HPLC-PDA/ESI-MS Analysis of Phenolic Compounds and Bioactivities of the Ethanolic Extract from Flowers of Moroccan Anacyclus clavatus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield of Polyphenols
2.2. Contents of Total Phenols (TPC) and Flavonoids (FC)
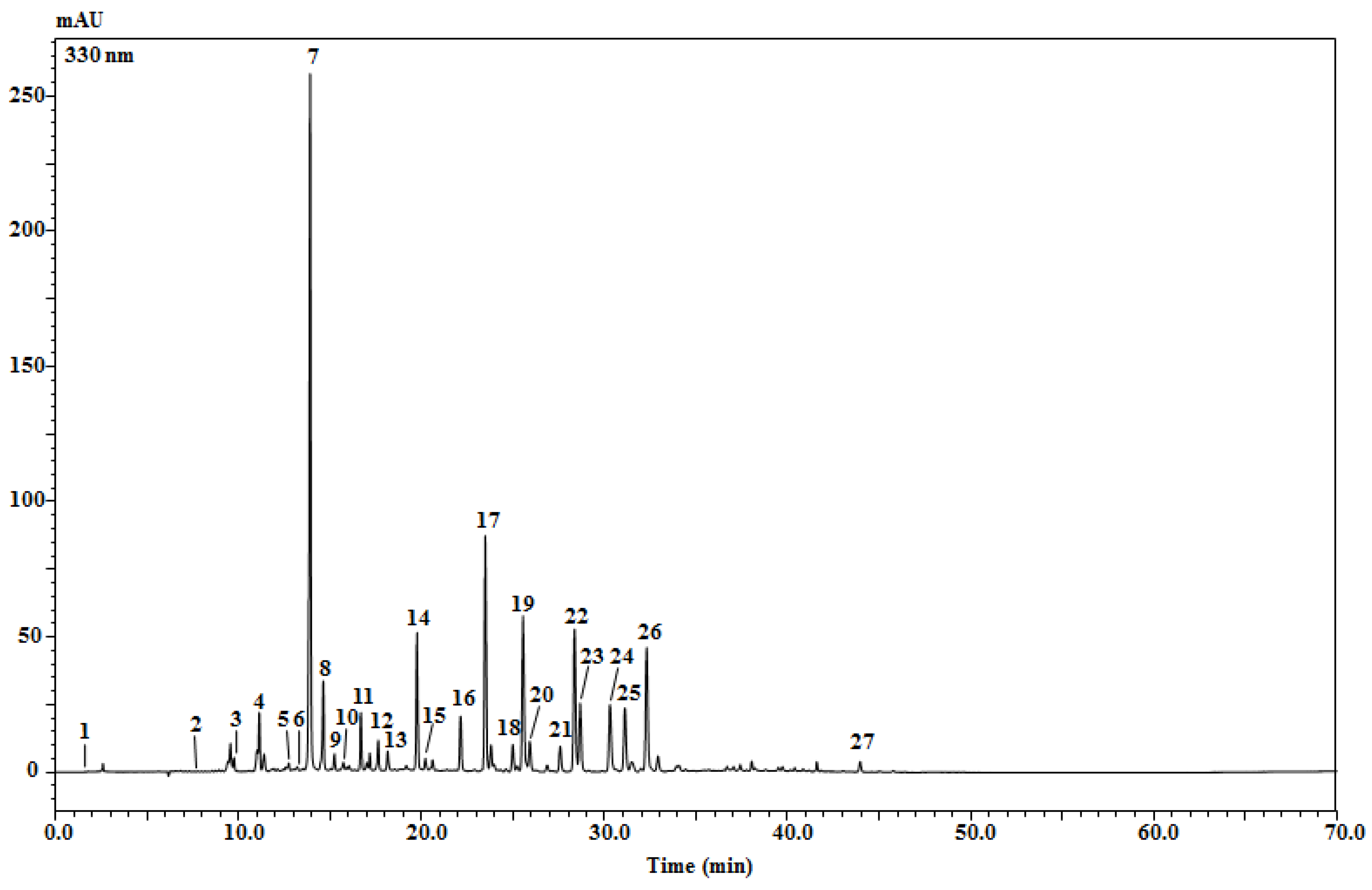
2.3. Identification of Phenolic Profile via an HPLC-PDA/ESI-MS Analysis
2.4. Antioxidant Capacity of Anacyclus clavatus Flower Extract Tested Using the Potassium Ferric Reducing Antioxidant Power (PFRAP) Assay
2.5. Antibacterial Capacity of Anacyclus clavatus Flower Extract
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Polyphenols from Anacyclus clavatus Flowers
3.3. Estimation of Total Phenol and Flavonoid Contents in Hydroethanolic Extract of Anacyclus clavatus Flowers
3.4. Identification of the Phenolic Composition of the Extract via Chromatographic Analysis Using HPLC-PDA-ESI-MS
3.4.1. Sample Preparation
3.4.2. HPLC-MS Conditions
3.4.3. Standard Employed
3.5. Antioxidant Capacity of the Extract Assessed Using Potassium Ferric Reducing Antioxidant Power (PFRAP) Assay
3.6. Antibacterial Activity
3.6.1. Bacterial Strains and Their Conditions of Growth
3.6.2. Broth Microdilution Method
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolnik, A.; Soluch, A.; Kowalska, I.; Olas, B. Antioxidant and Hemostatic Properties of Preparations from Asteraceae Family and Their Chemical Composition–Comparative Studies. Biomed. Pharmacother. 2021, 142, 111982. [Google Scholar] [CrossRef]
- Nikolić, M.; Stevović, S. Family Asteraceae as a Sustainable Planning Tool in Phytoremediation and Its Relevance in Urban Areas. Urban For. Urban Green. 2015, 14, 782–789. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Imtara, H.; El Moussaoui, A.; Khalis, H.; Es-Safi, I.; Al Kamaly, O.; Saleh, A.; Khalid Parvez, M.; Guemmouh, R.; Bari, A. Reproductive Biology of the Two Varieties of Anacyclus pyrethrum L.—Anacyclus pyrethrum Var. pyrethrum (L.) Link and Anacyclus pyrethrum Var. Depressus (Ball.) Maire—An Endemic Endangered Species. Plants 2022, 11, 2299. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Abd Rani, N.Z.; Husain, K. A Review on the Potential Use of Medicinal Plants From Asteraceae and Lamiaceae Plant Family in Cardiovascular Diseases. Front. Pharmacol. