Assisted Phytostabilization of Mine-Tailings with Prosopis laevigata (Fabaceae) and Biochar
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Characterization of the Corncobs/Coconut Fiber Biochar
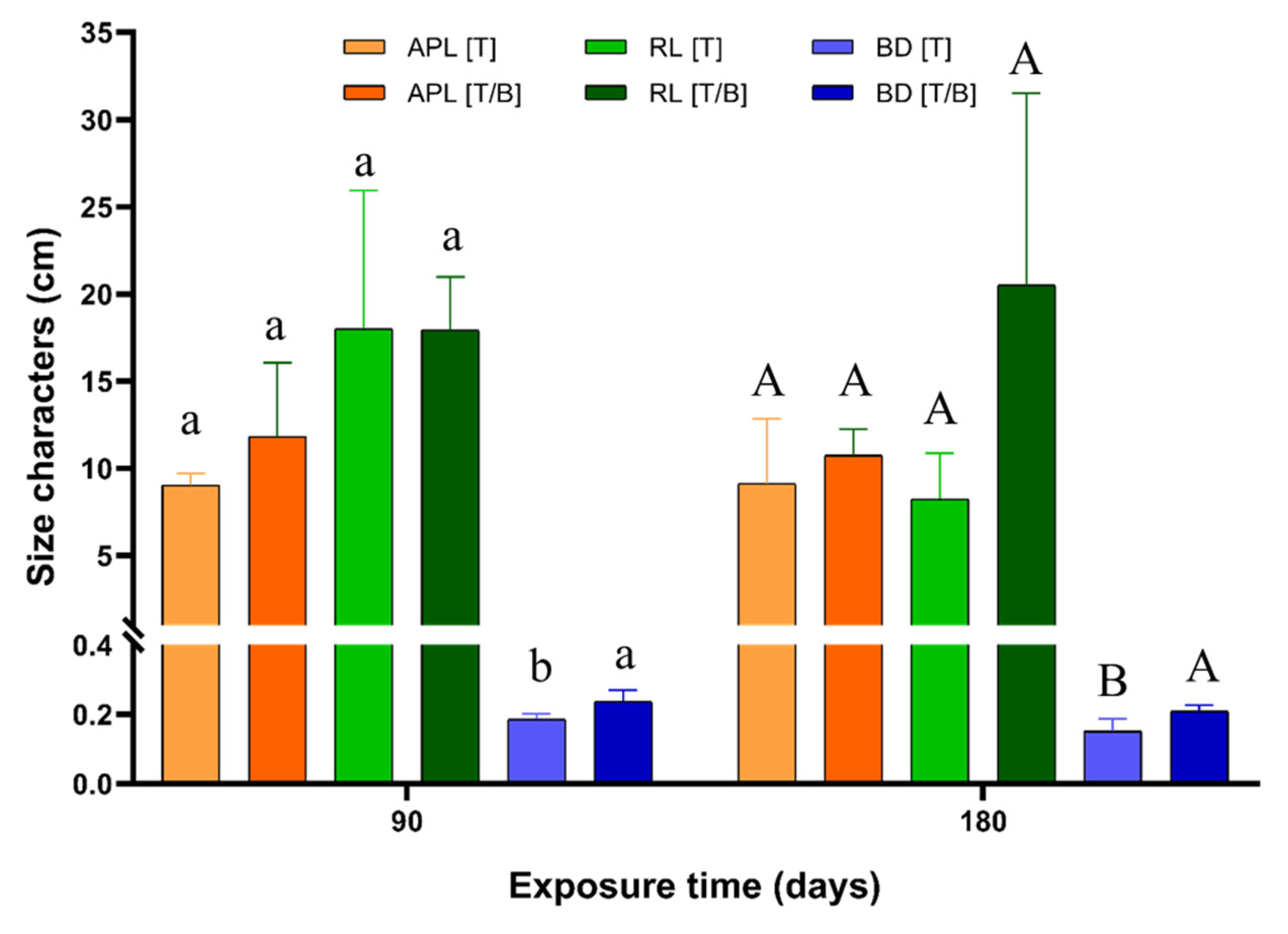
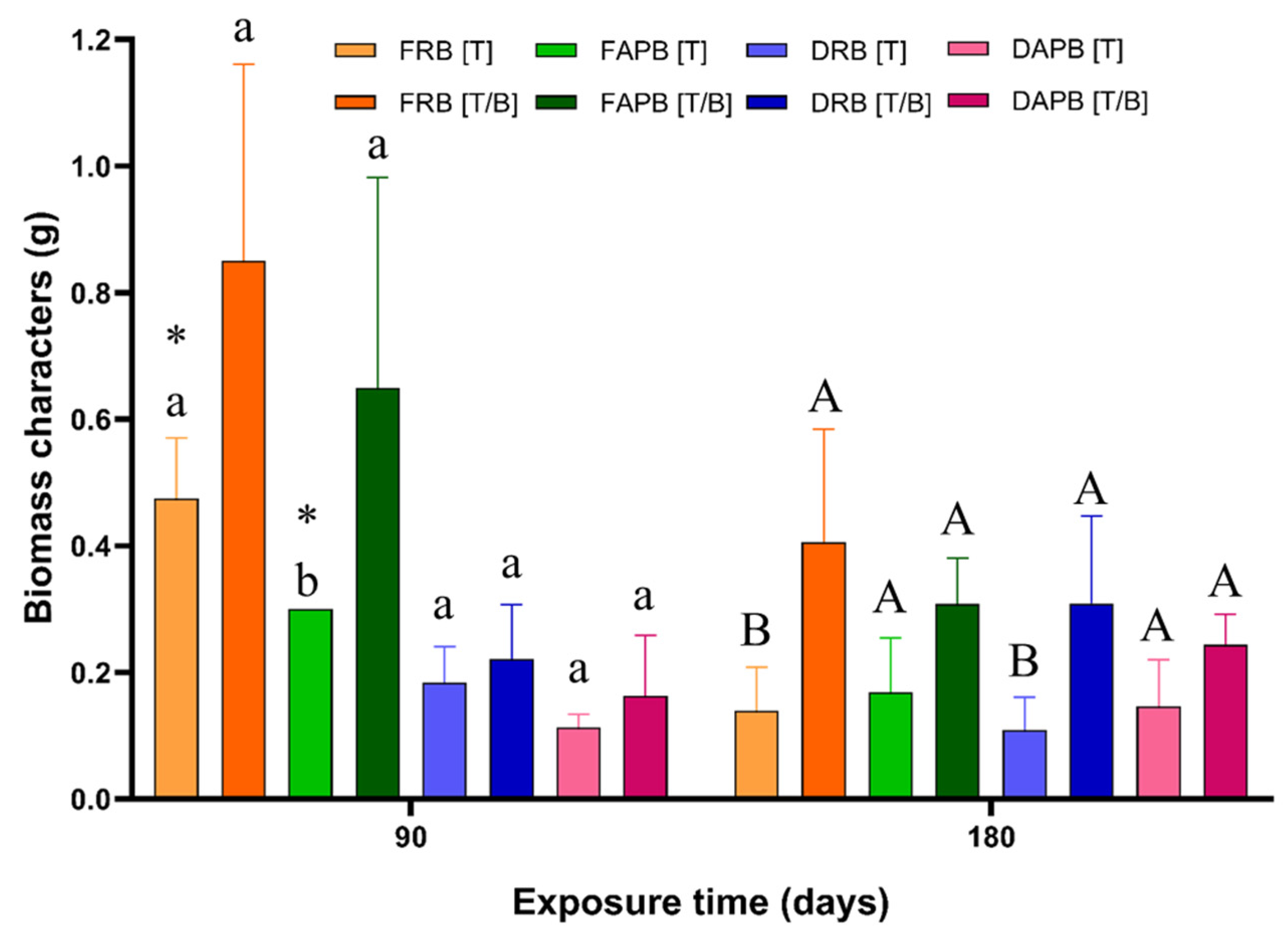
2.2. Effect on the Mine Tailing Exposure and the Biochar Addition over the Size Characters of P. laevigata
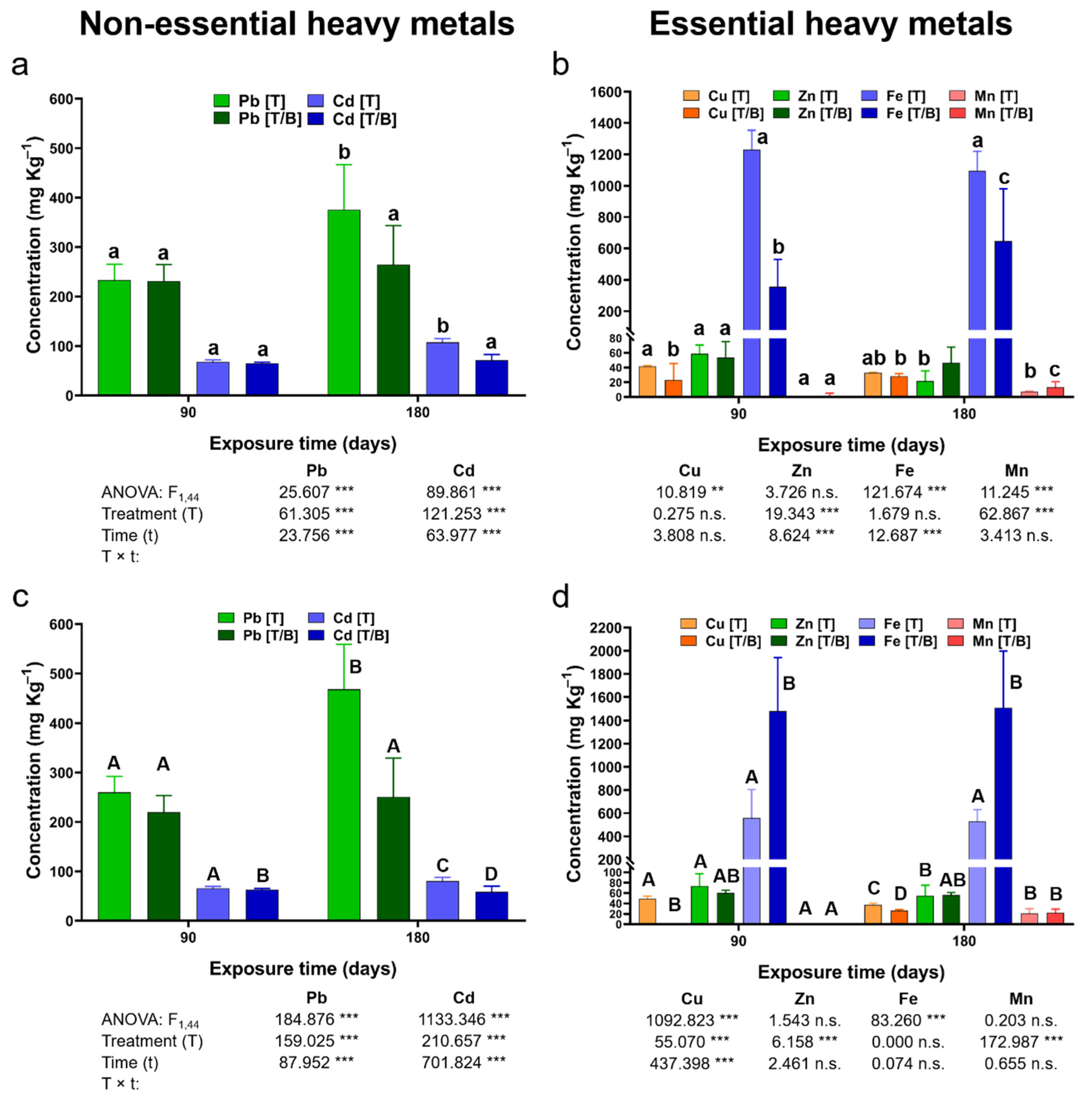
2.3. Bioaccumulation and Translocation Profiles of Nonessential Heavy Metals in P. laevigata
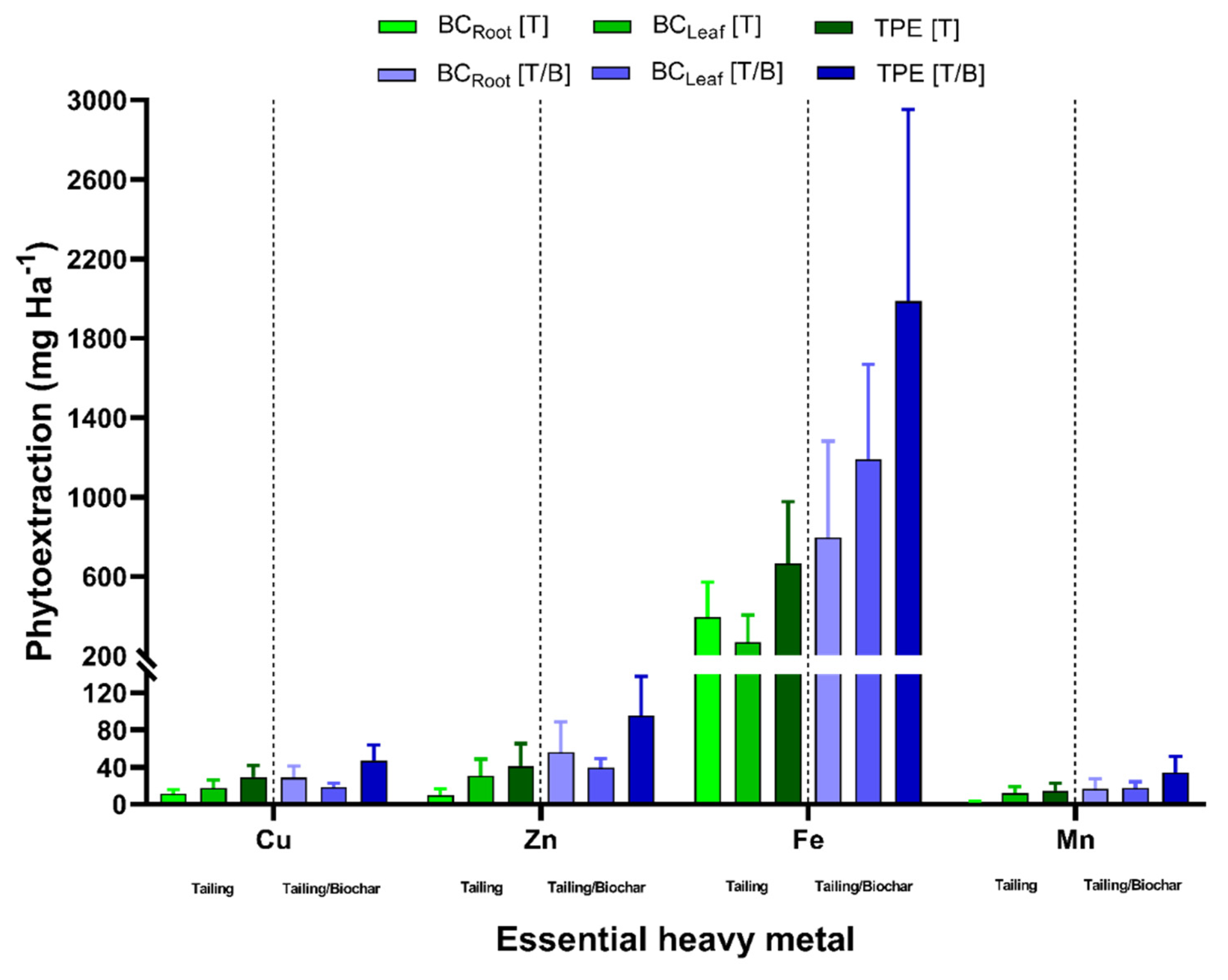
2.4. Bioaccumulation and Translocation Profiles of Essential Heavy Metals in P. laevigata
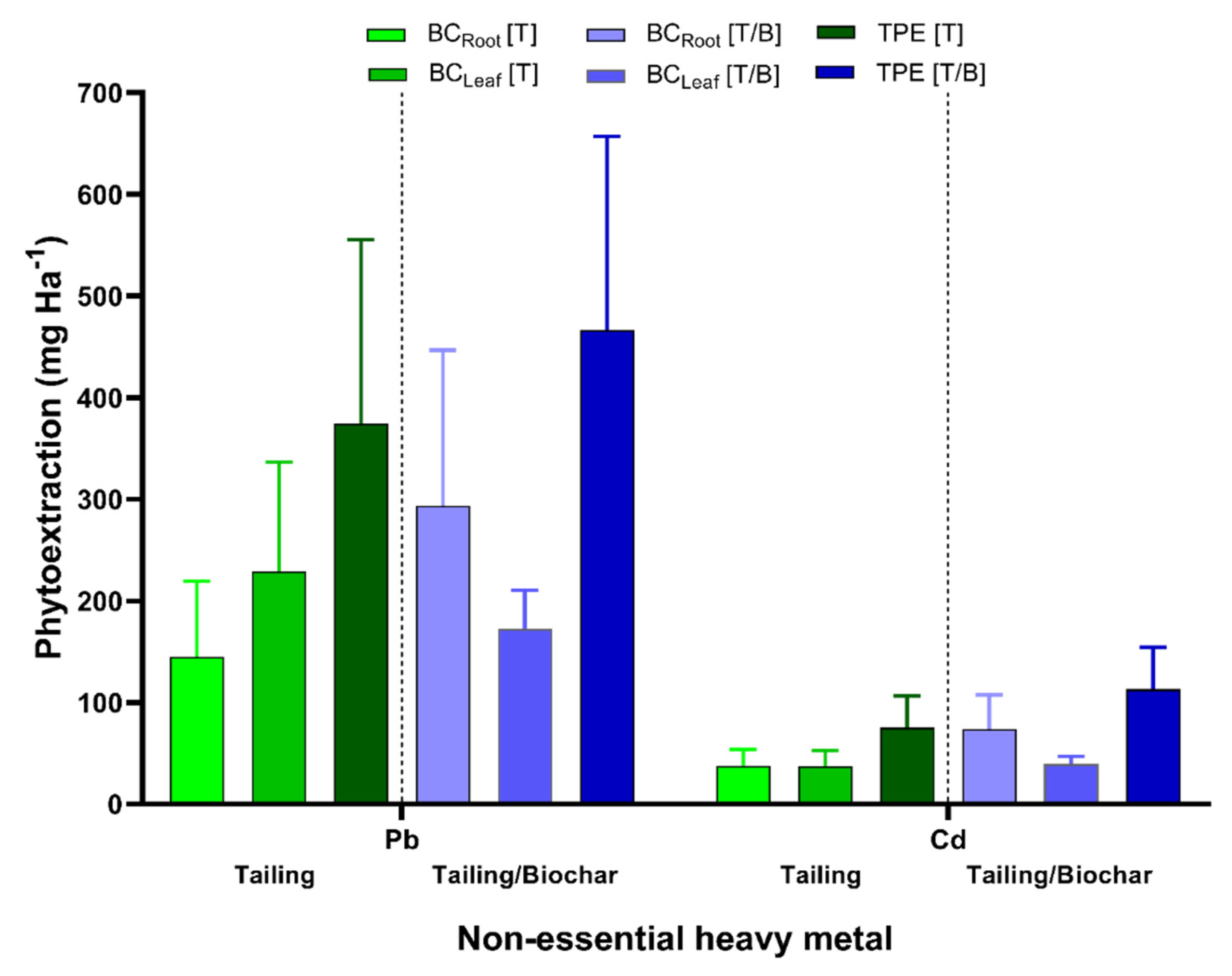
2.5. P. laevigata Total Heavy Metals Phytoextraction
3. Discussion
3.1. Physicochemical Properties of Biochar
3.2. Size Characters of P. laevigata
3.3. Bioaccumulation and Translocation of Nonessential Heavy Metals in P. laevigata
3.4. Bioaccumulation and Translocation of Essential Heavy Metals in P. laevigata
4. Materials and Methods
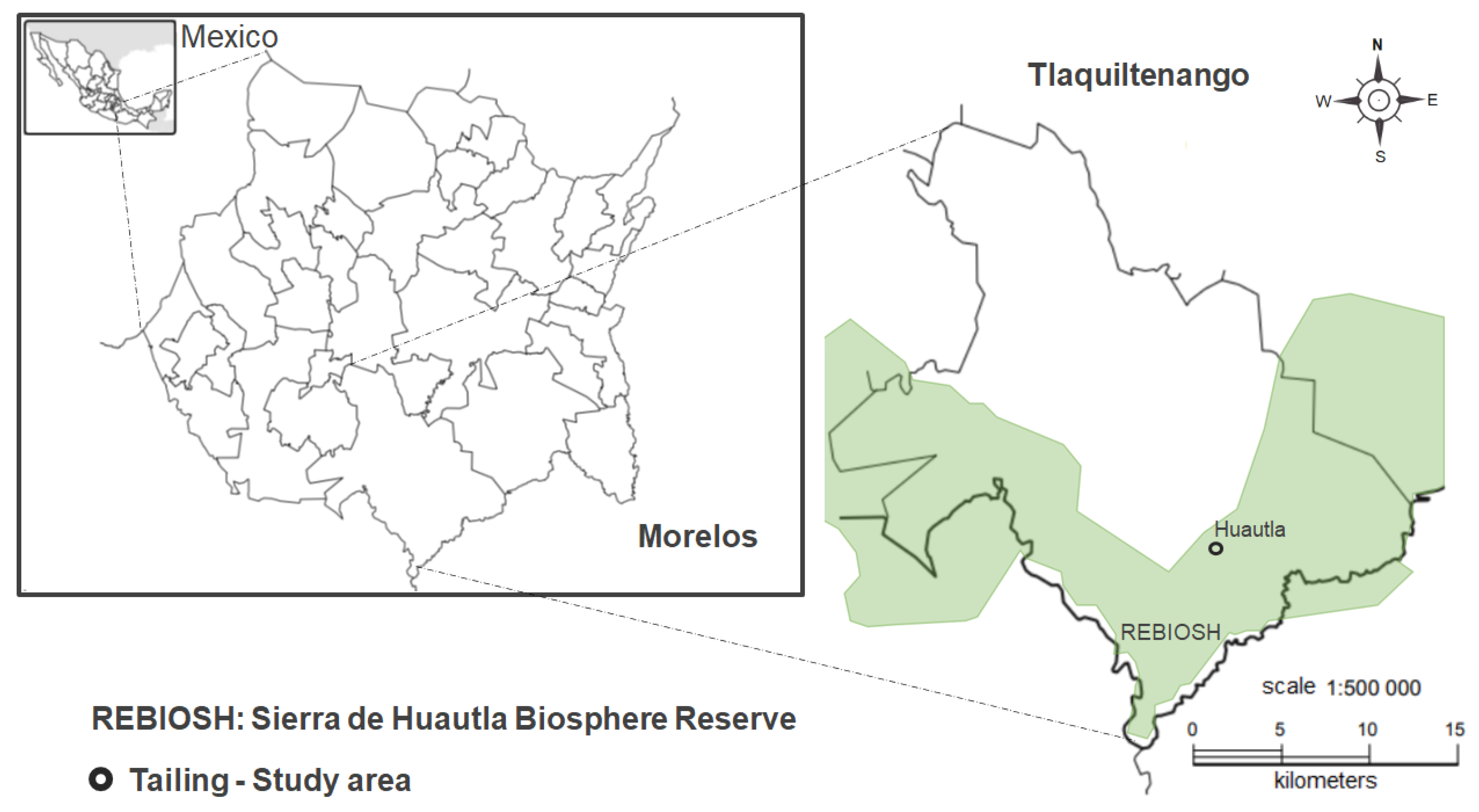
4.1. Study Site
4.2. Plant Species
4.3. Biochar Production and Physicochemical Characterization
4.4. In Situ Phytostabilization Design
4.5. Plants size and Biomass Determinations
4.6. Heavy Metal Determination
4.7. Heavy Metal Translocation Index and Total Phytoextraction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, F.P. Mining industry and sustainable development: Time for change. Food Energy Secur. 2017, 6, 61–77. [Google Scholar] [CrossRef]
- Gabarrón, M.; Zornoza, R.; Acosta, J.A.; Faz, Á.; Martínez-Martínez, S. Mining environments. Adv. Chem. Pollut. Environ. Manag. Prot. 2019, 4, 157–205. [Google Scholar] [CrossRef]
- Artiola, J.F.; Walworth, J.L.; Musil, S.A.; Crimmins, M.A. Soil and land pollution. In Environmental and Pollution Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–235. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Parvez Mahmud, M.A.; Saidur, R.A. Review on the impact of mining and mineral processing industries through life cycle assessment. J. Clean. Prod. 2019, 231, 1200–1217. [Google Scholar] [CrossRef]
