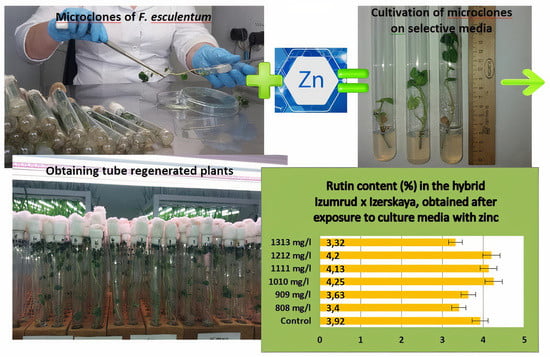
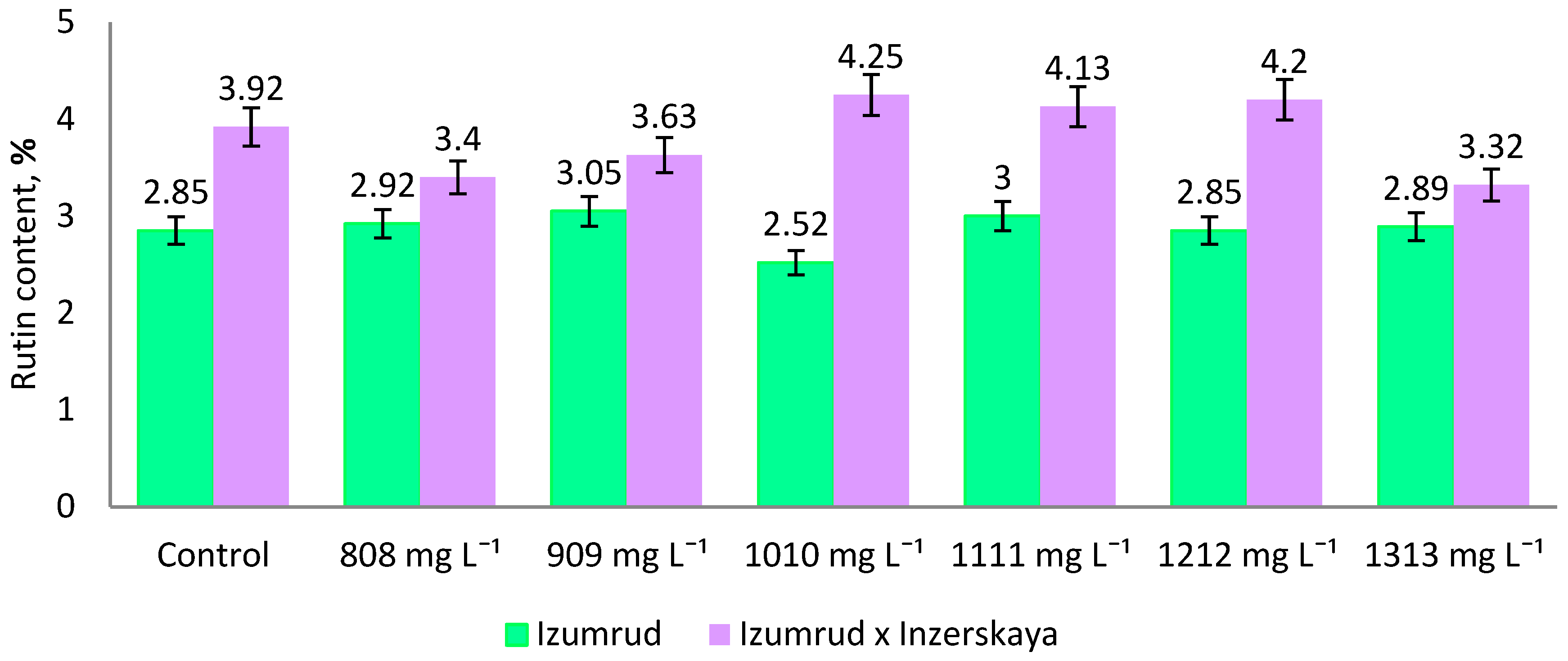
Study of the Effect of Selective Media with High Doses of Zinc on Regeneration Ability and Rutin Accumulation in Common Buckwheat In Vitro
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Original Plant Material
3.2. Introduction into In Vitro Culture and Obtaining Regenerant Buckwheat Plants
3.3. Composition of Selective Media and Experiments
3.4. Determination of Morphological Indicators of Regenerant Plants
3.5. Determination of Rutin Content
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shupletsova, O.N.; Shirokikh, I.G. Increase of barley tolerance to the toxicity of metals and osmotic stress using cell selection. Grain Econ. Russia 2015, 1, 57–62. [Google Scholar]
- Barsukova, E.N.; Klykov, A.G.; Chaikina, E.L. Use of a Tissue Culture Method to Develop New Forms of Fagopyrum esculentum Moench. Russ. Agric. Sci. 2019, 45, 503–506. [Google Scholar] [CrossRef]
- Barsukova, E.N.; Klykov, A.G.; Fisenko, P.V.; Borovaya, S.A.; Chaikina, E.L. Usage of the method of biotechnology in the selection of buckwheat plants in the Far East. Vestn. Far East Branch 2020, 4, 58–66. [Google Scholar] [CrossRef]
- Tumova, L.; Tuma, J. The effect of paraquat on flavonoid production in Fagopyrum esculentum cultures in vitro. Cer. Res. Commun. 2009, 7, 557–560. [Google Scholar] [CrossRef]
- Debski, H.; Wiczkowski, W.; Szawara-Nowak, D.; Baczek, N.; Chrzanowski, G.; Horbowicz, M. Effects of glyphosate and fluazifop-P-butyl on flavonoids content and growth of common buckwheat (Fagopyrum esculentum Moench). Fresen. Environ. Bul. 2018, 27, 91–97. [Google Scholar]
- Tomotake, H.; Yamamoto, N.; Yanaka, N.; Ohinata, H.; Yamazaki, R.; Kayashita, J.; Kato, N. High protein buckwheat flour suppresses hypercholesterolemia in rats and gallstone formation in mice by hypercholesterolemic diet and body fat in rats because of its low protein digestibility. Nutrition 2006, 22, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Naramoto, K.; Koyama, M. Blood-pressure-lowering effect of fermented buckwheat sprouts in spontaneously hypertensive rats. J. Funct. Foods 2013, 5, 406–415. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, K.J.; Park, K.J.; Yoon, B.R.; Lim, J.H.; Lee, O.H. Buckwheat (Fagopyrum esculentum M.) sprout treated with methyl jasmonate (MeJA) improved anti-adipogenic activity associated with the oxidative stress system in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2013, 14, 1428–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, D.A.; Schmidt, B.; Hiser, C.; Westley, E.; Ferguson-Miller, S. Membrane potential-controlled inhibition of cytochrome c oxidase by zinc. J. Biol. Chem. 2002, 277, 14894–14901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dineley, K.E.; Votyakova, T.V.; Reynolds, I.J. Zinc inhibition of cellular energy production: Implications for mitochondria and neurodegeneration. J. Neurochem. 2003, 85, 563–570. [Google Scholar] [CrossRef]
- Skugoreva, S.G.; Aschikhmina, T.Y.; Fokina, A.I.; Lyapina, E.I. Chemical grounds of toxic effect of heavy metals. Theor. Pract. Ecol. 2016, 1, 4–10. [Google Scholar]
- Gladkov, E.A.; Dolgikh, Y.I.; Biryukov, V.V.; Gladkova, O.V. Methods in biotechnology for obtaining plants resistant to heavy metals. Cell selection of lawn grass resistant to copper ions. Biotechnology 2006, 5, 63–66. [Google Scholar]
- Gladkov, E.A. Biotechnological methods of obtaining of Agrostis stolonifera plants resistant to Cd and Pb. Agric. Biol. 2008, 3, 83–87. [Google Scholar]
- Gladkov, E.A. Cell selection of Agrostis stolonifera plants possessing resistance to heavy metals and salinization. Biotechnology 2010, 6, 72–74. [Google Scholar]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro selection and biochemical characterization of zinc and manganese adapted callus lines in Brassica spp. Plant Sci. 1999, 137, 89–100. [Google Scholar] [CrossRef]
- Samantaray, S.; Rout, G.R.; Das, P. In vitro selection and regeneration of zinc tolerant calli from Setaria italica L. Plant Sci. 1999, 143, 201–209. [Google Scholar] [CrossRef]
- Lyubenova, L.; Nehnevajova, E.; Herzig, R.; Schröder, P. Response of antioxidant enzymes in Nicotiana tabacum clones during phytoextraction of heavy metals. Environ. Sci. Pollut. Res. 2009, 16, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Barsukova, E.N. Morphogenetic potential of in vitro common buckwheat callus under ionic stress conditions. In Food Biotechnology: Problems and Opportunities in the 21st Century, Proceedings of the III International Symposium, Vladivostok, 8–10 October 2008; Belkin, V.G., Ed.; TGEU Publications: Vladivostok, Russia; pp. 105–107.