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Sissi, S.; Di Giacomo, S.; Ferrante, C.; Angelini, P.; Macone, A.; Giusti, A.M.; Toniolo, C.; Vitalone, A.; Abdellah, A.; Larhsini, M.; et al. Characterization of the Phytochemical Composition and Bioactivities of Anacyclus maroccanus Ball. and Anacyclus radiatus Loisel Aerial Parts: Preliminary Evidence for the Possible Development of Moroccan Plants. Molecules 2022, 27, 692. [Google Scholar] [CrossRef] [PubMed]
- Bouriche, H.; Abdallah, K.; Kada, S.; Senator, A.; Demirtas, I. Phenolic Content, Anti-Inflammatory and Antioxidant Activities of Anacyclus clavatus Extracts. Environ. Exp. Biol. 2016, 14, 127–135. [Google Scholar] [CrossRef]
- Kalam, M.A.; Karim, M.S.; Anzar, M.A.; Sofi, G.; Ahmad, G.; Shahzad, A. Aqer Qerha (Anacyclus pyrethrum Dc.) a Nobel Drug of Unani System of Medicine—A Review. Int. J. Pharmacogn. 2015, 2, 116–122. [Google Scholar] [CrossRef]
- Pardo-de-Santayana, M.; Morales, R. Chamomiles in Spain The Dynamics of Plant Nomenclature. In Ethnobotany in the New Europe: People, Health and Wild Plant Resources; Berghahn Books: New York, NY, USA, 2010; pp. 283–307. [Google Scholar]
- Houicher, A.; Hamdi, M.; Hechachna, H.; Özogul, F. Chemical Composition and Antifungal Activity of Anacyclus valentinus Essential Oil from Algeria. Food Biosci. 2018, 25, 28–31. [Google Scholar] [CrossRef]
- Zaidi, S.M.A.; Pathan, S.A.; Singh, S.; Jamil, S.; Ahmad, F.J.; Khar, R.K. Anticonvulsant, Anxiolytic and Neurotoxicity Profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) Root Ethanolic Extract. Pharmacol. Pharm. 2013, 4, 535–541. [Google Scholar] [CrossRef]
- Sharma, V.; Thakur, M.; Chauhan, N.S.; Dixit, V.K. Evaluation of the Anabolic, Aphrodisiac and Reproductive Activity of Anacyclus pyrethrum DC in Male Rats. Sci. Pharm. 2009, 77, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating Activity of the Hot Water-Soluble Polysaccharide Extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef]
- Mifsud, S. Anacyclus clavatus (White Anacyclus): MaltaWildPlants.Com-The Online Flora of the Maltese Islands. Available online: http://maltawildplants.com/ASTR/Anacyclus_clavatus.php (accessed on 3 July 2021).
