The Role of γ-Aminobutyric Acid (GABA) in the Occurrence of Adventitious Roots and Somatic Embryos in Woody Plants
Abstract
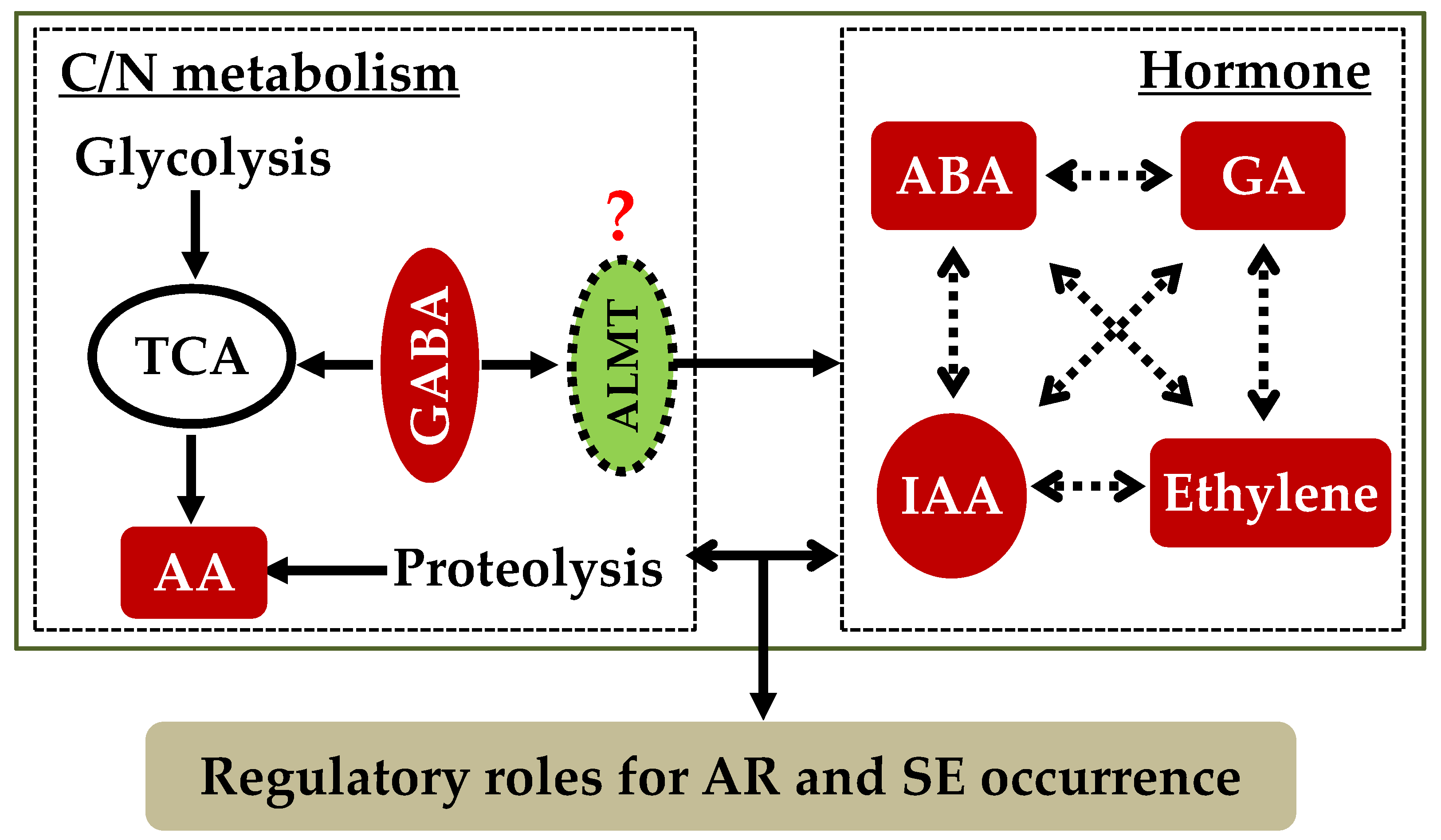
:1. Introduction
2. GABA and Phytohormones during AR Development
2.1. Auxin
2.2. Abscisic Acid and Ethylene
2.3. Gibberellin
3. GABA and Carbon/Nitrogen Metabolism during AR Development
3.1. Carbon Metabolism
3.2. Nitrogen Metabolism
4. GABA and Somatic Embryogenesis
5. Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.-W.; Xue, L.; Xu, S.; Feng, H.; An, L. Mediators, Genes and Signaling in Adventitious Rooting. Bot. Rev. 2009, 75, 230–247. [Google Scholar] [CrossRef]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious Roots and Lateral Roots: Similarities and Differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.B.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, T.; Ji, J.; Chen, W.; Yue, J.; Du, C.; Sun, J.; Chen, L.; Jiang, Z.; Shi, S. GABA negatively regulates adventitious root development in poplar. J. Exp. Bot. 2019, 71, 1459–1474. [Google Scholar] [CrossRef]
- Tahir, M.M.; Tong, L.; Fan, L.; Liu, Z.; Li, S.; Zhang, X.; Li, K.; Shao, Y.; Zhang, D.; Mao, J. Insights into the complicated networks contribute to adventitious rooting in transgenic MdWOX11 apple microshoots under nitrate treatments. Plant Cell Environ. 2022, 45, 3134–3156. [Google Scholar] [CrossRef]
- Druege, U.; Zerche, S.B.; Kadner, R.; Ernst, M. Relation between Nitrogen Status, Carbohydrate Distribution and Subsequent Rooting of Chrysanthemum Cuttings as Affected by Pre-harvest Nitrogen Supply and Cold-storage. Ann. Bot. 2000, 85, 687–701. [Google Scholar] [CrossRef] [Green Version]
- Zerche, S.; Haensch, K.-T.; Druege, U.; Hajirezaei, M.-R. Nitrogen remobilisation facilitates adventitious root formation on reversible dark-induced carbohydrate depletion in Petunia hybrida. BMC Plant Biol. 2016, 16, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Du, C.; Ji, J.; Xie, T.; Chen, W.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Inhibition of α-ketoglutarate dehydrogenase activity affects adventitious root growth in poplar via changes in GABA shunt. Planta 2018, 248, 963–979. [Google Scholar] [CrossRef]
- Druege, U. Overcoming Physiological Bottlenecks of Leaf Vitality and Root Development in Cuttings: A Systemic Perspective. Front. Plant Sci. 2020, 11, 907. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, T.; Yang, H.; Yang, S.; Wang, J. Overexpression of a SHORT-ROOT transcriptional factor enhances the auxin mediated formation of adventitious roots and lateral roots in poplar trees. Plant Sci. 2022, 323, 111408. [Google Scholar] [CrossRef] [PubMed]
- Mauriat, M.; Petterle, A.; Bellini, C.; Moritz, T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014, 78, 372–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, X.; Shen, F.; Li, W.; Qiu, C.; Wu, T.; Wang, Y.; Xu, X.; Han, Z.; Zhang, X. γ-Aminobutyric Acid Participates in the Adult-Phase Adventitious Rooting Recalcitrance. J. Plant Growth Regul. 2020, 40, 1981–1991. [Google Scholar] [CrossRef]
- Molina-Rueda, J.J.; Pascual, M.B.; Pissarra, J.; Gallardo, F. A putative role for γ-aminobutyric acid (GABA) in vascular development in pine seedlings. Planta 2014, 241, 257–267. [Google Scholar] [CrossRef]