- Barsukova, E.N.; Fisenko, P.P.; Efremova, O.S.; Romashova, M.V.; Khokhlova, N.I. Application of biotechnology methods at PrimSRIA. In Innovative Activity of Agricultural Science in the Far East; Chaikina, A.K., Ed.; Dalnauka Publications: Vladivostok, Russia, 2011; pp. 66–76. [Google Scholar]
- Moumou, Y.; Trotin, F.; Dubois, J.; Vasseur, J.; El-Boustani, E. Influence of culture conditions on polyphenol production by Fagopyrum esculentum tissue cultures. J. Nat. Prod. 1992, 55, 33–38. [Google Scholar] [CrossRef]
- Gumerova, E.A.; Utina, D.B.; Rumyantseva, N.I. Phenolic compounds and antioxidant activity in suspension and callus cultures of Fagopyrum tataricum. In Bioantioxidant: Proceedings of the VIII International Conference; Abstr. October 4–6, 2010; RUDN (Peoples’ Friendship University of Russia—RUDN) Publications: Moscow, Russia, 2010; p. 558. [Google Scholar]
- Gumerova, E.; Akulov, A.; Rumyantseva, N. Effect of methyl jasmonate on growth characteristics and accumulation of phenolic compounds in suspension culture of Tartary buckwheat. Russ. J. Plant Physiol. 2015, 62, 195–203. [Google Scholar] [CrossRef]
- Hou, S.; Sun, Z.; Linghu, B.; Wang, Y.; Huang, K.; Xu, D.; Han, Y. Regeneration of buckwheat plantlets from hypocotyl and the inuence of exogenous hormones on rutin content and rutin biosynthetic gene expression in vitro. Plant Cell Tiss. Org. Cult. 2015, 12, 1159–1167. [Google Scholar] [CrossRef]
- Zhu, Q.; Guo, T.; Sui, S.; Liu, G.; Lei, X.; Luo, L.; Li, M. Molecular cloning and characterization of a novel isoflavone reductase-like gene (FcIRL) from high Flavonoids-producing callus of Fagopyrum cymosum. Acta Pharm. Sin. 2009, 44, 809–819. [Google Scholar]
- Tumova, L.; Pichova, M.; Dusek, J. Fagopyrum esculentum in vitro. Ceska Slov. Farm. 2007, 56, 125–128. [Google Scholar] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tussue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Klykov, A.G.; Barsukova, E.N. Biotechnology and Selection of Buckwheat in the Russian Far East; LLC PSP95: Vladivostok, Russia, 2021; p. 352. [Google Scholar]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef]
- Rout, G.R.; Das, P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. Sustain. Agric. 2009, 23, 873–884. [Google Scholar] [CrossRef]
- Pande, S.D.; Iqbal, M.; Shrivastava, P.S. Effect of ZnSO4 and CuSO4 on regeneration and lepidium content in Lepidium sativum L. Biol. Plant. 2000, 43, 253–256. [Google Scholar]