- Mechergui, K.; Khaldi, S.; Jaouadi, W. Assessment of Phenology and Morphological Diversity of 3 Species of Asteraceae: Anacyclus clavatus, Chamaemelum fuscatum and Tanacetum parthenium. Asian J. Biol. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Hammami, S.; Salem, A.B.; Mastouri, M.; Falconieri, D.; Gorcii, M.; M’henni, M.F.; Marongiu, B.; Mighri, Z. Essential Oil Composition and Antimicrobial Activities of Aerial Parts from Tunisian Anacyclus clavatus (Desf.). J. Med. Plants Res. 2013, 7, 71–75. [Google Scholar]
- Aliboudhar, H.; Tigrine-Kordjani, N.; Hanifi, N.; Meklati, B.Y. Volatiles Profiling and Antioxidant Activity Evaluation of Different Parts of a Medicinal Plant: Anacyclus clavatus. J. Herbs Spices Med. Plants 2013, 19, 33–47. [Google Scholar] [CrossRef]
- Aliboudhar, H.; Tigrine-Kordjani, N. Effect of Extraction Technique on the Content and Antioxidant Activity of Crude Extract of Anacyclus clavatus Flowers and Their Essential Oil Composition. Nat. Prod. Res. 2014, 28, 2140–2149. [Google Scholar] [CrossRef]
- Aliboudhar, H.; Tigrine-Kordjani, N.; Youcef Meklati, B. Competition of Microwave-Assisted Hydro-Distillation in Highlighting Volatile Phytochemicals of Anacyclus clavatus Species. J. Essent. Oil Res. 2015, 27, 355–362. [Google Scholar] [CrossRef]
- Ben Chaabane, A.; Aubin, P. Catalogue des plantes d’un milieu salé dans la région de Marrakech (Ouljat-Oued-Tensift, Maroc). Publ. Société Linnéenne Lyon 1991, 60, 405–409. [Google Scholar] [CrossRef]
- Essakhi, D.; Benjelloun, M.; Errabhi, N.; Harchli, H.E.; Ghadraoui, L.E. Richesse spécifique en Orthoptères Acridiens du Moyen Atlas Marocain. Bull. L’institut Sci. Rabat Sect. Sci. Vie 2014, 41–48. [Google Scholar]
- Hachi, M.; Rahou, A.; Lamfadal, K. Contribution À Une Étude Floristique De « Aguelmouss–Moulay Bouaazza–Tiddass » (Plateau Central, Maroc). Eur. Sci. J. 2016, 12, 267. [Google Scholar] [CrossRef] [Green Version]
- Larbi, K.S.; Meddah, B.; Belkhodja, H.; Belmimoun, A.; Slimani, K.; Sonnet, P. Chemical Composition and Anti-Arthritic Activity of Anacyclus valentinus Extract on Adjuvant-Induced Arthritis in Rats. Int. J. Environ. Agric. Biotechnol. 2017, 2, 3025–3032. [Google Scholar] [CrossRef]
- Jawhari, F.Z.; Moussaoui, A.E.L.; Bourhia, M.; Imtara, H.; Saghrouchni, H.; Ammor, K.; Ouassou, H.; Elamine, Y.; Ullah, R.; Ezzeldin, E.; et al. Anacyclus pyrethrum Var. pyrethrum (L.) and Anacyclus pyrethrum Var. depressus (Ball) Maire: Correlation between Total Phenolic and Flavonoid Contents with Antioxidant and Antimicrobial Activities of Chemically Characterized Extracts. Plants 2021, 10, 149. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 243–259. ISBN 978-0-12-813768-0. [Google Scholar]
- Erez, M.E.; Dalar, A.; Fidan, M.; Pınar, S.M. Comprehensive Appraisement of Antioxidant Potential and Phytochemical Profile of Native Botanicals from Turkey. Food Meas. 2019, 13, 3230–3241. [Google Scholar] [CrossRef]
- FooDB. Available online: https://foodb.ca/ (accessed on 17 July 2022).