- Smertenko, A.; Bozhkov, P. Somatic embryogenesis: Life and death processes during apical–basal patterning. J. Exp. Bot. 2014, 65, 1343–1360. [Google Scholar] [CrossRef] [Green Version]
- Verdeil, J.L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef]
- Corredoira, E.; Merkle, S.A.; Martínez, M.T.; Toribio, M.; Canhoto, J.M.; Correia, S.I.; Ballester, A.; Vieitez, A.M. Non-Zygotic Embryogenesis in Hardwood Species. Crit. Rev. Plant Sci. 2019, 38, 29–97. [Google Scholar] [CrossRef]
- Corredoira, E.; Valladares, S.; Viéitez, A.M.; Ballester, A. Improved germination of somatic embryos and plant recovery of European chestnut. Vitr. Cell. Dev. Biol. Plant. 2008, 44, 307–315. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, J.; Xia, Y.; Kong, L.; OuYang, F.; Zhang, S.; Zhang, H.; Wang, J. Proteomic analysis of stress-related proteins and metabolic pathways in Picea asperata somatic embryos during partial desiccation. Plant Biotechnol J. 2017, 15, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Booz, M.R.; Kerbauy, G.B.; Guerra, M.P.; Pescador, R. The role of γ-aminobutyric acid (GABA) in somatic embryogenesis of Acca sellowiana Berg. (Myrtaceae). Braz. J. Plant Physiol. 2009, 21, 271–280. [Google Scholar] [CrossRef]
- Chen, T.; Yang, D.; Fan, R.; Zheng, R.; Lu, Y.; Cheng, T.; Shi, J.; Chen, J. γ-Aminobutyric acid a novel candidate for rapid induction in somatic embryogenesis of Liriodendron hybrid. Plant Growth Regul. 2022, 96, 293–302. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. The Metabolism and Functions of [gamma]-Aminobutyric Acid. Plant Physiol. 1997, 115, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z. GABA, a New Player in the Plant Mating Game. Dev. Cell 2003, 5, 185–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steward, F.C.; Thompson, J.F.; Dent, C.E. γ-aminobutyric acid: A constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar]
- Renault, H.; El Amrani, A.; Palanivelu, R.; Updegraff, E.P.; Yu, A.; Renou, J.-P.; Preuss, D.; Bouchereau, A.; Deleu, C. GABA Accumulation Causes Cell Elongation Defects and a Decrease in Expression of Genes Encoding Secreted and Cell Wall-Related Proteins in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 894–908. [Google Scholar] [CrossRef] [Green Version]
- Akama, K.; Akter, N.; Endo, H.; Kanesaki, M.; Endo, M.; Toki, S. An In Vivo Targeted Deletion of the Calmodulin-Binding Domain from Rice Glutamate Decarboxylase 3 (OsGAD3) Increases γ-Aminobutyric Acid Content in Grains. Rice 2020, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Chiu, G.; Bajwa, V.S. Strategies and tools for studying the metabolism and function of γ-aminobutyrate in plants. I. Pathw. Struct. Botany. 2012, 90, 651–668. [Google Scholar]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric Acid (GABA) Imparts Partial Protection from Heat Stress Injury to Rice Seedlings by Improving Leaf Turgor and Upregulating Osmoprotectants and Antioxidants. J. Plant Growth Regul. 2013, 33, 408–419. [Google Scholar] [CrossRef]