- Matinde, E.; Simate, G.S.; Ndlovu, S. Mining and Metallurgical Wastes: A Review of Recycling and Re-Use Practices. J. S. Afr. Inst. Min. Metall. 2018, 118, 825–844. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Cleaver, A.E.; Jamieson, H.E.; Rickwood, C.J.; Huntsman, P. Tailings dust characterization and impacts on surface water chemistry at an abandoned Zn-Pb-Cu-Au-Ag deposit. Appl. Geochem. 2021, 128, 104927. [Google Scholar] [CrossRef]
- Del Rio-Salas, R.; Ayala-Ramírez, Y.; Loredo-Portales, R.; Romero, F.; Molina-Freaner, F.; Minjarez-Osorio, C.; Pi-Puig, T.; Ochoa–Landín, L.; Moreno-Rodríguez, V. Mineralogy and geochemistry of rural road dust and nearby mine tailings: A case of ignored pollution hazard from an abandoned mining site in semi-arid zone. Nat. Resour. Res. 2019, 28, 1485–1503. [Google Scholar] [CrossRef]
- Dinu, C.; Vasile, G.G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.M. Translocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- Mussali-Galante, P.; Tovar-Sánchez, E.; Valverde, M.; Rojas del Castillo, E. Biomarkers of exposure for assessing environmental metal pollution: From molecules to ecosystems. Rev. Int. Contam. Ambient. 2013, 29, 117–140. [Google Scholar] [CrossRef]
- Sun, W.; Ji, B.; Khoso, S.A.; Tang, H.; Liu, R.; Wang, L.; Hu, Y. An extensive review on restoration technologies for mining tailings. Environ. Sci. Pollut. Res. 2018, 25, 33911–33925. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Khan, I.; Meng, K.; Zhao, Y.; Peng, C. A review on remediation technologies for heavy metals contaminated soil. Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 21–29. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Sarmadi, M.S.; Rezaee, M.; Khodadadi-Darban, A.; Yazdani, M.; Paz-Garcia, J.M. Comparison of Different Extracting agents for the recovery of Pb and Zn through electrokinetic remediation of mine tailings. J. Environ. Manag. 2021, 279, 111728. [Google Scholar] [CrossRef] [PubMed]
