Combined Transcriptomic and Metabolomic Analysis Reveals Insights into Resistance of Arabidopsis bam3 Mutant against the Phytopathogenic Fungus Fusarium oxysporum
Abstract
:1. Introduction
2. Results
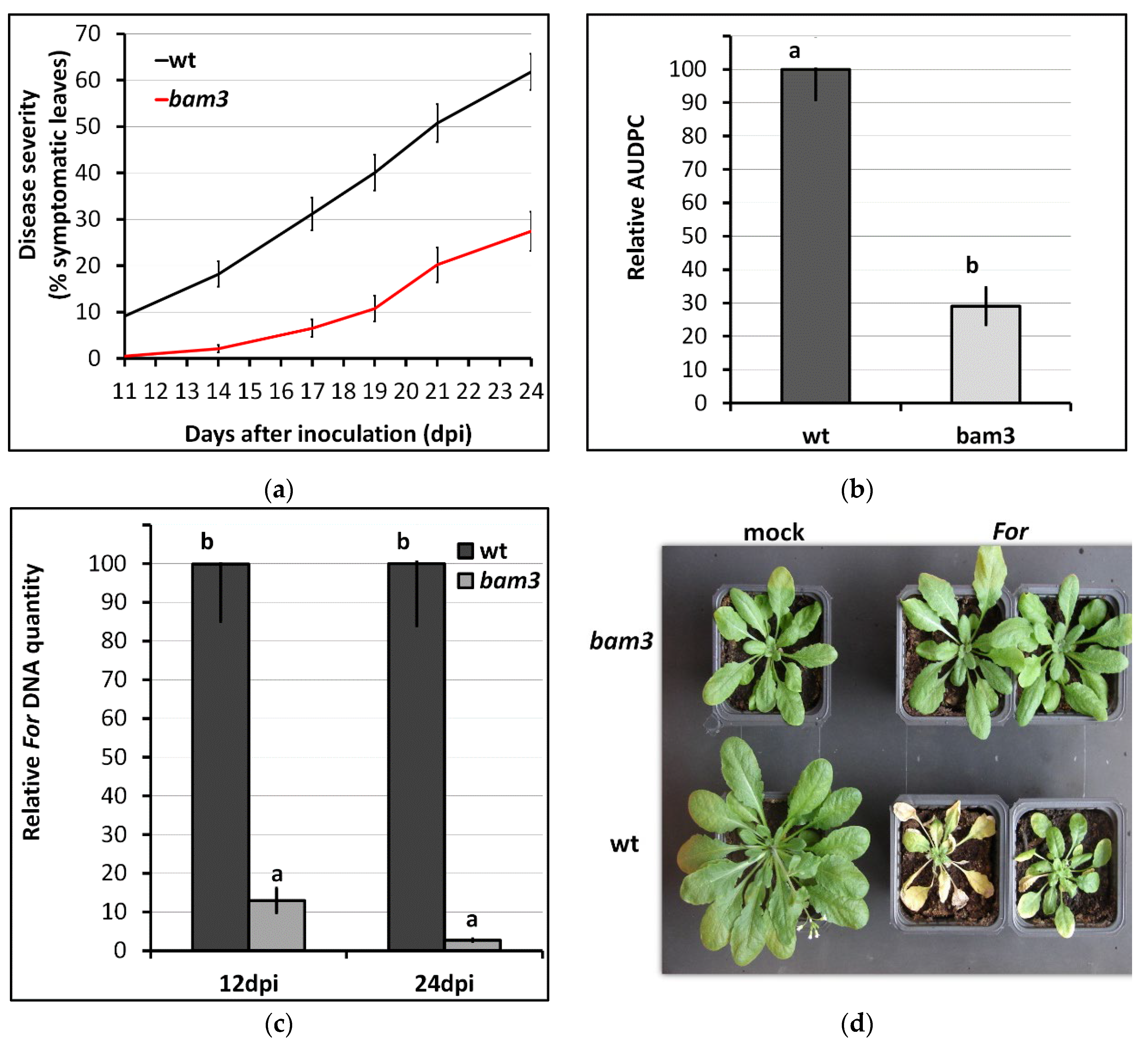
2.1. Disruption of BAM3 Gene Increased Resistance to F. oxysporum f. sp. raphani
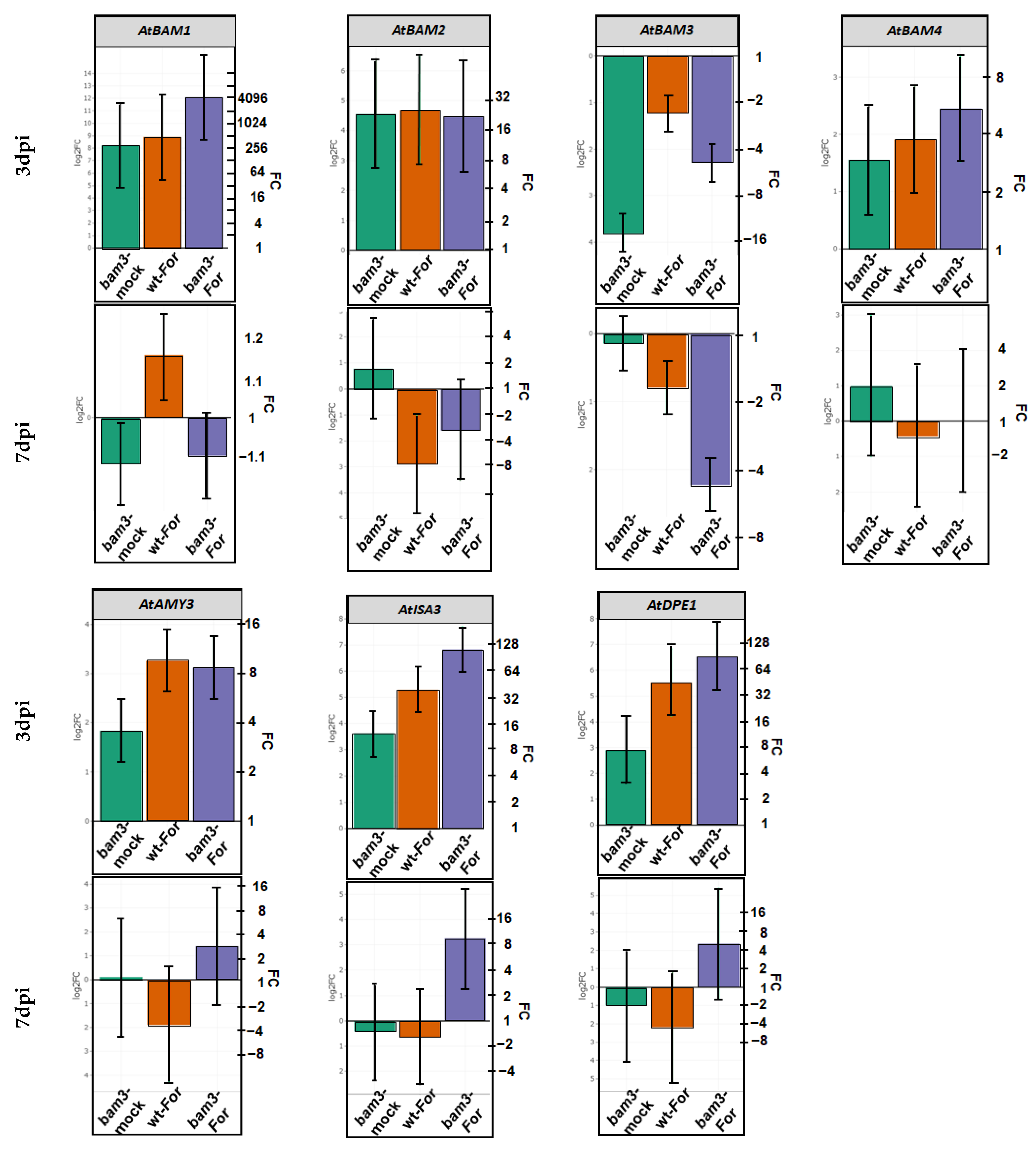
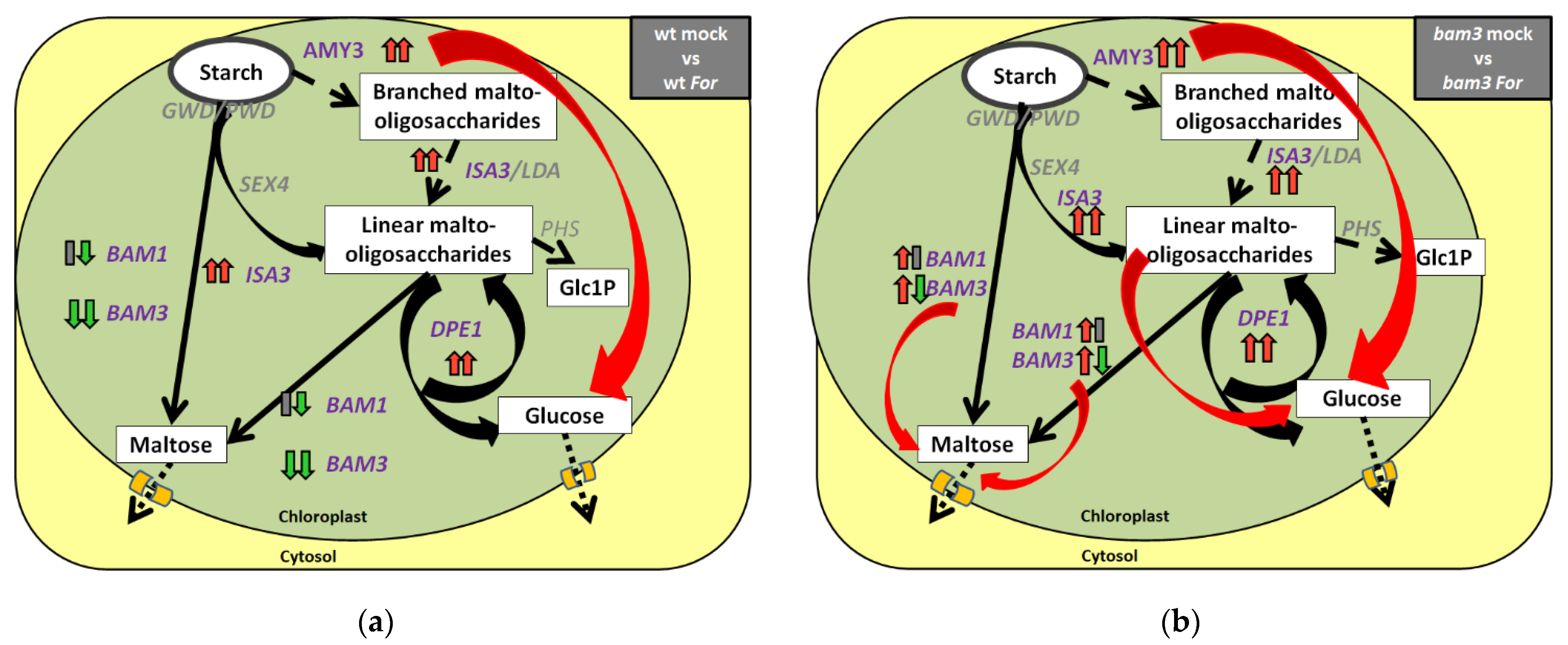
2.2. Expression Analysis of Starch-Degradation-Related Genes in bam3 Mutant in Response to F. oxysporum f. sp. raphani Inoculation
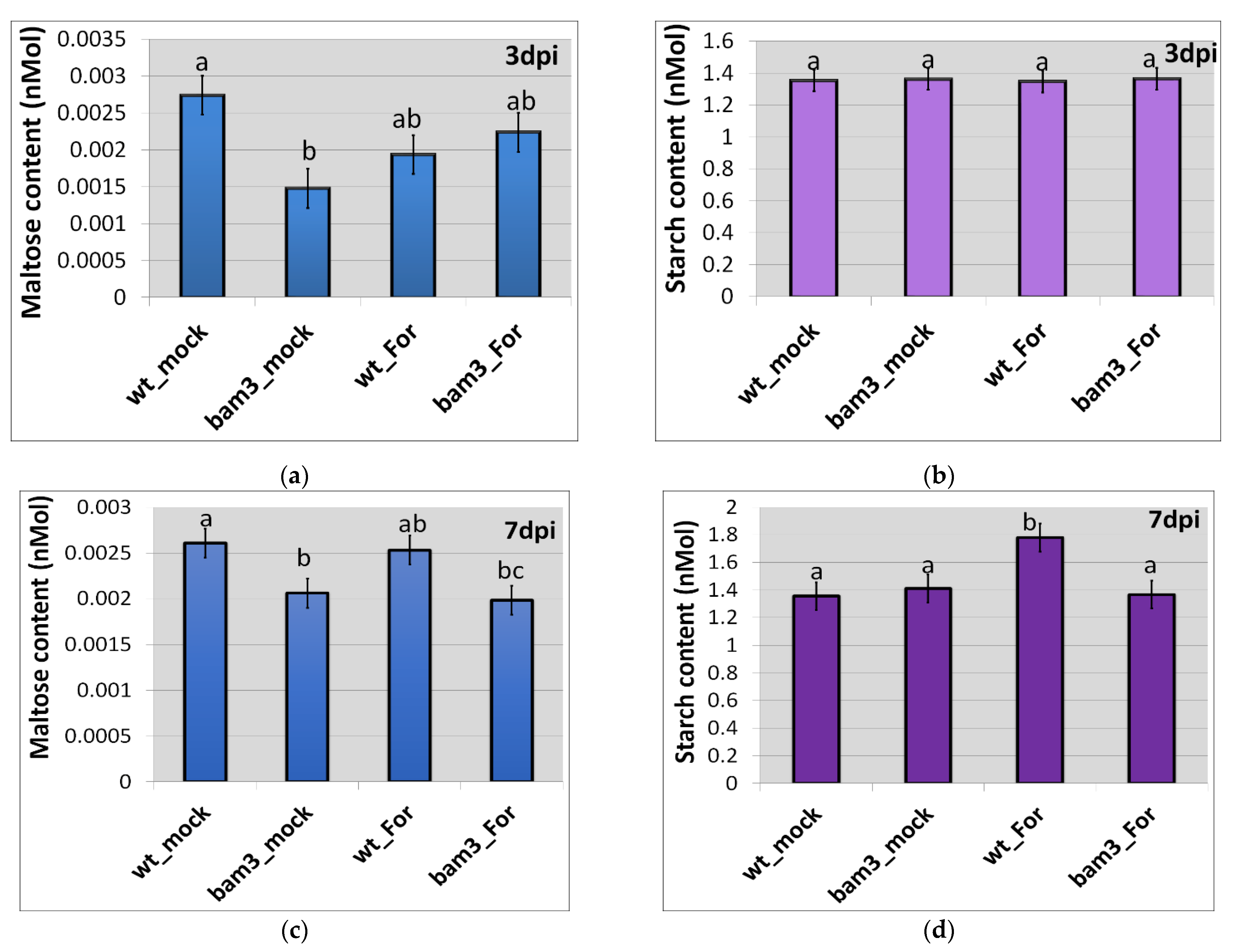
2.3. Maltose and Starch-Derived Glucose Contents in bam3 Mutant upon Inoculation with F. oxysporum f. sp. raphani
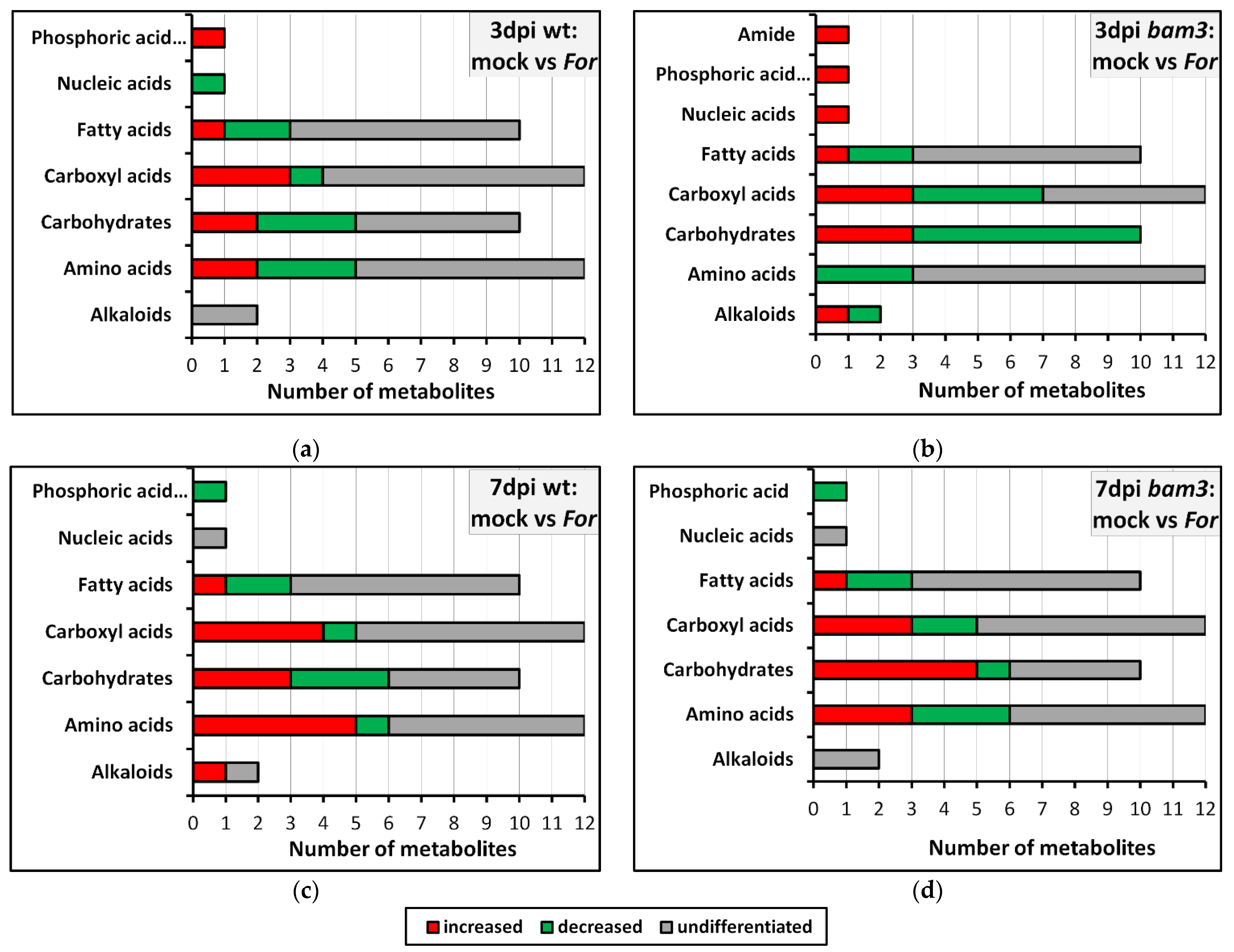
2.4. Metabolite Profiling of Inoculated and Noninoculated wt and bam3 Plants
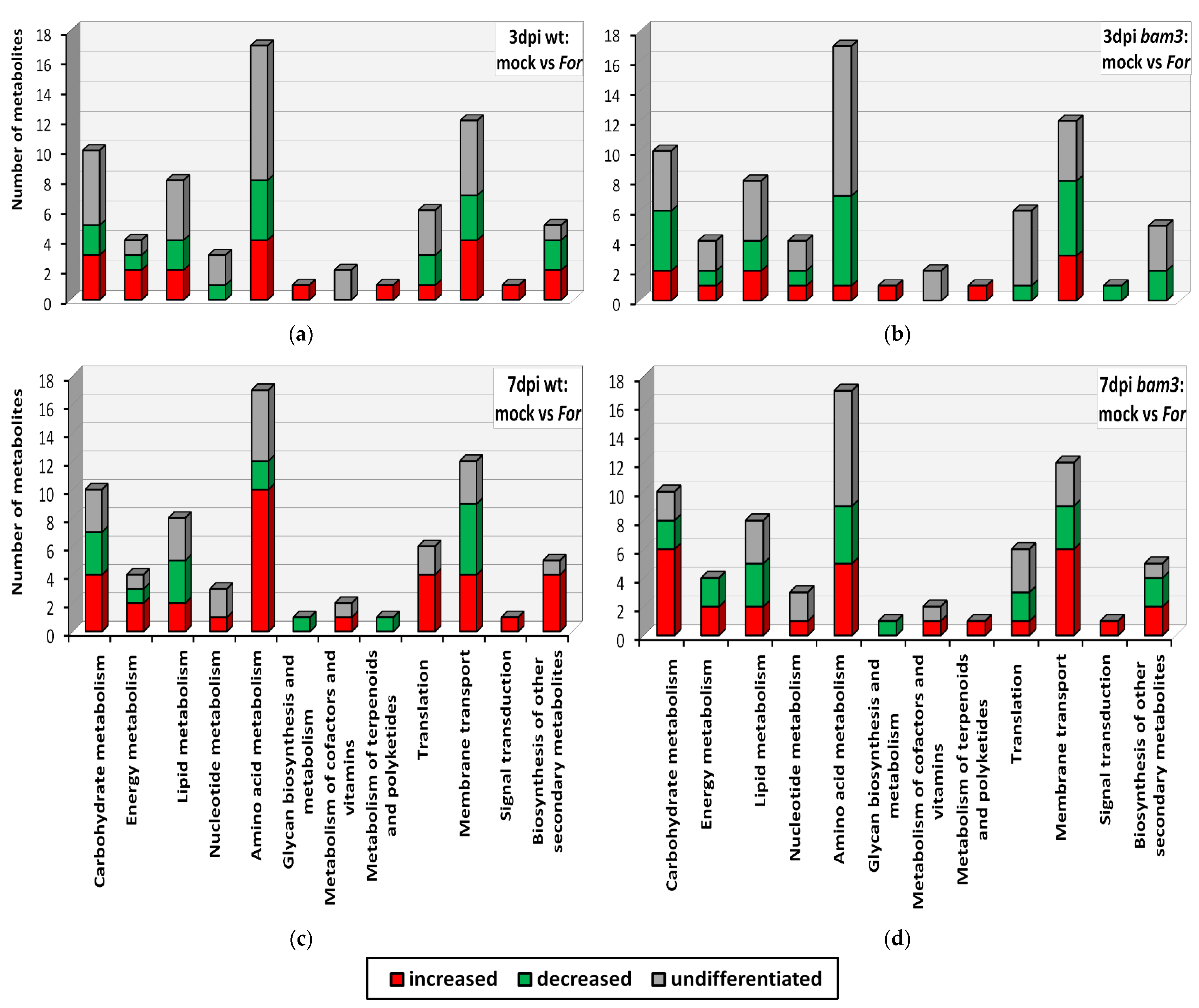
2.5. KEGG Classification and Enrichment Analysis of Differential Metabolites
2.6. Metabolic Biomarkers in Response to F. oxysporum f. sp. raphani in bam3 Mutants
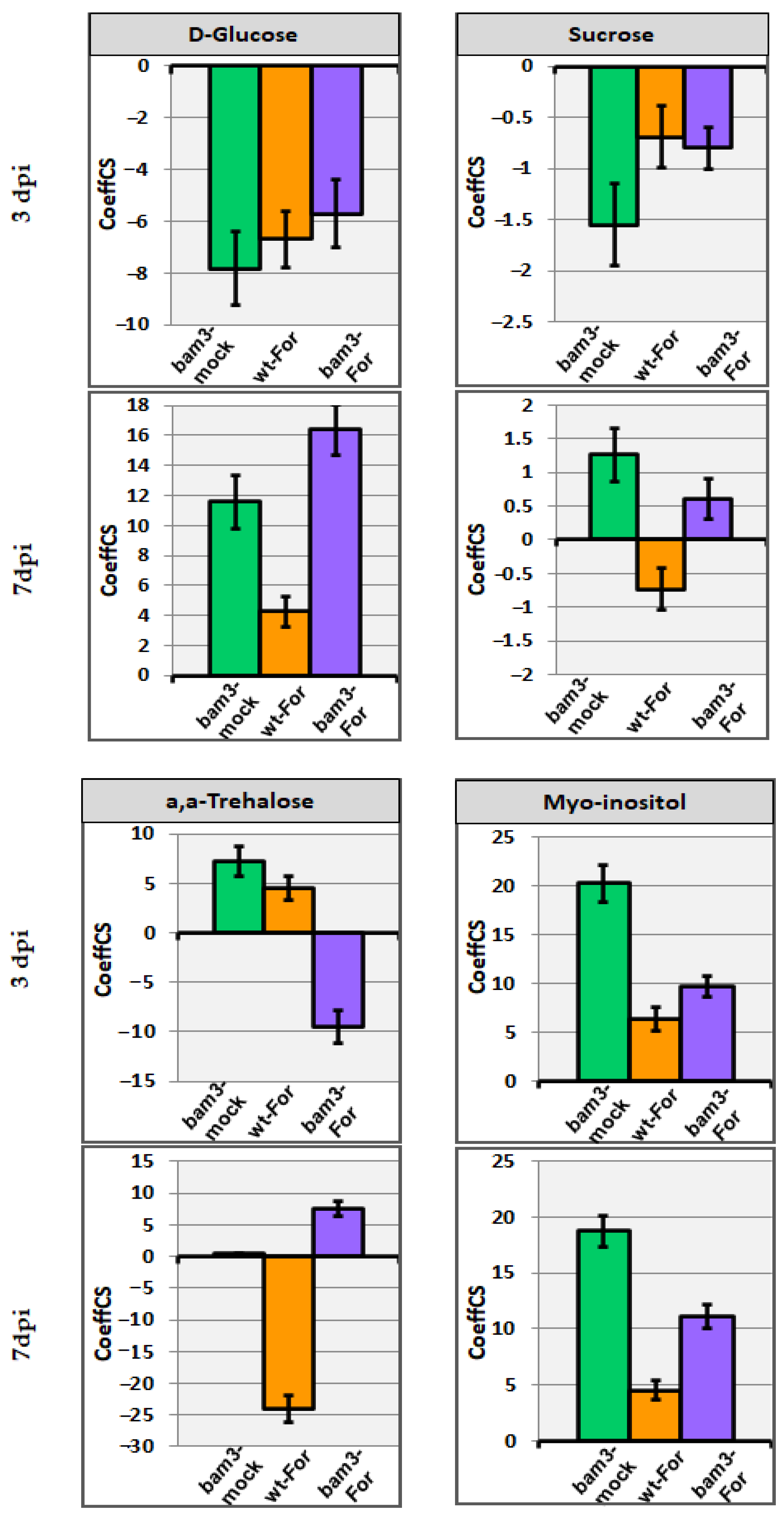
2.6.1. Carbohydrates and Related Biomarkers
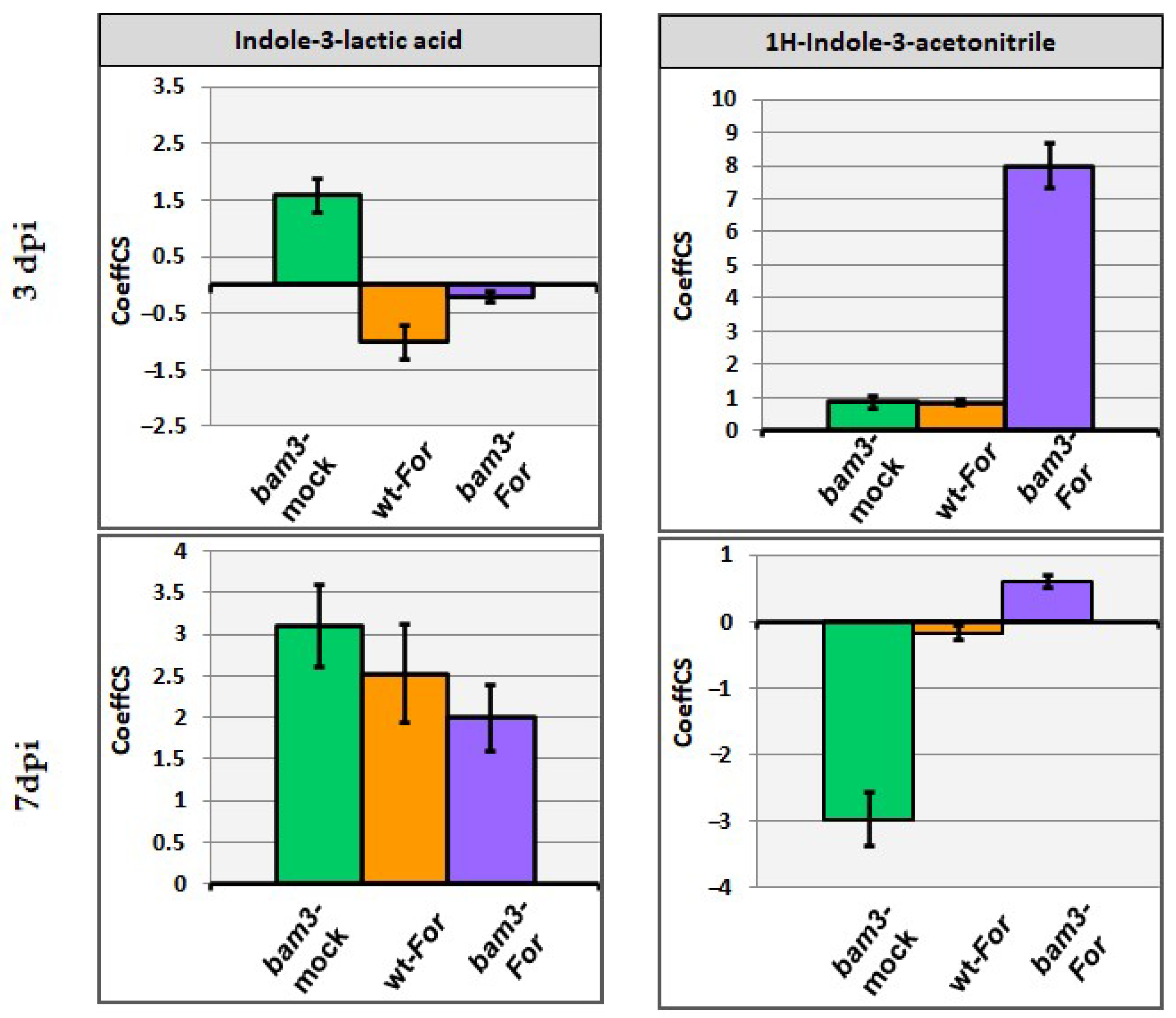
2.6.2. Indole-3-Lactic Acid and 1H-Indole-3-Acetonitrile as Biomarkers
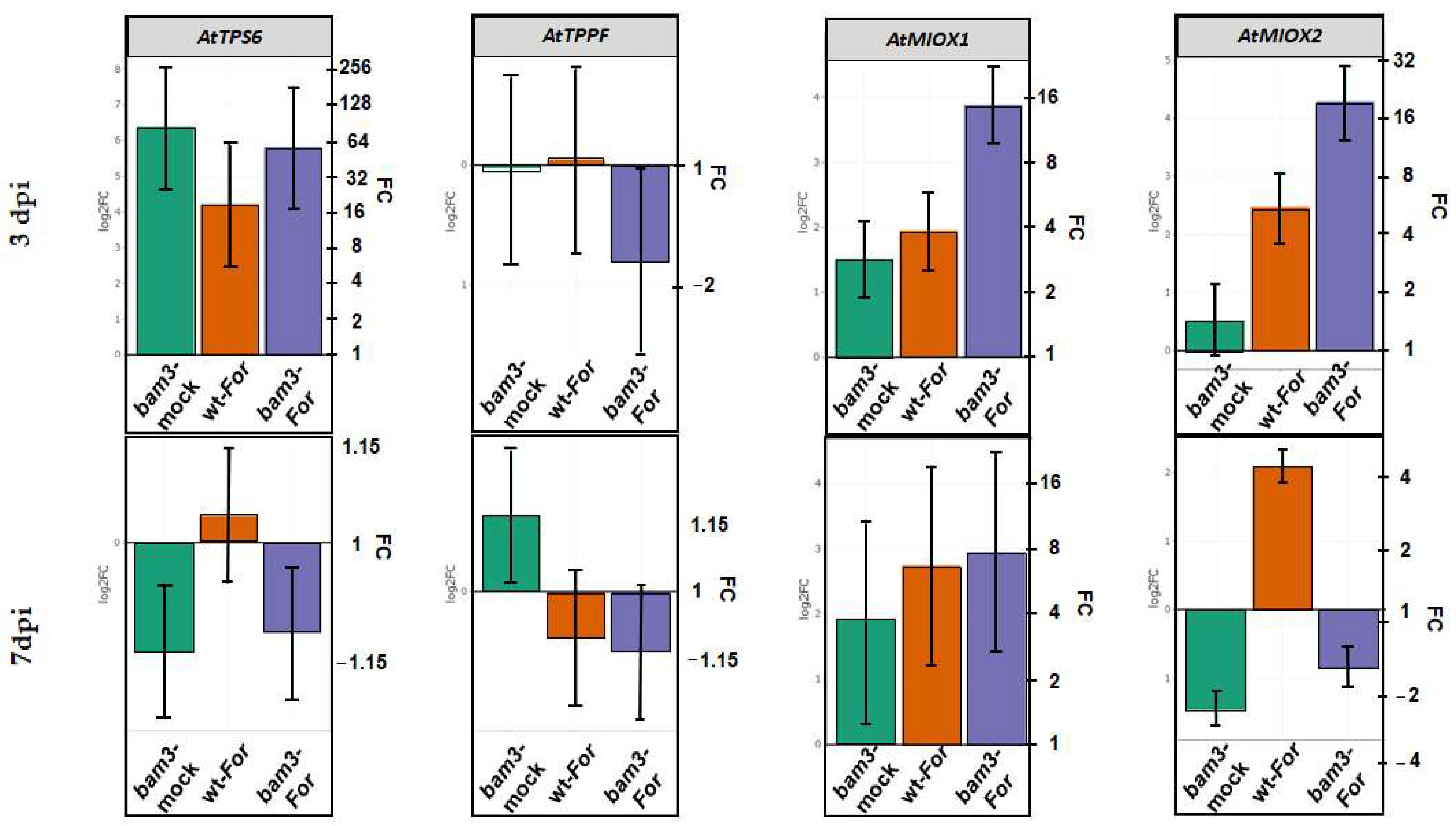
2.7. Expression of Arabidopsis Genes Related to Trehalose and Myo-Inositol Metabolism in bam3 Mutant in Response to F. oxysporum f. sp. raphani Inoculation
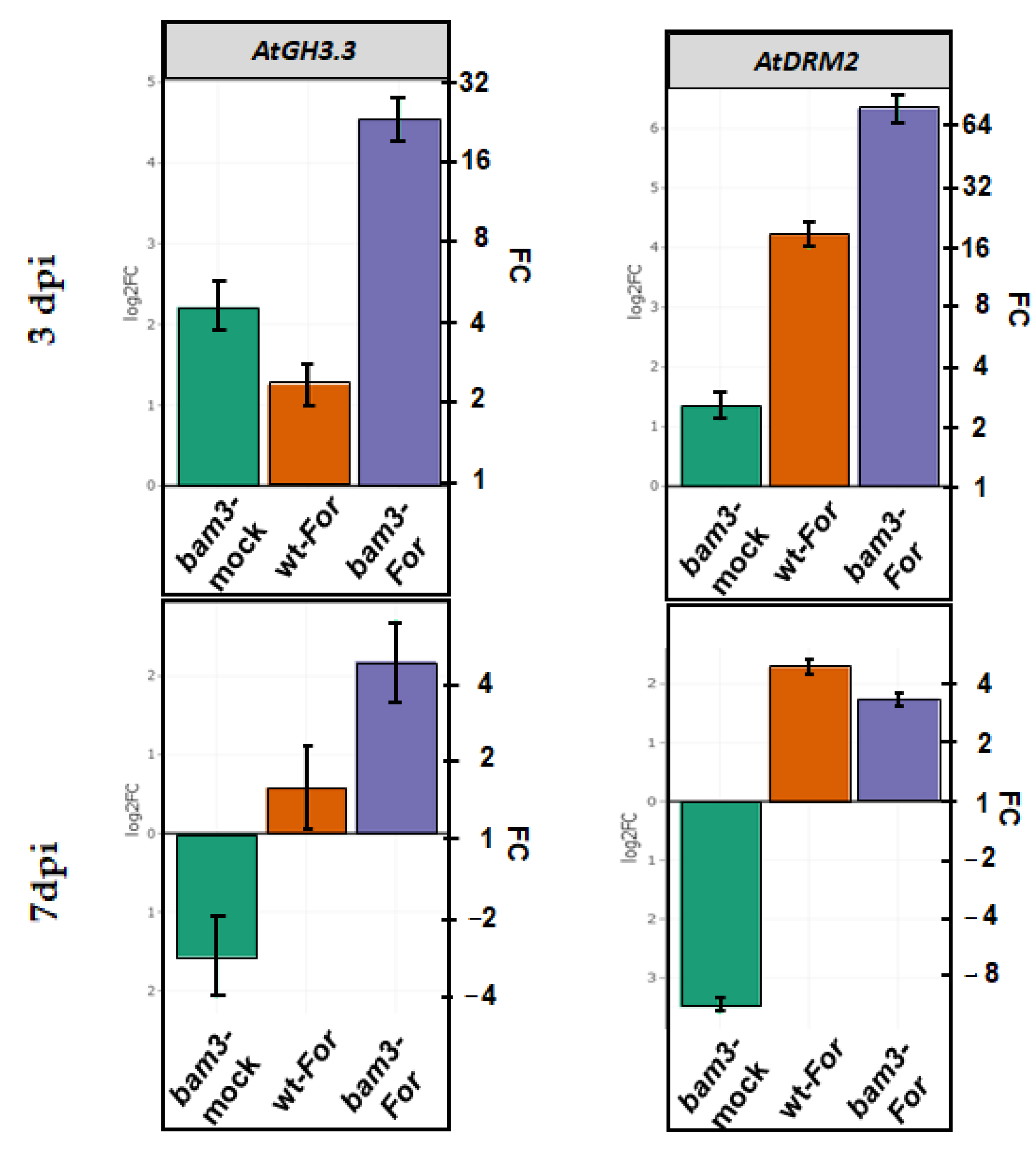
2.8. Expression of GH3.3 and DRM2 Genes Related to Auxin Metabolism in bam3 Mutants upon Inoculation with F. oxysporum f. sp. raphani
3. Discussion
4. Materials and Methods
4.1. Biological Material and Bioassays
4.2. Disease Assessment
4.3. Measurement of For Quantity in Inoculated Plants
4.4. Sampling and Metabolite Extraction