- Balfagón, D.; Gómez-Cadenas, A.; Rambla, J.L.; Granell, A.; de Ollas, C.; Bassham, D.C.; Mittler, R.; I Zandalinas, S. γ-Aminobutyric acid plays a key role in plant acclimation to a combination of high light and heat stress. Plant Physiol. 2022, 188, 2026–2038. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2019, 225, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shi, Z.; Xie, T.; Zhang, X.; Chen, W.; Du, C.; Sun, J.; Yue, J.; Zhao, X.; Jiang, Z.; et al. Responses of GABA shunt coupled with carbon and nitrogen metabolism in poplar under NaCl and CdCl2 stresses. Ecotoxicol. Environ. Saf. 2020, 193, 110322. [Google Scholar] [CrossRef] [PubMed]
- Alquraan, N.A.; Alajlouni, Z.I.; Qawasma, N.F. Physiological and biochemical characterization of the GABA shunt pathway in pea (Pisum sativum L.) seedlings under drought stress. Horticulturae 2021, 7, 125. [Google Scholar] [CrossRef]
- Fromm, H. GABA signaling in plants: Targeting the missing pieces of the puzzle. J. Exp. Bot. 2020, 71, 6238–6245. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; Mclauchlin, M.; Gilliham, M.; Tyerman, S.D. Aluminium-activated malate transporters can facilitate GABA transport. Plant Cell. 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [Green Version]
- Bao, H.; Chen, X.; Lv, S.; Jiang, P.; Feng, J.; Fan, P.; Nie, L.; Li, Y. Virus-induced gene silencing reveals control of reactive oxygen species accumulation and salt tolerance in tomato by γ-aminobutyric acid metabolic pathway. Plant Cell Environ. 2015, 38, 600–613. [Google Scholar] [CrossRef]
- Renault, H.; Amrani, A.E.; Berger, A.; Mouille, G.; Soubigou-Taconnat, L.; Bouchereau, A.; Deleu, C. γ-Aminobutyric acid transaminase deficiency impairs central carbon metabolism and leads to cell wall defects during salt stress in Arabidopsis roots. Plant Cell Environ. 2013, 36, 1009–1018. [Google Scholar] [CrossRef]
- Chen, W.; Meng, C.; Ji, J.; Li, M.-H.; Zhang, X.; Wu, Y.; Xie, T.; Du, C.; Sun, J.; Jiang, Z.; et al. Exogenous GABA promotes adaptation and growth by altering the carbon and nitrogen metabolic flux in poplar seedlings under low nitrogen conditions. Tree Physiol. 2020, 40, 1744–1761. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Fan, Y.; Liu, C.; Wang, H.; Li, Y.; Xin, Y.; Gai, Y.; Ji, X. Characterization of GABA-Transaminase Gene from Mulberry (Morus multicaulis) and Its Role in Salt Stress Tolerance. Genes 2022, 13, 501. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, J.; Cui, D.; Zhao, H.; Niu, Y.; Xu, M.; Jiang, G.; Zhao, Y.; Wang, W. Resistance against Ralstonia solanacearum in tomato depends on the methionine cycle and the γ-aminobutyric acid metabolic pathway. Plant J. 2019, 97, 1032–1047. [Google Scholar] [CrossRef]
- Ji, J.; Yue, J.; Xie, T.; Chen, W.; Du, C.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Roles of γ-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: Involving in abscisic acid and ethylene-signalling pathways. Planta 2018, 248, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sai, N.; Gilliham, M. The emerging role of GABA as a transport regulator and physiological signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen Tube Growth and Guidance Is Regulated by POP2, an Arabidopsis Gene that Controls GABA Levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akama, K.; Takaiwa, F. C-terminal extension of rice glutamate decarboxylase (OsGAD2) functions as an autoinhibitory domain and overexpression of a truncated mutant results in the accumulation of extremely high levels of GABA in plant cells. J. Exp. Bot. 2007, 58, 2699–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, J.M.; Singh, N.K.; Cherry, J.H.; Locy, R.D. Nitrate uptake and utilization is modulated by exogenous γ-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol. Biochem. 2010, 48, 443–450. [Google Scholar] [CrossRef]