- Manca, P.P.; Massacci, G.; Pintus, D.; Sogos, G. The flotation of sphalerite mine tailings as a remediation method. Miner. Eng. 2021, 165, 106862. [Google Scholar] [CrossRef]
- Verma, N.; Sharma, R. Bioremediation of toxic heavy metals: A patent review. Recent Pat. Biotechnol. 2017, 11, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahirwar, N.K.; Tiwari, J.; Pathak, J. Review on sources and effect of heavy metal in soil: Its bioremediation. Int. J. Res. Appl. Nat. Soc. Sci. 2018, 2008, 1–22. [Google Scholar]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A Review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Muro-González, D.A.; Mussali-Galante, P.; Valencia-Cuevas, L.; Flores-Trujillo, K.; Tovar-Sánchez, E. Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopis laevigata reveal its potential for phytoremediation. Environ. Sci. Pollut. Res. 2020, 27, 40187–40204. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, B.; Singh, D. Assessment of different multipurpose tree species for phytoextraction of lead from lead-contaminated soils. Bioremediat. J. 2020, 24, 215–230. [Google Scholar] [CrossRef]
- Sharma, P.; Ngo, H.H.; Khanal, S.; Larroche, C.; Kim, S.H.; Pandey, A. Efficiency of transporter genes and proteins in hyperaccumulator plants for metals tolerance in wastewater treatment: Sustainable technique for metal detoxification. Environ. Technol. Innov. 2021, 23, 101725. [Google Scholar] [CrossRef]
- Cameselle, C.; Gouveia, S.; Urréjola, S. Benefits of phytoremediation amended with DC electric field. Application to soils contaminated with heavy metals. Chemosphere 2019, 229, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Martínez, M.; Mussali-Galante, P.; Hernández-Plata, I.; Valencia-Cuevas, L.; Flores-Morales, A.; Ortiz-Hernández, L.; Flores-Trujillo, K.; Ramos-Quintana, F.; Tovar-Sánchez, E. Heavy metal bioaccumulation and morphological changes in Vachellia Campechiana (Fabaceae) reveal its potential for phytoextraction of Cr, Cu, and Pb in mine tailings. Environ. Sci. Pollut. Res. 2020, 27, 11260–11276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef]