4.5. Chemical Analyses and Data Processing
4.6. Quality Control of Metabolomic Analyses
4.7. Statistical Analyses
4.8. RNA Isolation and Gene-Expression Analysis
4.9. Quantification of Maltose and Starch-Derived Glucose Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Pietro, A.; García-MacEira, F.I.; Méglecz, E.; Roncero, M.I. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C. Fusarium Wilt of Banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.; Karley, A.J.; Gregory, P.J.; Brennan, R.M. Editorial: Crop Traits for Defense against Pests and Disease: Durability, Breakdown and Future Prospects. Front. Plant Sci. 2017, 8, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, C.M.; Esenthil-Kumar, M.; Etzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Streb, S.; Zeeman, S.C. Starch Metabolism in Arabidopsis. Arab. Book 2012, 10, e0160. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D. Involvement of five catalytically active Arabidopsis β-amylases in leaf starch metabolism and plant growth. Plant Direct 2020, 4, e00199. [Google Scholar] [CrossRef] [Green Version]
- Ceusters, N.; Frans, M.; Ende, W.V.D.; Ceusters, J. Maltose Processing and Not β-Amylase Activity Curtails Hydrolytic Starch Degradation in the CAM Orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1386. [Google Scholar] [CrossRef]
- Fulton, D.C.; Stettler, M.; Mettler, T.; Vaughan, C.K.; Li, J.; Francisco, P.; Gil, M.; Reinhold, H.; Eicke, S.; Messerli, G.; et al. β-AMYLASE4, a Noncatalytic Protein Required for Starch Breakdown, Acts Upstream of Three Active β-Amylases in Arabidopsis Chloroplasts. Plant Cell 2008, 20, 1040–1058. [Google Scholar] [CrossRef] [Green Version]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef] [PubMed]