- Agraval, V.; Sharma, K. Phytotoxic effects of Cu, Zn, Cd and Pb on in vitro regeneration and concomitant protein changes in Holarrhena antidysenterica. Biol. Plant. 2006, 50, 307310. [Google Scholar]
- Titov, A.F.; Kaznina, N.M.; Talanova, V.V. Heavy Metals and Plants; Karelian Scientific Center of RAS: Petrozavodsk, Russia, 2014; p. 194. [Google Scholar]
- Van Assche, F.; Clijsters, H. Inhibition of photosynthesis in Phaseolus vulgaris by treatment with toxic concentration of zinc: Effect on ribulose—1,5-bisphosphate carboxylase/oxygenase. J. Plant Physiol. 1986, 125, 355–360. [Google Scholar] [CrossRef]
- Stiborova, M.; Hromadkova, R.; Leblova, S. Effect of ions of heavy metals on the photosynthesis characteristics of maize (Zea mays L.). Biologia 1986, 41, 1221–1228. [Google Scholar]
- Garty, J.; Karary, Y.; Harel, J. Effect of low pH, heavy metals and anions on chlorophyll degradation in the lichen Ramalina duriaei (De Not) Bagl. Exp. Bot. 1992, 32, 229–241. [Google Scholar] [CrossRef]
- Ahmad, N.; Alatar, A.A.; Faisal, M.; Khan, M.I.; Fatima, N.; Anis, M.; Hegazy, A.K. Effect of copper and zinc on the in vitro regeneration of Rauvolfia serpentine. Biol. Plant. 2015, 59, 11–17. [Google Scholar] [CrossRef]
- Akin, B. In vitro Germination and Phytoremediation Potential of Endemic Plant Species Verbascum phrygium Bornm. Growing under Zinc Stress. Pol. J. Environ. Stud. 2021, 30, 1513–1520. [Google Scholar] [CrossRef]
- Gatti, E. Micropropagation of Ailanthus altissima and in vitro heavy metal tolerance. Biol. Plant. 2008, 52, 146148. [Google Scholar] [CrossRef] [Green Version]
- Naik, P.M.; Godbole, M.; Nagella, P.; Murthy, H.N. The Effect of Heavy Metals on In Vitro Adventitious Shoot Production and Bacoside A Content in Bacopa monnieri (L). Mapana J. Sci. 2015, 14, 1–10. [Google Scholar] [CrossRef]
- Kalisova, I.; Pinchoschava, J.; Kafka, Z. Accumulation of heavy metals by in vitro cultures of plants. Water Air Soil Pollut. 2003, 3, 269276. [Google Scholar]
- Kreft, I. Buchweizen Slowenien. In Das Buchweizen Buch: Mit Rezepten aus Aller Welt; Kreft, I., Ries, C., Zewen, C., Eds.; 2 aufl.; Islek ohne Grenzen EWIV: Arzfeld, Germany, 2007; pp. 71–79. [Google Scholar]
- Germ, M.; Gaberščik, A. The effect of environmental factors on buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 273–281. [Google Scholar] [CrossRef]
