Subterrânea No Estado De São Paulo—2014; CETESB: São Paulo, Brazil, 2014. [Google Scholar]
- Susheela, A.K. Fluorosis management programme in India. Curr. Sci. 1999, 77, 1250–1256. [Google Scholar]
- Vikas, C.; Kushwaha, R.; Ahmad, W.; Prasannakumar, V.; Reghunath, R. Genesis and geochemistry of high fluoride bearing groundwater from a semi-arid terrain of NW India. Environ. Earth Sci. 2013, 68, 289–305. [Google Scholar] [CrossRef]
- Abiye, T.; Bybee, G.; Leshomo, J. Fluoride concentrations in the arid Namaqualand and the Waterberg groundwater, South Africa: Understanding the controls of mobilization through hydrogeochemical and environmental isotopic approaches. Groundw. Sustain. Dev. 2018, 6, 112–120. [Google Scholar] [CrossRef]
- Martins, V.T.S.; Pino, D.S.; Bertolo, R.; Hirata, R.; Babinski, M.; Pacheco, D.F.; Rios, A.P. Who to blame for groundwater fluoride anomaly in São Paulo, Brazil? Hydrogeochemistry and isotopic evidence. Appl. Geochem. 2018, 90, 25–38. [Google Scholar] [CrossRef]
- Sant’Anna-Santos, B.F.; Azevedo, A.A.; Alves, T.G.; Campos, N.V.; Oliva, M.A.; Valente, V.M.M. Effects of emissions from an aluminium smelter in a tree tropical species sensitive to fluoride. Water Air Soil Pollut. 2014, 225, 1817. [Google Scholar] [CrossRef]
- Miller, G.W. The effect of fluoride on higher plants: With special emphasis on early physiological and biochemical disorders. Fluoride 1993, 26, 3–22. [Google Scholar]
- Chaves, A.L.F.; Silva, E.A.M.; Azevedo, A.A.; Cano, M.A.O.; Matsuoka, K. Ação do flúor dissolvido em chuva simulada sobre a estrutura foliar de Panicum maximum Jacq. (colonião) e Chloris gayana Kunth. (capim-Rhodes)—Poaceae. Acta Bot. Bras. 2002, 16, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Pita-Barbosa, A.; Sant’Anna-Santos, B.F.; Silva, K.L.F.; Azevedo, A.A.; Rocha, D.I. Efeitos fitotóxicos do fluoreto na morfoanatomia foliar de Brachiaria brizantha (Hochst. ex A. Rich.) Stapf e Brachiaria decumbens Stapf (Poaceae). Acta Bot. Bras. 2009, 23, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.R.; Vasconcelos-Filho, S.C.; Mendes, G.C.; Rehn, L.S.; Rodrigues, D.A.; Rodrigues, C.L.; Müller, C. Fluoride in simulated rain affects the morphoanatomy and physiology of Eugenia dysenterica (Mart.) DC. Ecol. Indic. 2017, 82, 189–195. [Google Scholar] [CrossRef]
- Kamaluddin, M.; Zwiazek, J.J. Fluoride inhibits root water transport and affects leaf expansion and gas exchange in aspen (Populus tremuloides) seedlings. Physiol. Plant. 2003, 117, 368–375. [Google Scholar] [CrossRef]
- Rodrigues, A.A.; Vasconcelos-Filho, S.C.; Müller, C.; Rodrigues, D.A.; Mendes, G.C.; Rehn, L.S.; Costa, A.C.; Vital, R.G.; Sales, J.F. Sapindus saponaria bioindicator potential concerning potassium fluoride exposure by simulated rainfall: Anatomical and physiological traits. Ecol. Indic. 2018, 89, 552–558. [Google Scholar] [CrossRef]
- Zouari, M.; Elloumi, N.; Bellassoued, K.; Ahmed, C.B.; Krayen, M.; Delmail, D.; Elfeki, A.; Rouina, B.B.; Abdallh, F.B.; Labrousse, P. Enzymatic antioxidant responses and mineral status in roots and leaves of olive plants subjected to fluoride stress. South Afri. J. Bot. 2017, 111, 44–49. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patra, P.K.; Mondal, B. Uptake of fluoride by two paddy (Oryza sativa L.) varieties treated with fluoride-contaminated water. Paddy Water Environ. 2013, 11, 619–623. [Google Scholar] [CrossRef]