- Formela-Luboińska, M.; Chadzinikolau, T.; Drzewiecka, K.; Jeleń, H.; Bocianowski, J.; Kęsy, J.; Labudda, M.; Jeandet, P.; Morkunas, I. The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. Int. J. Mol. Sci. 2020, 21, 4133. [Google Scholar] [CrossRef] [PubMed]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-Phosphate: Connecting Plant Metabolism and Development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, G.; Aeschbacher, R.A.; Müller, J.; Boller, T.; Wiemken, A. Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: Identification by functional complementation of the yeast tps2 mutant. Plant J. 1998, 13, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, B.N.; Harper, C. The roles of auxin during interactions between bacterial plant pathogens and their hosts. J. Exp. Bot. 2017, 69, 245–254. [Google Scholar] [CrossRef]
- Ostrowski, M.; Mierek-Adamska, A.; Porowińska, D.; Goc, A.; Jakubowska, A. Cloning and biochemical characterization of indole-3-acetic acid-amino acid synthetase PsGH3 from pea. Plant Physiol. Biochem. 2016, 107, 9–20. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Zhang, N.; Zhong, T.; Wang, C.; Xu, M.-L.; Ye, J.-R. Identification and characterization of the GH3 gene family in maize. J. Integr. Agric. 2016, 15, 249–261. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Liu, X.-T.; Chen, Y.; Xu, X.-J.; Yu, B.; Zhang, S.-Q.; Li, Q.; He, Z.-H. Arabidopsis Acetyl-Amido Synthetase GH3.5 Involvement in Camalexin Biosynthesis through Conjugation of Indole-3-Carboxylic Acid and Cysteine and Upregulation of Camalexin Biosynthesis Genes. J. Integr. Plant Biol. 2012, 54, 471–485. [Google Scholar] [CrossRef]