- Ohnishi, O. Search for the wild ancestor of buckwheat. III. The wild ancestor or cultivated common buckwheat, and of Tartary buckwheat. Econ. Bot. 1998, 52, 123–133. [Google Scholar] [CrossRef]
- Vinod, K.; Awasthi, G.; Chauhan, P.K. Cu and Zn tolerance and responses of the Biochemical and Physiochemical system of Wheat. J. Stress Physiol. Biochem. 2012, 8, 203–213. [Google Scholar]
- Kazantseva, V.V.; Goncharuk, E.A.; Fesenko, A.N.; Shirokova, A.V.; Zagoskina, N.V. Features of the phenolicsformation in seedlings of different varieties of buckwheat (Fagopyrum esculentum Moench). Agric. Biol. 2015, 50, 611–619. [Google Scholar] [CrossRef]
- Kalashnikova, E.A. Biology basics of plant cell selection. Doklady TAA 2003, 275, 110–112. [Google Scholar]
- Pasqualini, V.; Robles, C.; Garzino, S.; Bousquet-Melou, A.; Bonin, G. Phenolic compounds content in Pinus halepensis Mill. Needles: A bioindicator of air pollution. Chemosphere 2003, 52, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Yadav, P.; Sharma, A.; Kumar Thukral, A.; Kumar, V.; Kaur Kohli, S.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef]
- Nanda, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Ikram, K.; Abdelhakim, R.Y.H.; Topcuoglu, B.; Badiaa, O.; Houria, T. Accumulation of polyphenols and flavonoids in Atriplex canescens (Pursh) Nutt stressed by heavy metals (zinc, lead and cadmium). Malays. J. Fundam. Appl. Sci. 2020, 16, 334–337. [Google Scholar] [CrossRef]
- Keziah, J.E.J.; Sharmila, S.; Jeyanthi Rebecca, L. Effect of heavy metals and UV irradiation on the production of flavonoids in Indigofera tinctoria. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 104–107. [Google Scholar]
- Goncharuk, E.A.; Zagoskina, N.V. The reaction of cells of fiber flax (Linum usitatissimum L.) cultivars with contrasting resistance to cadmium ions. Bull. Kharkov. Natl. Agrar. Inst. Biol. 2016, 3, 27–38. [Google Scholar]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Zhivukhina, E.A. The effect of cadmium ions on in vitro culture of tea plant (Camellia sinensis L.). Bull. Kharkov. Natl. Agrar. Inst. Biol. 2015, 3, 29–37. [Google Scholar]