- de Athayde, A.E.; de Araujo, C.E.S.; Sandjo, L.P.; Biavatti, M.W. Metabolomic Analysis among Ten Traditional “Arnica” (Asteraceae) from Brazil. J. Ethnopharmacol. 2021, 265, 113149. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Püssa, T.; Valner, C.; Malmiste, B.; Arak, E. Content of Essential Oil, Terpenoids and Polyphenols in Commercial Chamomile (Chamomilla recutita L. Rauschert) Teas from Different Countries. Food Chem. 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Crupi, P.; Bleve, G.; Tufariello, M.; Corbo, F.; Clodoveo, M.L.; Tarricone, L. Comprehensive Identification and Quantification of Chlorogenic Acids in Sweet Cherry by Tandem Mass Spectrometry Techniques. J. Food Compos. Anal. 2018, 73, 103–111. [Google Scholar] [CrossRef]
- Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Oulad El Majdoub, Y.; Alibrando, F.; Cacciola, A.; Spadaro, V.; Mondello, L.; Germanò, M.P.; Cacciola, F. Chemical Characterization of Anthemis parlatoreana Fresh and Dried Aerial Parts by GC and LC Chromatographic Techniques and Evaluation of the Antioxidant Properties. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1–12. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Cechinel-Filho, V.; Meira-Quintão, N.L.; Peña-Rodríguez, L.M. Bioactive Dicaffeoylquinic Acid Derivatives from the Root Extract of Calea urticifolia. Rev. Bras. Farmacogn. 2018, 28, 339–343. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Ji, R.; Quan, Q.; Guo, X.; Zhang, J.; Song, Y.; Zhu, M.; Tan, P.; Han, J.; Liu, Y. Simultaneous Determination of Five N-Alkylamides in the Root of Anacyclus pyrethrum by HPLC and Profiling of Components in Its Methanolic Root Extract by UPLC/Q-TOF-MS. Rev. Bras. Farmacogn. 2019, 29, 152–161. [Google Scholar] [CrossRef]
- Yahiaoui, H.; Howes, M.J.; Ohiomakhare, S.; M’Hamedi, A.; Chazot, P.L. Algerian Anacyclus pyrethrum Aqueous Extract: Novel Antioxidant and Neuroprotectant Activity of a Chemically Profiled Aqueous Extract. South Asian J. Exp. Biol. 2017, 7, 262–270. [Google Scholar] [CrossRef]
- Dif, A.; Yasmina, B.M.; Ammam, A.; Slimani, M. Chemical Composition and Protective Effect of Anacyclus valentinus against Cisplatin-Induced Hepatotoxicity in Wistar Rats. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e5290. [Google Scholar] [CrossRef]
- Greger, H. Comparative Phytochemistry and Systematics of Anacyclus. Biochem. Syst. Ecol. 1978, 6, 11–17. [Google Scholar] [CrossRef]
- Fanali, C.; Della Posta, S.; Vilmercati, A.; Dugo, L.; Russo, M.; Petitti, T.; Mondello, L.; De Gara, L. Extraction, Analysis, and Antioxidant Activity Evaluation of Phenolic Compounds in Different Italian Extra-Virgin Olive Oils. Molecules 2018, 23, 3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; El Mansouri, F.; Kabach, I.; Oulad El Majdoub, Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L.; et al. Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules 2021, 26, 3134. [Google Scholar] [CrossRef]