- Gkizi, D.; Santos-Rufo, A.; Rodríguez-Jurado, D.; Paplomatas, E.J.; Tjamos, S.E. The β-amylase genes: Negative regulators of disease resistance forVerticillium dahliae. Plant Pathol. 2015, 64, 1484–1490. [Google Scholar] [CrossRef]
- Perlikowski, D.; Wiśniewska, H.; Kaczmarek, J.; Góral, T.; Ochodzki, P.; Kwiatek, M.; Majka, M.; Augustyniak, A.; Kosmala, A. Alterations in Kernel Proteome after Infection with Fusarium culmorum in Two Triticale Cultivars with Contrasting Resistance to Fusarium Head Blight. Front. Plant Sci. 2016, 7, 1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horrer, D.; Flütsch, S.; Pazmino, D.; Matthews, J.; Thalmann, M.; Nigro, A.; Leonhardt, N.; Lawson, T.; Santelia, D. Blue Light Induces a Distinct Starch Degradation Pathway in Guard Cells for Stomatal Opening. Curr. Biol. 2016, 26, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flütsch, S.; Wang, Y.; Takemiya, A.; Vialet-Chabrand, S.R.M.; Klejchová, M.; Nigro, A.; Hills, A.; Lawson, T.; Blatt, M.R.; Santelia, D. Guard Cell Starch Degradation Yields Glucose for Rapid Stomatal Opening in Arabidopsis. Plant Cell 2020, 32, 2325–2344. [Google Scholar] [CrossRef] [PubMed]
- Gamir, J.; Pastor, V.; Sánchez-Bel, P.; Agut, B.; Mateu, D.; García-Andrade, J.; Flors, V. Starch degradation, abscisic acid and vesicular trafficking are important elements in callose priming by indole-3-carboxylic acid in response to Plectosphaerella cucumerina infection. Plant J. 2018, 96, 518–531. [Google Scholar] [CrossRef] [Green Version]
- O′Neill, E.C.; Stevenson, C.E.; Tantanarat, K.; Latousakis, D.; Donaldson, M.I.; Rejzek, M.; Nepogodiev, S.A.; Limpaseni, T.; Field, R.A.; Lawson, D.M. Structural Dissection of the Maltodextrin Disproportionation Cycle of the Arabidopsis Plastidial Disproportionating Enzyme 1 (DPE1). J. Biol. Chem. 2015, 290, 29834–29853. [Google Scholar] [CrossRef] [Green Version]