- Grobelak, A. Organic soil amendments in the phytoremediation process. Phytoremediation Manag. Environ. Contam. 2016, 4, 21–39. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Hanus-Fajerska, E.; MuszyŃska, E.; Ciarkowska, K. Natural organic amendments for improved phytoremediation of polluted soils: A review of recent progress. Pedosphere 2016, 26, 1–12. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Guarnieri, S.F.; do Nascimento, E.C.; Costa Junior, R.F.; de Faria, J.L.B.; de Almeida Lobo, F. Coconut fiber biochar alters physical and chemical properties in sandy soils. Acta Sci. Agron. 2021, 43, e51801. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Hui, D. Effects of biochar application on soil properties, plant biomass production, and soil greenhouse gas emissions: A mini-review. Agric. Sci. 2021, 12, 213–236. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals—A meta- analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef]
- Puga, A.P.; Melo, L.C.A.; de Abreu, C.A.; Coscione, A.R.; Paz-Ferreiro, J. Leaching and fractionation of heavy metals in mining soils amended with biochar. Soil Tillage Res. 2016, 164, 25–33. [Google Scholar] [CrossRef]
- Keshavarzifard, M.; Moore, F.; Sharifi, R. The Influence of physicochemical parameters on bioavailability and bioaccessibility of heavy metals in sediments of the intertidal zone of Asaluyeh region, Persian Gulf, Iran. Geochemistry 2019, 79, 178–187. [Google Scholar] [CrossRef]
- Palacios, A.; Rodríguez, R.; Hernández, M.D.L.L.; Jiménez, E.; Tirado, D. Potential distribution of Prosopis laevigata (Humb. et Bonpl. Ex Willd) M. C. Johnston Based on an ecological niche model. Rev. Mex. Ciencias For. 2016, 7, 35–36. [Google Scholar] [CrossRef]
- Hernández-Madrigal, F.; Contreras-Negrete, G.; Aguilar-Romero, R.; Pineda-García, F.; González-Rodríguez, A. Differentiation in seed mass and seedling biomass allocation in Prosopis laevigata throughout its distribution range in mexico is associated to water availability. Bot. Sci. 2020, 100, 274–290. [Google Scholar] [CrossRef]