- Fina, B.L.; Lupo, M.; Dri, N.; Lombarte, M.; Rigalli, A. Comparison of fluoride effects on germination and growth of Zea mays, Glycine max and Sorghum vulgare. J. Sci. Food Agr. 2016, 96, 3679–3687. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Bibia, H.; Munir, I.; Ahmad, M.N.; Zia, A.; Mustafa, G.; Ullah, I.; Khan, I. Fluoride toxicity and its effect on two varieties of Solanum lycopersicum. Fluoride 2018, 51, 267–277. [Google Scholar]
- Ming-Ho, Y. Effects of fluoride on growth and soluble sugars in germinating mung bean (Vigna radiata) seeds. Fluoride 1996, 29, 3–6. [Google Scholar]
- Baunthiyal, M.; Ranghar, S. Accumulation of Fluoride by Plants: Potential for Phytoremediation. Clean Soil Air Water 2015, 43, 127–132. [Google Scholar] [CrossRef]
- Kadiresan, K.; Khanal, P.R. Rethinking irrigation for global food security. Irrig. Drain. 2018, 67, 8–11. [Google Scholar] [CrossRef]
- Hurtado, C.; Domínguez, C.; Pérez-Babace, L.; Cañameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef]
- Margenat, A.; Matamoros, V.; Díez, S.; Cañameras, N.; Comas, J.; Bayona, J.M. Occurrence and bioaccumulation of chemical contaminants in lettuce grown in peri-urban horticulture. Sci. Total Environ. 2018, 637, 1166–1174. [Google Scholar] [CrossRef] [Green Version]
- Dala-Paula, B.M.; Custódio, F.B.; Knupp, E.A.; Palmieri, H.E.; Silva, J.B.B.; Glória, M.B.A. Cadmium, copper and lead levels in different cultivars of lettuce and soil from urban agriculture. Environ. Pollut. 2018, 242, 383–389. [Google Scholar] [CrossRef]
- Park, J.; Yoon, J.H.; Depuydt, S.; Oh, J.W.; Jo, Y.M.; Kim, Y.; Brown, M.T.; Han, T. The sensitivity of an hydroponic lettuce root elongation bioassay to metals, phenol and wastewaters. Ecotoxicol. Environ. Saf. 2016, 126, 147–153. [Google Scholar] [CrossRef]
- Mtisi, M.; Gwenzi, W. Evaluation of the phytotoxicity of coal ash on lettuce (Lactuca sativa L.) germination, growth and metal uptake. Ecotoxicol. Environ. Saf. 2019, 170, 750–762. [Google Scholar] [CrossRef]
- Sachan, P.; Lal, N. Effect of sodium fluoride on germination, seedling growth and photosynthetic pigments in Cicer arietinum L. and Hordeum vulgare L. MOJ Ecol. Environ. Sci. 2018, 3, 300–304. [Google Scholar] [CrossRef]
- Meharg, A.A. Integrated tolerance mechanisms: Constitutive and adaptive plant responses to elevated metal concentrations in the environment. Plant Cell Environ. 1994, 17, 989–993. [Google Scholar] [CrossRef]
- Datta, J.K.; Maitra, A.; Mondal, N.K.; Banerjee, A. Studies on the impact of fluoride toxicity on germination and seedling growth of gram seed (Cicer arietinum L. cv. Anuradha). J. Stress Physiol. Biochem. 2012, 8, 194–202. [Google Scholar]
- Chakrabarti, S.; Patra, P.K.; Mandal, B.; Mahato, D. Effect of sodium fluoride on germination, seedling growth, and biochemistry of Bengal gram (Cicer arieninum). Fluoride 2012, 45, 257–262. [Google Scholar]
- Ahmed, M.R.; Yasmin, J.; Collins, W.; Cho, B.K. X-ray CT image analysis for morphology of muskmelon seed in relation to germination. Biosyst. Eng. 2018, 175, 183–193. [Google Scholar] [CrossRef]
- Pereira, D.F.; Bugatti, P.H.; Lopes, F.M.; Souza, A.L.S.M.; Saito, P.T.M. Contributing to agriculture by using soybean seed data from the tetrazolium test. Data Brief 2019, 23, 103652. [Google Scholar] [CrossRef]
- Farnese, F.S.; Oliveira, J.A.; Saiva, E.A.S.; Menezes-Silva, P.E.; Silva, A.A.; Campos, F.V.; Ribeiro, C. The involvement of nitric oxide in integration of plant physiological and ultrastructural adjustments in response to arsenic. Front. Plant Sci. 2017, 8, 516. [Google Scholar] [CrossRef]