- Deleu, C.; Faes, P.; Niogret, M.-F.; Bouchereau, A. Effects of the inhibitor of the γ-aminobutyrate-transaminase, vinyl-γ-aminobutyrate, on development and nitrogen metabolism in Brassica napus seedlings. Plant Physiol. Biochem. 2013, 64, 60–69. [Google Scholar] [CrossRef]
- Shi, S.Q.; Shi, Z.; Jiang, Z.P.; Qi, L.W.; Sun, X.M.; Li, C.X.; Liu, J.F.; Xiao, W.F.; Zhang, S.G. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010, 33, 149–162. [Google Scholar] [CrossRef]
- Kathiresan, A.; Tung, P.; Chinnappa, C.C.; Reid, D.M. γ-Aminobutyric acid stimulates ethylene biosynthesis in sunflower. Plant Physiol. 1997, 115, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhu, J.; Sun, L.; Cheng, Y.; Hou, J.; Fan, Y.; Ge, Y. Exogenous γ-aminobutyric acid maintains fruit quality of apples through regulation of ethylene anabolism and polyamine metabolism. Plant Physiol. Biochem. 2021, 169, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dong, Y.; Zhu, L.; Hao, Z.; Hu, L.; Hu, X.; Wang, G.; Cheng, T.; Shi, J.; Chen, J. The role of γ-aminobutyric acid in aluminum stress tolerance in a woody plant, Liriodendron chinense × tulipifera. Hortic. Res. 2021, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Chen, W.; Wu, Y.; Wang, G.; Zhao, J.; Sun, J.; Ji, J.; Yan, D.; Jiang, Z.; Shi, S. Effects of GABA and Vigabatrin on the Germination of Chinese Chestnut Recalcitrant Seeds and Its Implications for Seed Dormancy and Storage. Plants 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Liao, J.; Xia, X.; Xiong, F.; Li, Y.; Shen, J.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W. Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous γ-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol. 2019, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Yadav, R.; Bhatt, B.P. Effects of mother tree ages, different rooting mediums, light conditions and auxin treatments on rooting behaviour of Dalbergia sissoo branch cuttings. J. For. Res. 2011, 22, 53–57. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Huang, K.; Cheng, Z.-M. Auxin regulation and MdPIN expression during adventitious root initiation in apple cuttings. Hortic. Res. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2005, 223, 604–612. [Google Scholar] [CrossRef]
- Negi, S.; Sukumar, P.; Liu, X.; Cohen, J.D.; Muday, G.K. Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J. 2009, 61, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Dawood, T.; Yang, X.; Visser, E.J.; Beek, T.A.T.; Kensche, P.R.; Cristescu, S.M.; Lee, S.; Floková, K.; Nguyen, D.; Mariani, C.; et al. A Co-Opted Hormonal Cascade Activates Dormant Adventitious Root Primordia upon Flooding in Solanum dulcamara. Plant Physiol. 2016, 170, 2351–2364. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Pang, D.; Ruan, L.; Liang, J.; Zhang, Q.; Qian, Y.; Zhang, Y.; Bai, P.; Wu, L.; Cheng, H.; et al. Integrated transcriptome and hormonal analysis of naphthalene acetic acid-induced adventitious root formation of tea cuttings (Camellia sinensis). BMC Plant Biol. 2022, 22, 1–19. [Google Scholar] [CrossRef]