- Buendía, L.; Orozco, J.; Cruz, F.; Barrera, C.E.; Vernon, E.J. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010, 101, 5862–5867. [Google Scholar] [CrossRef]
- Buendía, L.; Orozco, J.; Estrada, M.; Barrera, C.; Vernon, E.; Cruz, F. In vitro lead and nickel accumulation in mesquite (Prosopis laevigata) seedlings. Rev. Mex. Ing. Química 2012, 9, 1–9. [Google Scholar]
- Alcalá-Jauregui, J.; Rodriguez-Ortiz, J.C.; Hernández-Montoya, A.; Filippini, M.F.R.; Martinez-Carretero, E.E.; Diaz-Flores, P.E. Capacity of Two vegetative species of heavy metal accumulation. Rev. FCA UNCUYO 2018, 50, 1853–8665. [Google Scholar]
- Ramírez, V.; Baez, A.; López, P.; Bustillos, R.; Villalobos, M.Á.; Carreño, R.; Contreras, J.L.; Muñoz-Rojas, J.; Fuentes, L.E.; Martínez, J.; et al. Chromium hyper-tolerant Bacillus sp. Mh778713 assists phytoremediation of heavy metals by mesquite trees (Prosopis laevigata). Front. Microbiol. 2019, 10, 1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buendía, L.; Cruz, F.; Rodríguez, M.E.; Barrera, C.E.; Hernández, C.; Orozco, J. In vitro simultaneous accumulation of multiple heavy metals by Prosopis laevigata seedlings cultures. Rev. Mex. Ing. Química 2019, 18, 1167–1177. [Google Scholar] [CrossRef]
- Pipíška, M.; Krajčíková, E.K.; Hvostik, M.; Frišták, V.; Ďuriška, L.; Černičková, I.; Kaňuchová, M.; Conte, P.; Soja, G. Biochar from wood chips and corn cobs for adsorption of thioflavin T and erythrosine B. Materials 2022, 15, 1492. [Google Scholar] [CrossRef]
- Oluk, I.; Nagawa, C.B.; Banadda, N.; Tumutegyereize, P.; Owusu, P.A. Development of maize cob-based biochar filter for water purification. Water Environ. J. 2021, 35, 349–358. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.L.; Zhang, J.; Zheng, L.; Chen, D.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Wang, H.; Wu, W. Coconut-fiber biochar reduced the bioavailability of lead but increased its translocation rate in rice plants: Elucidation of immobilization mechanisms and significance of iron plaque barrier on roots using spectroscopic techniques. J. Hazard. Mater. 2020, 389, 122117. [Google Scholar] [CrossRef] [PubMed]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Forms and solubility of plant nutrient elements in tropical plant waste biochars. J. Plant Nutr. Soil Sci. 2015, 178, 732–740. [Google Scholar] [CrossRef]