- Granot, D.; David-Schwartz, R.; Kelly, G. Hexose Kinases and Their Role in Sugar-Sensing and Plant Development. Front. Plant Sci. 2013, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Blázquez, M.A.; Santos, E.; Flores, C.; Martínez-Zapater, J.M.; Salinas, J.; Gancedo, C. Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J. 1998, 13, 685–689. [Google Scholar] [CrossRef]
- Chary, S.N.; Hicks, G.R.; Choi, Y.G.; Carter, D.; Raikhel, N.V. Trehalose-6-Phosphate Synthase/Phosphatase Regulates Cell Shape and Plant Architecture in Arabidopsis. Plant Physiol. 2008, 146, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.C.M.; Hejazi, M.; Fettke, J.; Steup, M.; Feil, R.; Krause, U.; Arrivault, S.; Vosloh, D.; Figueroa, C.M.; Ivakov, A.; et al. Feedback Inhibition of Starch Degradation in Arabidopsis Leaves Mediated by Trehalose 6-Phosphate. Plant Physiol. 2013, 163, 1142–1163. [Google Scholar] [CrossRef] [Green Version]
- Hulsmans, S.; Rodriguez, M.S.; De Coninck, B.; Rolland, F. The SnRK1 Energy Sensor in Plant Biotic Interactions. Trends Plant Sci. 2016, 21, 648–661. [Google Scholar] [CrossRef]
- Chaliha, C.; Rugen, M.D.; Field, R.A.; Kalita, E. Glycans as Modulators of Plant Defense Against Filamentous Pathogens. Front. Plant Sci. 2018, 9, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, W.; Ren, W.; Kong, L.; Zhang, W.; Wang, T. Transcriptomic Profiling Analysis of Arabidopsis thaliana Treated with Exogenous Myo-Inositol. PLoS ONE 2016, 11, e0161949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaouch, S.; Noctor, G. Myo -inositol abolishes salicylic acid-dependent cell death and pathogen defence responses triggered by peroxisomal hydrogen peroxide. New Phytol. 