- Hauvermale, A.L.; Steber, C.M. GA signaling is essential for the embryo-to-seedling transition during Arabidopsis seed germination, a ghost story. Plant Signal. Behav. 2020, 15, 1705028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busov, V.; Meilan, R.; Pearce, D.W.; Rood, S.B.; Ma, C.; Tschaplinski, T.J.; Strauss, S.H. Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 2006, 224, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Scagel, C. Changes in Cutting Composition during Early Stages of Adventitious Rooting of Miniature Rose Altered by Inoculation with Arbuscular Mycorrhizal Fungi. J. Am. Soc. Hortic. Sci. 2004, 129, 624–634. [Google Scholar] [CrossRef] [Green Version]
- Zerche, S.; Druege, U. Nitrogen content determines adventitious rooting in Euphorbia pulcherrima under adequate light independently of pre-rooting carbohydrate depletion of cuttings. Sci. Hortic. 2009, 121, 340–347. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Batushansky, A.; Kirma, M.; Grillich, N.; Toubiana, D.; Pham, P.A.; Balbo, I.; Fromm, H.; Galili, G.; Fernie, A.R.; Fait, A. Combined Transcriptomics and Metabolomics of Arabidopsis thaliana Seedlings Exposed to Exogenous GABA Suggest Its Role in Plants Is Predominantly Metabolic. Mol. Plant 2014, 7, 1065–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kim, S.; Kim, S.; Shim, I.S. Production of γ-Aminobutyric acid and its supplementary role in the TCA cycle in rice (Oryza sativa L.) seedlings. J. Plant Growth Regul. 2020, 40, 78–90. [Google Scholar] [CrossRef]
- I Gibson, S. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Druege, U.; Zerche, S.; Kadner, R. Nitrogen- and Storage-affected Carbohydrate Partitioning in High-light-adapted Pelargonium Cuttings in Relation to Survival and Adventitious Root Formation under Low Light. Ann. Bot. 2004, 94, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Druege, U.; Kadner, R. Response of post-storage carbohydrate levels in pelargonium cuttings to reduced air temperature during rooting and the relationship with leaf senescence and adventitious root formation. Postharvest Biol. Technol. 2008, 47, 126–135. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [PubMed]
| Factors | Plant Species | Response | References | |
|---|---|---|---|---|
| Phytohormones | Auxin | Malus xiaojinensis | High GABA concentrations inhibited AR formation and inhibited the expression of PINs (auxin export carriers), therefore perturbing polar auxin transport. | [13] |
| Populus alba × Populus glandulosa cv. ‘84K’ | GABA (10 mM) delayed the initiation of AR primordia by 1–3 d and was accompanied by a large endogenous IAA accumulation at 1–3 d compared to the control. | [5] | ||
| Ethylene | Helianthus annuus | GABA could stimulate ethylene biosynthesis. | [50] | |
| Caragana intermedia | GABA could increase ethylene biosynthesis under NaCl stress. | [49] | ||
| Populus tomentosa | GABA had a positive effect on the increase in ethylene under NaCl stress. | [43] | ||
| Malus domestica cv. Golden Delicious | GABA caused a decrease in ethylene during apple storage. | [51] | ||
| Populus alba × Populus glandulosa cv. ‘84K’ | GABA decreased the ethylene level at 1 d as well as the addition of γ-vinyl-γ-aminobutyric acid (VGB) during AR formation. | [5] | ||