- Kimetu, J.M.; Lehmann, J.; Ngoze, S.O.; Mugendi, D.N.; Kinyangi, J.M.; Riha, S.; Verchot, L.; Recha, J.W.; Pell, A.N. Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 2008, 11, 726–739. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H.; Cleveland, C.C. Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: A meta-analysis. Sci. Total Environ. 2019, 654, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Mašek, O.; Brownsort, P. Biochar production. In An Assessment of the Benefits and Issues Associated with the Application of Biochar to Soil; Shackley, S., Sohi, S., Eds.; UK Biochar Research Centre: Edinburgh, UK, 2011; pp. 37–44. [Google Scholar]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef] [PubMed]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Macri, C.; Miard, F.; Hattab-Hambli, N.; Motelica-Heino, M.; Morabito, D.; Bourgerie, S. Effect of Biochar amendments on As and Pb mobility and phytoavailability in contaminated mine technosols phytoremediated by Salix. J. Geochem. Explor. 2017, 182, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Bini, C.; Wahsha, M.; Fontana, S.; Maleci, L. Effects of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J. Geochemical Explor. 2012, 123, 101–108. [Google Scholar] [CrossRef]
- Maldonado-Magaña, A.; Favela-Torres, E.; Rivera-Cabrera, F.; Volke-Sepulveda, T.L. Lead bioaccumulation in Acacia farnesiana and Its effect on lipid peroxidation and glutathione production. Plant Soil 2011, 339, 377–389. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Saffari, N.; Hajabbasi, M.A.; Shirani, H.; Mosaddeghi, M.R.; Owens, G. Influence of corn residue biochar on water retention and penetration resistance in a calcareous sandy loam soil. Geoderma 2021, 383, 114734. [Google Scholar] [CrossRef]