2010, 188, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.H.; Raynaud, C.; Tcherkez, G.; Blanchet, S.; Massoud, K.; Domenichini, S.; Henry, Y.; Soubigou-Taconnat, L.; Lelarge-Trouverie, C.; Saindrenan, P.; et al. Crosstalks between Myo-Inositol Metabolism, Programmed Cell Death and Basal Immunity in Arabidopsis. PLoS ONE 2009, 4, e7364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosblech, A.; König, S.; Stenzel, I.; Grzeganek, P.; Feussner, I.; Heilmann, I. Phosphoinositide and Inositolpolyphosphate Signalling in Defense Responses of Arabidopsis thaliana Challenged by Mechanical Wounding. Mol. Plant 2008, 1, 249–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.M.; Otto, B.; Brearley, C.A.; Carr, J.P.; Hanke, D.E. A role for inositol hexakisphosphate in the maintenance of basal resistance to plant pathogens. Plant J. 2008, 56, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.; Hofmann, U.; Ludwig-Müller, J. Indole-3-Acetic Acid Is Synthesized by the Endophyte Cyanodermella asteris via a Tryptophan-Dependent and -Independent Way and Mediates the Interaction with a Non-Host Plant. Int. J. Mol. Sci. 2021, 22, 2651. [Google Scholar] [CrossRef]
- Peat, T.; Böttcher, C.; Newman, J.; Lucent, D.; Cowieson, N.; Davies, C. Crystal Structure of an Indole-3-Acetic Acid Amido Synthetase from Grapevine Involved in Auxin Homeostasis. Plant Cell 2012, 24, 4525–4538. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-E.; Park, J.-Y.; Kim, Y.-S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.-Y.; Kim, J.; Lee, Y.-H.; Park, C.-M. GH3-mediated Auxin Homeostasis Links Growth Regulation with Stress Adaptation Response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef] [Green Version]