- Cai, H.; Dong, Y.; Peng, C.; Li, Y.; Xu, W.; Li, D.; Wan, X. Fluoride-induced responses in the chlorophyll content and the antioxidant system in tea leaves (camellia sinensis). Res. Rep. Fluoride 2017, 50, 59–78. [Google Scholar]
- Reddy, M.P.; Kaur, M. Sodium fluoride induced growth and metabolic changes in Salicornia brachiata Roxb. Water Air Soil Pollut. 2008, 188, 171–179. [Google Scholar] [CrossRef]
- Elloumi, N.; Zouari, M.; Mezghani, I.; Abdallah, F.B.; Woodward, S.; Kallel, M. Adaptive biochemical and physiological responses of Eriobotrya japonica to fluoride air pollution. Ecotoxicology 2017, 26, 991–1001. [Google Scholar] [CrossRef]
- Dan, T.V.; KrishnaRaj, S.; Saxena, P.K. Metal tolerance of scented geranium (Pelargonium sp. ‘Frensham’): Effects of cadmium and nickel on chlorophyll fluorescence kinetics. Int. J. Phytoremediat. 2000, 2, 91–104. [Google Scholar] [CrossRef]
- Hoshika, Y.; Watanabe, M.; Inada, N.; Koike, T. Model-based analysis of avoidance of ozone stress by stomatal closure in Siebold’s beech (Fagus crenata). Ann. Bot. 2013, 112, 1149–1158. [Google Scholar] [CrossRef]
- Sharma, R.; Kaur, R. Insights into fluoride-induced oxidative stress and antioxidant defences in plants. Acta Physiol. Plant. 2018, 40, 181. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Biochar alleviates fluoride toxicity and oxidative stress in safflower (Carthamus tinctorius L.) seedlings. Chemosphere 2019, 223, 406–415. [Google Scholar] [CrossRef]
- Singh, A.; Banerjee, A.; Roychoudhury, A. Differential responses of Vigna radiata and Vigna mungo to fluoride-induced oxidative stress and amelioration via exogenous application of sodium nitroprusside. J. Plant Growth Regul. 2021, 40, 2342–2357. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; Hullebusch, E.D.V.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Environ. 2016, 563, 693–703. [Google Scholar] [CrossRef]
- Jain, G.; Gould, K.S. Are betalain pigments the functional homologues of anthocyanins in plants? Environ. Exp. Bot. 2015, 119, 48–53. [Google Scholar] [CrossRef]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- Garrec, J.P.; Oberlin, J.C.; Ligeon, E.; Bisch, A.M.; Fourcy, A. Fluoride-calcium interaction in polluted fir needles. Fluoride 1974, 7, 78–83. [Google Scholar]
- Arnesen, A.K.M. Availability of Fluoride in plants grown in contaminated soils. Plant Soil 1997, 191, 13–25. [Google Scholar] [CrossRef]
- Stevens, D.P.; McLaughlin, M.J.; Alston, A.M. Phytotoxicity of aluminium-fluoride complex and their uptake from solution culture by Avena sativa and Lycopersicon esculentum. Plant Soil 1997, 192, 81–93. [Google Scholar] [CrossRef]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Dąbrowski, P.; Cetner, M.D.; Samborska, I.A.; Łukasik, I.; Brestic, M.; Panchal, B.M. A comparison between different chlorophyll content meters under nutrient deficiency conditions. J. Plant Nutr. 2017, 40, 1024–1034. [Google Scholar] [CrossRef]
- Anjos, T.B.O.; Louback, E.; Azevedo, A.A.; Silva, L.C. Sensibility of Spondias purpurea L. (Anacardiaceae) exposed to fluoridesimulated fog. Ecol. Indic. 2018, 90, 154–163. [Google Scholar] [CrossRef]
- Louback, E.; Pereira, T.A.R.; de Souza, S.R.; Oliveira, J.A.de.; Silva, L.C. Vegetation damage in the vicinity of an aluminum smelter in Brazil. Ecol. Indic. 2016, 67, 193–203. [Google Scholar] [CrossRef]