- Dhar, S.A.; Sakib, T.U.; Hilary, L.N. Effects of Pyrolysis temperature on production and physicochemical characterization of biochar derived from coconut fiber biomass through slow pyrolysis process. Biomass Convers. Biorefinery 2020, 12, 2631–2647. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, J.; Chen, R. Is There a strategy i iron uptake mechanism in maize? Plant Signal. Behav. 2018, 13, e1161877. [Google Scholar] [CrossRef] [Green Version]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, L.; Wang, S.L.; Wu, Z.; Wu, W.; Niazi, N.K.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.; Ok, Y.S. Sorption mechanisms of lead on silicon-rich biochar in aqueous solution: Spectroscopic investigation. Sci. Total Environ. 2019, 672, 572–582. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.L.; Zheng, L.; Chen, D.; Wu, Z.; Xie, Y.; Wu, W.; Niazi, N.K.; Ok, Y.S.; Rinklebe, J. Sorption of lead in soil amended with coconut fiber biochar: Geochemical and spectroscopic investigations. Geoderma 2019, 350, 52–60. [Google Scholar] [CrossRef]
- Eissa, M.A. Effect of Cow manure biochar on heavy metals uptake and translocation by zucchini (Cucurbita pepo L). Arab. J. Geosci. 2019, 12, 48. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Mn homeostasis in plants: Regulation of Mn Transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Farooq Qayyum, M.; Abbas, F.; Hannan, F.; Rinklebe, J.; Sik Ok, Y. Effect of Biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Abidine Triqui, Z.E.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468, 598–608. [Google Scholar] [CrossRef]
- Meers, E.; Ruttens, A.; Hopgood, M.; Lesage, E.; Tack, F.M.G. Potential of Brassic rapa, Cannabis sativa, Helianthus annuus and Zea mays for phytoextraction of heavy metals from calcareous dredged sediment derived soils. Chemosphere 2005, 61, 561–572. [Google Scholar] [CrossRef]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Felker, P.; Cannell, G.H.; Clark, P.R.; Osborn, J.F.; Nash, P. Biomass production of Prosopis species (mesquite), Leucaena, and other leguminous trees grown under heat/drought stress. For. Sci. 1983, 29, 592–606. [Google Scholar]