- Rae, G.M.; David, K.; Wood, M. The Dormancy Marker DRM1/ARP Associated with Dormancy but a Broader Role In Planta. Dev. Biol. J. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Roy, S.; Saxena, S.; Sinha, A.; Nandi, A.K. DORMANCY/AUXIN ASSOCIATED FAMILY PROTEIN 2 of Arabidopsis thaliana is a negative regulator of local and systemic acquired resistance. J. Plant Res. 2020, 133, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kidd, B.N.; Kadoo, N.Y.; Dombrecht, B.; Tekeoglu, M.; Gardiner, D.M.; Thatcher, L.F.; Aitken, E.A.B.; Schenk, P.M.; Manners, J.M.; Kazan, K. Auxin Signaling and Transport Promote Susceptibility to the Root-Infecting Fungal Pathogen Fusarium oxysporum in Arabidopsis. Mol. Plant-Microbe Interact.® 2011, 24, 733–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazan, K.; Manners, J.M. Linking development to defense: Auxin in plant–pathogen interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-L.; Yang, Y.; He, Z. Roles of Plant Hormones and Their Interplay in Rice Immunity. Mol. Plant 2013, 6, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiol. Plant. 2021, 174, e13546. [Google Scholar] [CrossRef]
- Leeman, M.; van Pelt, J.A.; Ouden, F.M.D.; Heinsbroek, M.; Bakker, P.A.H.M.; Schippers, B. Induction of systemic resistance by Pseudomonas fluorescens in radish cultivars differing in susceptibility to fusarium wilt, using a novel bioassay. Eur. J. Plant Pathol. 1995, 101, 655–664. [Google Scholar] [CrossRef]
- Pantelides, I.; Tjamos, S.E.; Pappa, S.; Kargakis, M.; Paplomatas, E.J. The ethylene receptor ETR1 is required for Fusarium oxysporum pathogenicity. Plant Pathol. 2013, 62, 1302–1309. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990; Available online: https://www.cabdirect.org/cabdirect/abstract/19912305030 (accessed on 15 June 2020).