- Rodrigues, D.A.; Vasconcelos-Filho, S.C.; Rodrigues, A.A.; Müller, C.; Farnese, F.S.; Costa, A.C.; Teles, E.M.G.; Rodrigues, C.L. Byrsonima basiloba as a bioindicator of simulated air pollutants: Morphoanatomical and physiological changes in response to potassium fluoride. Ecol. Indic. 2018, 89, 301–308. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Fluorides, Hydrogen Fluoride, and Fluorine; Draft for Public Comment; US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2001. [Google Scholar]
- Ekstrand, J.; Ehrnebo, M. Absorption of fluoride from fluoride dentrifices. Caries Res. 1980, 14, 96–102. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the tolerable upper intake level of fluoride. EFSA J. 2005, 192, 1–65. [Google Scholar]
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Regras para Análise de Sementes; SNDA/DNDV/CLAV: Brasília, Brazil, 2009; 399p. [Google Scholar]
- Maguire, J.D. Speed of germination aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Caporale, A.G.; Sommella, A.; Lorito, M.; Lombardi, N.; Azam, S.M.G.G.; Pigna, M.; Ruocco, M. Trichoderma spp. alleviate phytotoxicity in lettuce plants (Lactuca sativa L.) irrigated with arsenic-contaminated water. J. Plant Physiol. 2014, 171, 1378–1384. [Google Scholar] [PubMed]
- Rodrigues, D.A.; Sales, J.F.; Vasconcelos-Filho, S.C.; Rodrigues, A.A.; Costa, A.C.; Rodrigues, C.L.; Silva, H.L.; Müller, C. Spondias mombin, a potential bioindicator of potassium fluoride pollution. Ecol. Indic. 2020, 114, 106314. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 2009, 47, 215–222. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. Environ. Exp. Bot. 2009, 66, 203–211. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Byophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Cakmak, L.; Horst, W.J. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxide activity in root tip of soybean (Glycine max). Plant Physiol. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Gay, C.; Gebicki, J.M. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal. Biochem. 2000, 284, 217–220. [Google Scholar] [CrossRef]
- Abdallah, F.B.; Goffart, J.P. Potential indicators based on leaf flavonoids content for the evaluation of potato crop nitrogen status. In Proceedings of the 11th ICPA, Indianapolis, MI, USA; 15–18 July 2012; pp. 1–18.
- Embrapa. Manual de Análises Químicas de Solos, Plantas E Fertilizantes, 2nd ed.; Silva, F.C., Ed.; EMBRAPA Informação Tecnológica: Brasília, Brazil, 2009. [Google Scholar]
- Karnovsky, M.J.A. Formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- O’Brien, T.P.; Feder, N.; Mccully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; Mcgraw-Hill Book: New York, NY, USA, 1940; 523p. [Google Scholar]
- Jensen, W.A. Botamical Histochemistry: Principles and Practice; W.H. Freeman and Company: San Francisco, CA, USA, 1962; 408p. [Google Scholar]

| KF (mg L−1) | TG (%) | GRI | RL (cm) |
| 0 | 93.00 ± 1.29 | 23.25 ± 0.32 | 3.31 ± 0.34 |
| 10 | 91.50 ± 2.22 | 22.88 ± 0.55 | 3.23 ± 0.27 |
| 20 | 86.00 ** ± 1.83 | 21.50 ** ± 0.46 | 2.91 ± 0.31 |
| 30 | 84.50 ** ± 0.96 | 21.13 ** ± 0.24 | 2.59 * ± 0.15 |
| One-Way ANOVA | |||
| F (t-test) | 6.3077 ** | 6.3077 ** | 3.5409 * |
| p | 0.0081 | 0.0081 | 0.0185 |
| Chlorophyll a Fluorescence | |||||
| KF (mg L−1) | Fv/Fm | YII | ETR | qL | YNPQ |
| 0 | 0.83 ± 0.015 | 0.19 ± 0.008 | 101.5 ± 4.39 | 0.19 ± 0.03 | 0.60 ± 0.01 |
| 10 | 0.83 ± 0.003 | 0.13 ** ± 0.005 | 69.2 ** ± 2.80 | 0.13 ± 0.03 | 0.65 ± 0.03 |
| 20 | 0.81 ± 0.016 | 0.11 ** ± 0.017 | 57.7 ** ± 9.42 | 0.12 ± 0.05 | 0.65 ± 0.03 |