- Vangronsveld, J.; Cunningham, S.D. Introduction to the concepts. In Metal-Contaminated Soils: In-Situ Inactivation and Phytorestoration; Vangronsveld, J., Cunningham, S.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 1–15. [Google Scholar]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Colpaert, J.; Van Tichelen, K. Reclamation of a bare industrial area contaminated by non-ferrous metals: Physicochemical and biological evaluation of the durability of soil treatment and revegetation. Environ Pollut. 1996, 94, 131–140. [Google Scholar] [CrossRef]
- IBI. International Biochar Initiative, Comparison of European Biochar Certificate Version 4. 8 and IBI Biochar Standards Version 2.0. 2014. Available online: https://www.biochar-international.org/wp-content/uploads/2018/04/IBI-EBC_comparison_Oct2014.pdf (accessed on 1 December 2022).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2010. [Google Scholar]
- R Core Team. R: A Language and environment for statistical computing. R Found. Stat. Comput. 2020. Available online: https://www.R-project.org (accessed on 3 June 2022).
- Statsoft, Inc. Statistica for Windows; Statsoft, Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Zamora, J.; Mussali-Galante, P.; Rodríguez, A.; Castrejón-Godínez, M.L.; Valencia-Cuevas, L.; Tovar-Sánchez, E. Assisted Phytostabilization of Mine-Tailings with Prosopis laevigata (Fabaceae) and Biochar. Plants 2022, 11, 3441. https://doi.org/10.3390/plants11243441
Ramírez-Zamora J, Mussali-Galante P, Rodríguez A, Castrejón-Godínez ML, Valencia-Cuevas L, Tovar-Sánchez E. Assisted Phytostabilization of Mine-Tailings with Prosopis laevigata (Fabaceae) and Biochar. Plants. 2022; 11(24):3441. https://doi.org/10.3390/plants11243441
Chicago/Turabian StyleRamírez-Zamora, Juan, Patricia Mussali-Galante, Alexis Rodríguez, María Luisa Castrejón-Godínez, Leticia Valencia-Cuevas, and Efraín Tovar-Sánchez. 2022. "Assisted Phytostabilization of Mine-Tailings with Prosopis laevigata (Fabaceae) and Biochar" Plants 11, no. 24: 3441. https://doi.org/10.3390/plants11243441