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant. Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Czechowski, T.; Stitt, M.; Altmann, T.; Udvardi, M.; Scheible, W.-R. Genome-Wide Identification and Testing of Superior Reference Genes for Transcript Normalization in Arabidopsis. Plant Physiol. 2005, 139, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, J.; Jiang, J.; Chen, S.; Guan, Z.; Liao, Y.; Chen, F. Reference genes for normalizing transcription in diploid and tetraploid Arabidopsis. Sci. Rep. 2015, 4, 6781. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A Metabolic Profiling Strategy for the Dissection of Plant Defense against Fungal Pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliferis, K.A.; Jabaji, S. FT-ICR/MS and GC-EI/MS Metabolomics Networking Unravels Global Potato Sprout′s Responses to Rhizoctonia solani Infection. PLoS ONE 2012, 7, e42576. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikstrőm, C. Multi and Megavariate Data Analysis: Basic Principles and Applications; Umetrics Academy: Malmö, Sweden, 2013. [Google Scholar]
- Campbell, E.J.; Schenk, P.M.; Kazan, K.; Penninckx, I.A.; Anderson, J.; Maclean, D.J.; Cammue, B.P.; Ebert, P.; Manners, J.M. Pathogen-Responsive Expression of a Putative ATP-Binding Cassette Transporter Gene Conferring Resistance to the Diterpenoid Sclareol Is Regulated by Multiple Defense Signaling Pathways in Arabidopsis. Plant Physiol. 2003, 133, 1272–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, R.; Stiller, J.; Powell, J.; Rusu, A.; Manners, J.M.; Kazan, K. Fusarium oxysporum Triggers Tissue-Specific Transcriptional Reprogramming in Arabidopsis thaliana. PLoS ONE 2015, 10, e0121902. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, L.F.; Cevik, V.; Grant, M.; Zhai, B.; Jones, J.D.G.; Manners, J.M.; Kazan, K. Characterization of aJAZ7activation-tagged Arabidopsis mutant with increased susceptibility to the fungal pathogen Fusarium oxysporum. J. Exp. Bot. 2016, 67, 2367–2386. [Google Scholar] [CrossRef] [Green Version]
- Fallath, T.; Kidd, B.N.; Stiller, J.; Davoine, C.; Björklund, S.; Manners, J.M.; Kazan, K.; Schenk, P.M. MEDIATOR18 and MEDIATOR20 confer susceptibility to Fusarium oxysporum in Arabidopsis thaliana. PLoS ONE 2017, 12, e0176022. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Baek, W.; Lim, C.W.; Lee, S.C. Functional analysis of the pepper protein phosphatase, CaAIPP1, and its interacting partner CaAIRF1: Modulation of ABA signalling and the drought stress response. Plant Cell Environ. 2017, 40, 2359–2368. [Google Scholar] [CrossRef]
- Steibel, J.P.; Poletto, R.; Coussens, P.M.; Rosa, G.J. A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics 2009, 94, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H.; Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package 2022, Version, 1 (7), p. 2. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 8 November 2022).
- Ritchie, M.E.; Belinda, P.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 19 July 2011).
- Smirnova, J.; Fernie, A.R.; Spahn, C.M.; Steup, M. Photometric assay of maltose and maltose-forming enzyme activity by using 4-alpha-glucanotransferase (DPE2) from higher plants. Anal. Biochem. 2017, 532, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Liu, Y.-J.; Wang, C.-L.; Zeng, Q.-Y. Molecular Evolution of Trehalose-6-Phosphate Synthase (TPS) Gene Family in Populus, Arabidopsis and Rice. PLoS ONE 2012, 7, e42438. [Google Scholar] [CrossRef] [PubMed]
- Lisso, J.; Schröder, F.; Müssig, C. EXO modifies sucrose and trehalose responses and connects the extracellular carbon status to growth. Front. Plant Sci. 2013, 4, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endres, S.; Tenhaken, R. Down-regulation of the myo-inositol oxygenase gene family has no effect on cell wall composition in Arabidopsis. Planta 2011, 234, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D.; Storm, A.R.; Badley, E.M.; Lehman, M.D.; Platt, S.M.; Saunders, L.K.; Schmitz, J.M.; Torres, C.E. β-Amylase1 and β-Amylase3 Are Plastidic Starch Hydrolases in Arabidopsis That Seem to Be Adapted for Different Thermal, pH, and Stress Conditions. Plant Physiol. 2014, 166, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.-Q.; Liu, L.-P.; Binari, R.; Hardy, R.; Shim, H.-S.; Cavallaro, A.; Booker, M.; Pfeiffer, B.D.; Markstein, M.; Wang, H.; et al. A Drosophila Resource of Transgenic RNAi Lines for Neurogenetics. Genetics 2009, 182, 1089–1100. [Google Scholar] [CrossRef] [Green Version]
- Guan, C.; Huang, Y.-H.; Cui, X.; Liu, S.-J.; Zhou, Y.-Z.; Zhang, Y.-W. Overexpression of gene encoding the key enzyme involved in proline-biosynthesis (PuP5CS) to improve salt tolerance in switchgrass (Panicum virgatum L.). Plant Cell Rep. 2018, 37, 1187–1199. [Google Scholar] [CrossRef]
- Liu, L.; Sonbol, F.-M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [Green Version]
- Poupin, M.J.; Greve, M.; Carmona, V.; Pinedo, I. A Complex Molecular Interplay of Auxin and Ethylene Signaling Pathways Is Involved in Arabidopsis Growth Promotion by Burkholderia phytofirmans PsJN. Front. Plant Sci. 2016, 7, 492. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogeropoulou, E.; Aliferis, K.A.; Tjamos, S.E.; Vloutoglou, I.; Paplomatas, E.J. Combined Transcriptomic and Metabolomic Analysis Reveals Insights into Resistance of Arabidopsis bam3 Mutant against the Phytopathogenic Fungus Fusarium oxysporum. Plants 2022, 11, 3457. https://doi.org/10.3390/plants11243457
Kalogeropoulou E, Aliferis KA, Tjamos SE, Vloutoglou I, Paplomatas EJ. Combined Transcriptomic and Metabolomic Analysis Reveals Insights into Resistance of Arabidopsis bam3 Mutant against the Phytopathogenic Fungus Fusarium oxysporum. Plants. 2022; 11(24):3457. https://doi.org/10.3390/plants11243457
Chicago/Turabian StyleKalogeropoulou, Eleni, Konstantinos A. Aliferis, Sotirios E. Tjamos, Irene Vloutoglou, and Epaminondas J. Paplomatas. 2022. "Combined Transcriptomic and Metabolomic Analysis Reveals Insights into Resistance of Arabidopsis bam3 Mutant against the Phytopathogenic Fungus Fusarium oxysporum" Plants 11, no. 24: 3457. https://doi.org/10.3390/plants11243457