- Arena, K.; Trovato, E.; Cacciola, F.; Spagnuolo, L.; Pannucci, E.; Guarnaccia, P.; Santi, L.; Dugo, P.; Mondello, L.; Dugo, L. Phytochemical Characterization of Rhus coriaria L. Extracts by Headspace Solid-Phase Micro Extraction Gas Chromatography, Comprehensive Two-Dimensional Liquid Chromatography, and Antioxidant Activity Evaluation. Molecules 2022, 27, 1727. [Google Scholar] [CrossRef]
- Selles, C.; El, M.; Dib, M.; Allali, H.; Boufeldja, T. Evaluation of Antimicrobial and Antioxidant Activities of Solvent Extracts of Anacyclus pyrethrum L., from Algeria. Mediterr. J. Chem. 2012, 2, 408–415. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Zovko Končić, M.; Kremer, D.; Karlović, K.; Kosalec, I. Evaluation of Antioxidant Activities and Phenolic Content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010, 48, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Chroho, M.; Bouymajane, A.; Aazza, M.; Oulad El Majdoub, Y.; Cacciola, F.; Mondello, L.; Zair, T.; Bouissane, L. Determination of the Phenolic Profile, and Evaluation of Biological Activities of Hydroethanolic Extract from Aerial Parts of Origanum compactum from Morocco. Molecules 2022, 27, 5189. [Google Scholar] [CrossRef] [PubMed]
- Chroho, M.; Bouymajane, A.; Oulad El Majdoub, Y.; Cacciola, F.; Mondello, L.; Aazza, M.; Zair, T.; Bouissane, L. Phenolic Composition, Antioxidant and Antibacterial Activities of Extract from Flowers of Rosa damascena from Morocco. Separations 2022, 9, 247. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar]




| Extraction Yield | Total Phenol Content (TPC) | Flavonoid Content (FC) | EC50 Extract (PFRAP) mg/mL | EC50 Ascorbic Acid (PFRAP) mg/mL |
|---|---|---|---|---|
| 35.03 (%) | 9.53 ± 0.48 GAE/g dm | 1.31 ± 0.06 mg QE/g dm | 0.91 ± 0.04 mg/mL | 0.03 ± 0.11 mg/mL |
| Peak N° | Compound | tR (min) | UV (nm) | [M-H]− | [M + H]+ | Fragments | Quantity (mg/Kg) Extract ± sd | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Citric acid | 1.94 | 274 | 191 | - | - | Nq | [27] |
| 2 | Protocatechuic acid | 7.54 | 204, 259, 293 | 153 | - | - | Nq | [27] |
| 3 | Caffeoylquinic acid isomer | 9.76 | 215, 325 | 353 | - | 191(-), 179(-) | 570.12 ± 9.02 | [1,27,28,29,30] |
| 4 | Caffeoylquinic acid isomer | 11.15 | 215, 323 | 353 | - | 191(-), 179(-) | 1373.20 ± 5.54 | [1,27,28,29,30] |
| 5 | p-Coumaroylquinic acid | 12.75 | 203, 311 | 337 | - | 191(-) | 500.64 ± 2.13 | [1,27] |
| 6 | Dicaffeoylquinic acid isomer | 13.22 | 195, 212, 316 | 515 | - | 163(+) | 479.04 ± 4.52 | [1,27,28] |
| 7 | Caffeoylquinic acid isomer | 13.92 | 222, 236, 288, 333 | 353 | 355 | 191(-); 181(+) | 21,280.34 ± 731.00 | [1,27,28,29,30] |
| 8 | Caffeoylquinic acid isomer | 14.64 | 217, 325 | 353 | 355 | 191(-); 181(+) | 3173.78 ± 68.33 | [1,27,28,29,30] |
| 9 | Caffeoylquinic acid isomer | 15.25 | 195, 216, 295, 322 | 353 | 355 | 191(-); 181(+) | 811.51 ± 30.87 | [1,27,28,29,30] |
| 10 | Caffeoylquinic acid isomer | 15.74 | 199, 324 | 353 | 355 | 191(-); 181(+) | 647.46 ± 39.32 | [1,27,28,29,30] |
| 11 | p-Coumaroylquinic acid | 16.69 | 211, 223, 311 | 337 | 339 | 191(-); 165(+) | 1943.88 ± 62.20 | [1,27] |
| 12 | Dicaffeoylquinic acid isomer | 17.64 | 215, 321 | 515 | - | 163(+); 181(+) | 1300.07 ± 54.54 | [1,27,28] |
| 13 | Feruloylquinic acid | 18.16 | 271, 330 | 367 | 369 | 193(-) | 1003.77 ± 34.96 | [1] |