| 30 | 0.82 ± 0.009 | 0.13 ** ± 0.013 | 70.5 ** ± 7.24 | 0.12 ± 0.03 | 0.62 ± 0.03 |
| One-Way ANOVA | |||||
| F (t-test) | 0.6919 NS | 8.3466 ** | 8.3466 ** | 1.0504 NS | 1.1407 NS |
| P | 0.5744 | 0.0028 | 0.0028 | 0.4059 | 0.3721 |
| Gas exchange | |||||
| KF (mg L−1) | A | gS | E | Ci/Ca | A/Ci |
| 0 | 17.45 ± 3.07 | 0.68 ± 0.17 | 9.48 ± 2.05 | 0.83 ± 0.03 | 0.0524 ± 0.009 |
| 10 | 14.57 ± 1.45 | 0.67 ± 0.09 | 9.54 ± 1.03 | 0.87 ± 0.02 | 0.0410 ± 0.005 |
| 20 | 13.60 ± 3.00 | 0.68 ± 0.20 | 9.68 ± 2.18 | 0.87 ± 0.02 | 0.0407 ± 0.009 |
| 30 | 14.88 ± 1.66 | 0.63 ± 0.11 | 9.59 ± 1.44 | 0.86 ± 0.01 | 0.0450 ± 0.005 |
| One-Way ANOVA | |||||
| F (t-test) | 0.4643 NS | 0.0268 NS | 0.0023 NS | 0.7642 NS | 0.6260 NS |
| p | 0.7125 | 0.9938 | 0.9998 | 0.5356 | 0.6118 |
| KF (mg L−1) | Chl (µg/cm²) | Flv | Anth | MDA (nmol g−1 FM) | H2O2 (nmol g−1 FM) |
|---|---|---|---|---|---|
| 0 | 14.58 ± 1.16 | 0.33 ± 0.01 | 0.60 ± 0.02 | 4.03 ± 0.27 | 5.46 ± 0.83 |
| 10 | 11.82 ± 0.50 | 0.32 ± 0.03 | 0.59 ± 0.01 | 5.82 * ± 0.26 | 5.32 ± 0.71 |
| 20 | 10.77 * ± 0.19 | 0.31 ± 0.01 | 0.60 ± 0.02 | 6.04 * ± 0.33 | 5.49 ± 0.42 |
| 30 | 11.50 * ± 0.86 | 0.31 ± 0.04 | 0.60 ± 0.01 | 6.26 * ± 0.68 | 6.97 ± 0.56 |
| One-Way ANOVA | |||||
| F (t-test) | 4.6145 * | 0.1135 NS | 0.0884 NS | 5.9450 * | 1.4373 NS |
| p | 0.013 | 0.9512 | 0.9656 | 0.01 | 0.2806 |
| KF (mg L−1) | NBI | Ca (g kg−1) | Mg (g Kg−1) | K (g Kg−1) |
|---|---|---|---|---|
| 0 | 42.63 ± 2.27 | 14.93 ± 0.56 | 5.85 ± 0.57 | 52.00 ± 0.82 |
| 10 | 34.46 ** ± 0.83 | 15.25 ± 0.42 | 5.80 ± 0.08 | 51.83 ± 1.50 |
| 20 | 35.91 ** ± 1.78 | 14.10 ± 0.57 | 5.55 ± 0.23 | 51.80 ± 1.38 |
| 30 | 35.43 ** ± 3.33 | 14.63 ± 0.67 | 5.45 ± 0.17 | 51.98 ± 1.37 |
| One-Way ANOVA | ||||
| F (t-test) | 5.1728 ** | 0.7517 NS | 0.3631 NS | 0.0062 NS |
| p | 0.0082 | 0.5422 | 0.7809 | 0.9993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida Rodrigues, A.; Almeida Rodrigues, D.; de Fátima Sales, J.; Carvalho Vasconcelos Filho, S.; Carlos Costa, A.; Lino Rodrigues, C.; Alves da Silva, A.; Domingos, M.; Müller, C. Morphoanatomical, Physiological, and Biochemical Indicators in Lactuca sativa L. Germination and Growth in Response to Fluoride. Plants 2022, 11, 3406. https://doi.org/10.3390/plants11233406
Almeida Rodrigues A, Almeida Rodrigues D, de Fátima Sales J, Carvalho Vasconcelos Filho S, Carlos Costa A, Lino Rodrigues C, Alves da Silva A, Domingos M, Müller C. Morphoanatomical, Physiological, and Biochemical Indicators in Lactuca sativa L. Germination and Growth in Response to Fluoride. Plants. 2022; 11(23):3406. https://doi.org/10.3390/plants11233406
Chicago/Turabian StyleAlmeida Rodrigues, Arthur, Douglas Almeida Rodrigues, Juliana de Fátima Sales, Sebastião Carvalho Vasconcelos Filho, Alan Carlos Costa, Cássia Lino Rodrigues, Adinan Alves da Silva, Marisa Domingos, and Caroline Müller. 2022. "Morphoanatomical, Physiological, and Biochemical Indicators in Lactuca sativa L. Germination and Growth in Response to Fluoride" Plants 11, no. 23: 3406. https://doi.org/10.3390/plants11233406
APA StyleAlmeida Rodrigues, A., Almeida Rodrigues, D., de Fátima Sales, J., Carvalho Vasconcelos Filho, S., Carlos Costa, A., Lino Rodrigues, C., Alves da Silva, A., Domingos, M., & Müller, C. (2022). Morphoanatomical, Physiological, and Biochemical Indicators in Lactuca sativa L. Germination and Growth in Response to Fluoride. Plants, 11(23), 3406. https://doi.org/10.3390/plants11233406