| Populus alba × Populus glandulosa cv. ‘84K’ | The activity of the GABA shunt was increased after treatment with succinyl phosphate, and the growth of ARs (including root length and number) was significantly inhibited at 12 d. Ethylene was also reduced in the stems. | [9] | ||
| Abscisic Acid (ABA) | Populus tomentosa | GABA had a positive effect on the increase in ABA under salt stress. | [43] | |
| Populus alba × Populus glandulosa cv. ‘84K’ | GABA increased the ABA level in the early phase of AR formation. | [5] | ||
| Gibberellin (GA) | Populus alba × Populus glandulosa cv. ‘84K’ | GABA negatively regulated AR formation, which was accompanied by a high GA accumulation. | [5] | |
| Carbon/nitrogen metabolism | Populus alba × Populus glandulosa cv. ‘84K’ | GABA (10 mM) inhibited poplar AR formation and growth at 12 d, which was accompanied by a significant reduction in the sugar content and a significant increase in the amino acid and organic acid levels in roots. | [5] | |
| Populus alba × Populus glandulosa cv. ‘84K’ | Succinyl phosphate inhibited AR growth at 12 d; meanwhile, the sugars in the stems and roots significantly decreased, and the amino acids and malates significantly increased. | [9] | ||
| Liriodendron chinense× tulipifera | GABA and the GABA transaminase (GABA-T) inhibitor (AOA or Vir) increased the effusion of malate and citrate as well as the relative root elongation rate. | [52] | ||
| Castanea mollissima | GABA (10 mM) inhibited the germination of chestnut seeds and the growth of early primary roots with a change in the carbon and nitrogen balance. | [53] | ||
| Camellia sinensis | GABA induced interactions between photosynthesis, amino acid biosynthesis, and the carbon and nitrogen metabolism and improved cold tolerance. | [54] | ||
| Somatic embryogenesis occurrence | Acca sellowiana | GABA (10 µM) promoted the induction of somatic embryos and decreased the rate of abnormal ones. | [20] | |
| Liriodendron hybrid | GABA (~60 µM) enhanced the induction and maturation of somatic embryos and increased the root length of plantlets germinated from somatic embryos. | [21] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, L.; Zhao, Y.; Shi, X.; Chen, R.; Yan, J.; Li, X.; Jiang, Z.; Wang, J.; Shi, S. The Role of γ-Aminobutyric Acid (GABA) in the Occurrence of Adventitious Roots and Somatic Embryos in Woody Plants. Plants 2022, 11, 3512. https://doi.org/10.3390/plants11243512
Pei L, Zhao Y, Shi X, Chen R, Yan J, Li X, Jiang Z, Wang J, Shi S. The Role of γ-Aminobutyric Acid (GABA) in the Occurrence of Adventitious Roots and Somatic Embryos in Woody Plants. Plants. 2022; 11(24):3512. https://doi.org/10.3390/plants11243512
Chicago/Turabian StylePei, Lu, Yue Zhao, Xinru Shi, Rongrong Chen, Jiawei Yan, Xu Li, Zeping Jiang, Junhui Wang, and Shengqing Shi. 2022. "The Role of γ-Aminobutyric Acid (GABA) in the Occurrence of Adventitious Roots and Somatic Embryos in Woody Plants" Plants 11, no. 24: 3512. https://doi.org/10.3390/plants11243512
APA StylePei, L., Zhao, Y., Shi, X., Chen, R., Yan, J., Li, X., Jiang, Z., Wang, J., & Shi, S. (2022). The Role of γ-Aminobutyric Acid (GABA) in the Occurrence of Adventitious Roots and Somatic Embryos in Woody Plants. Plants, 11(24), 3512. https://doi.org/10.3390/plants11243512