| 14 | Myricetin-hexoside | 19.76 | 202, 259, 358 | 479 | 481 | 319(+) | 2410.94 ± 128.95 | [27] |
| 15 | Luteolin-dihexoside | 20.21 | 206, 254, 344 | 623 | 625 | 447(+); 287(+) | 170.30 ± 11.20 | [27] |
| 16 | Apigenin 7-O-diglucuronide | 22.13 | 201, 266, 336 | 621 | 623 | 447(+) | 420.53 ± 24.21 | [27] |
| 17 | Apigenin 7-glucuronosyl-glucoside | 23.49 | 202, 266, 336 | 607 | 609 | 269(-); 271(+) | 1773.14 ± 81.88 | [27] |
| 18 | Quercetin O-hexoside | 23.80 | 202, 255, 369 | 463 | 465 | 303(+) | 558.33 ± 8.31 | [1] |
| 19 | Kaempferol-7-O-glucoside | 25.55 | 206, 257, 365 | 447 | 449 | 285 (-) | 2508.73 ± 114.46 | [27,31] |
| 20 | Patuletin 3-O-glucoside | 25.91 | 202, 259, 355 | 493 | 495 | 333(+) | 711.03 ± 5.33 | [31] |
| 21 | Dicaffeoylquinic acid isomer | 27.81 | 216, 325 | 515 | - | 181(+) | 1449.69 ± 12.16 | [32] |
| 22 | Dicaffeoylquinic acid isomer | 28.36 | 216, 325 | 515 | - | 181(+); 163(+) | 6365.15 ± 279.25 | [1,27,28] |
| 23 | Dicaffeoylquinic acid isomer | 28.67 | 217, 328 | 515 | 353 | 191(-); 163(+) 181(+) | 3303.98 ± 146.74 | [1,27,28] |
| 24 | Apigenin-7-O-glucoside | 30.30 | 201, 266, 336 | 431 | 433 | 271(+) | 682.88 ± 25.72 | [31] |
| 25 | Apigenin 7-O-glucuronide | 31.11 | 201, 266, 336 | 445 | 447 | 269(-) | 636.41 ± 23.07 | [27] |
| 26 | Dicaffeoylquinic acid isomer | 32.30 | 217, 327 | 515 | - | 163(+); 181(+) | 5815.54 ± 252.78 | [1,27,28] |
| 27 | Apigenin | 43.96 | 214, 266, 335 | 269 | 271 | - | 80.49 ± 2.05 | Std |
| Bacteria | MIC | MBC | MBC/MIC |
|---|---|---|---|
| Escherichia coli | 20.83 ± 0.12 | 166.66 ± 0.12 | 8 |
| Salmonella typhimirium | 41.66 ± 0.15 | 166.66 ± 0.17 | 4 |
| Staphyloccocus aureus | 20.83 ± 0.20 | 83.33 ± 0.12 | 4 |
| Listeria monocytogenes | 41.66 ± 0.13 | 166.66 ± 0.16 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chroho, M.; Aazza, M.; Bouymajane, A.; Majdoub, Y.O.E.; Cacciola, F.; Mondello, L.; Zair, T.; Bouissane, L. HPLC-PDA/ESI-MS Analysis of Phenolic Compounds and Bioactivities of the Ethanolic Extract from Flowers of Moroccan Anacyclus clavatus. Plants 2022, 11, 3423. https://doi.org/10.3390/plants11243423
Chroho M, Aazza M, Bouymajane A, Majdoub YOE, Cacciola F, Mondello L, Zair T, Bouissane L. HPLC-PDA/ESI-MS Analysis of Phenolic Compounds and Bioactivities of the Ethanolic Extract from Flowers of Moroccan Anacyclus clavatus. Plants. 2022; 11(24):3423. https://doi.org/10.3390/plants11243423
Chicago/Turabian StyleChroho, Mounia, Mustapha Aazza, Aziz Bouymajane, Yassine Oulad El Majdoub, Francesco Cacciola, Luigi Mondello, Touriya Zair, and Latifa Bouissane. 2022. "HPLC-PDA/ESI-MS Analysis of Phenolic Compounds and Bioactivities of the Ethanolic Extract from Flowers of Moroccan Anacyclus clavatus" Plants 11, no. 24: 3423. https://doi.org/10.3390/plants11243423
APA StyleChroho, M., Aazza, M., Bouymajane, A., Majdoub, Y. O. E., Cacciola, F., Mondello, L., Zair, T., & Bouissane, L. (2022). HPLC-PDA/ESI-MS Analysis of Phenolic Compounds and Bioactivities of the Ethanolic Extract from Flowers of Moroccan Anacyclus clavatus. Plants, 11(24), 3423. https://doi.org/10.3390/plants11243423