- Zafari, S.; Sharifi, M.; Ahmadian Chashmi, N.; Mur, L.A. Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic metabolism and related heavy metal tolerance mechanism in Kandelia obovata under Cd and Zn stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Li, X.; Li, P.N.; Xu, H.; Woo, S.-H.; Cheol, H.P.; Sang, U.P. Differential efficiencies of flavonoid biosynthesis genes and accumulation of phenolic compounds in common buckwheat (Fagopyrum esculentum). J. Agric. Food Chem. 2010, 58, 12176–12181. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Jia, H.; Sun, X.; Shangguan, L.; Mu, Q.; Wang, B.; Fang, J. Comparative transcriptome analysis of grapevine in response to copper stress. Sci. Rep. 2015, 5, 17749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilba, V.A. Questions on determination of soybean rhizobium quantity in soil. In Microbiological and Biochemical Research on Soils, Proceedings of the Scientific Conference, Kyiv, 28–31 October 1969; Urozhai Publications: Kyiv, Ukraine, 1971; pp. 51–55. [Google Scholar]
- Barsukova, E.N. Micropropagation of regenerated buckwheat plants in vitro. In Questions on Scientific Support for Agricultural Production in Primorie; Primorsky SRIA Publications: Khabarovsk, Russia, 1997; pp. 27–31. [Google Scholar]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench). Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Klykov, A.G.; Moiseenko, L.M.; Gorovoy, P.G. Biological Resourses of Species of the Genus Fagopyrum Mill. (Buckwheat) in the Russian Far East; Dalnauka: Vladivostok, Russia, 2018; pp. 77–80. [Google Scholar]
- Halafian, A. STATISTICS 6. Statistical Analysis of Data, 3rd ed.; Binom-Press: Moscow, Russia, 2007; p. 512. [Google Scholar]

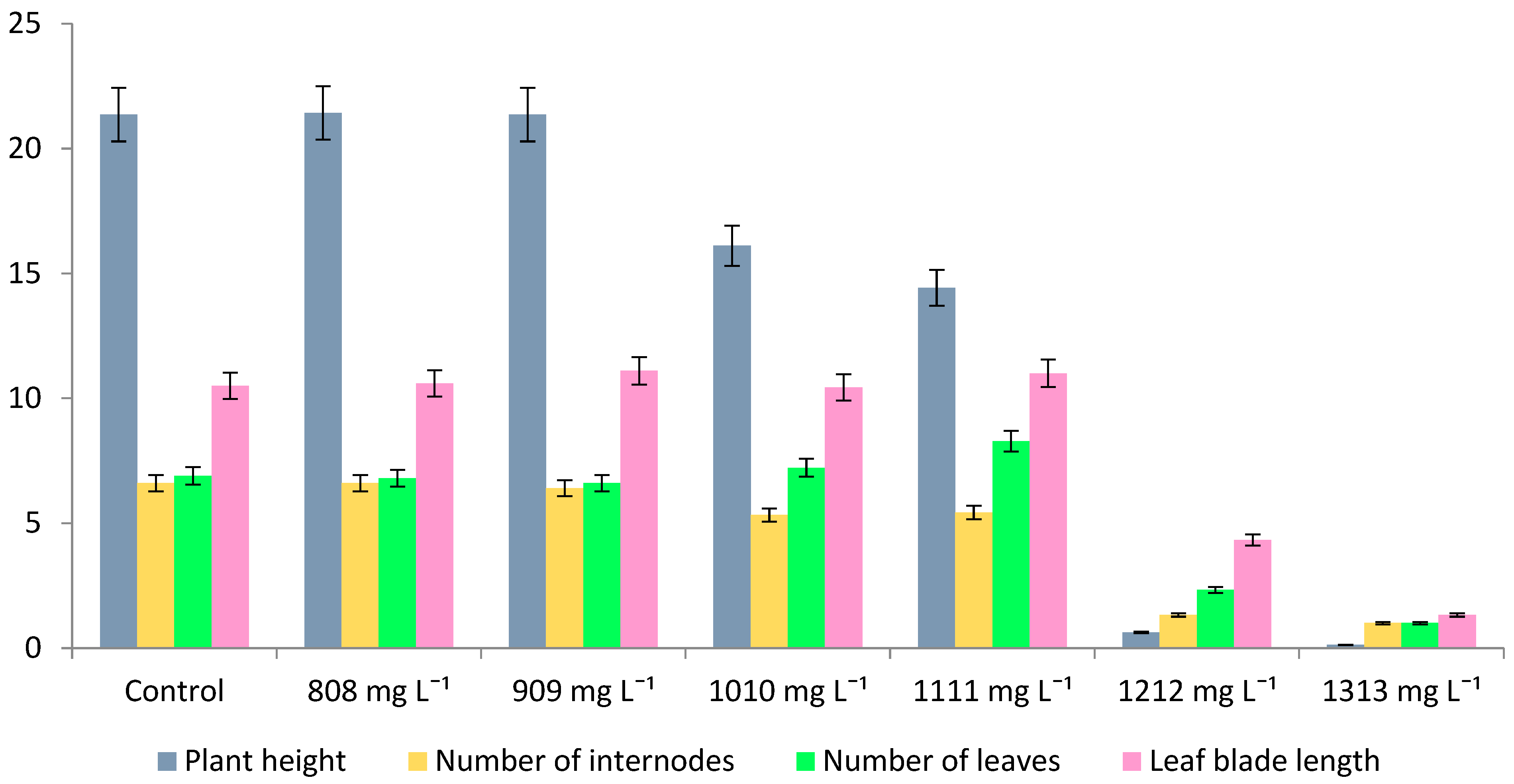


| ZnSO4 × 7H2O Treatment | Plant Height (cm) | Number of Internodes (pcs.) | Number of Leaves (pcs.) | Leaf Blade Length (mm) | Roots (+/−) | Leaf Color |
|---|---|---|---|---|---|---|
| Izumrud | ||||||
| Control 8.6 mg L−1 | 5.98 ± 4.70 b | 3.20 ± 0.60 b | 5.10 ± 0.30 c | 14.40 ± 2.20 d | + | green |
| 808 mg L−1 | 0.86 ± 0.32 a | 1.43 ± 0.79 a | 2.86 ± 0.90 b | 3.57 ± 0.79 ac | − | green and yellow |
| 909 mg L−1 | 0.64 ± 0.30 a | 1.22 ± 0.44 a | 2.78 ± 0.67 b | 5.22 ± 2.17 c | − | yellow |
| 1010 mg L−1 | 0.43 ± 0.24 a | 1.00 ± 0.00 a | 2.40 ± 0.97 ab | 7.50 ± 3.31 b | − | yellow |
| 1111 mg L−1 | 0.16 ± 0.07 a | 1.00 ± 0.00 a | 2.13 ± 1.13 a | 3.38 ± 1.68 ac | − | yellow |
| 1212 mg L−1 | 0.21 ± 0.08 a | 1.00 ± 0.00 a | 1.88 ± 1.13 a | 2.71 ± 0.95 a | − | brown and yellow |
| 1313 mg L−1 | 0.26 ± 0.17 a | 1.00 ± 0.00 a | 2.25 ± 1.16 ab | 2.50 ± 1.20 a | − | brown and yellow |
| Izumrud × Inzerskaya | ||||||
| Control 8.6 mg L−1 | 5.43 ± 3.77 b | 3.63 ± 0.52 b | 5.38 ± 0.52 c | 13.63 ± 4.34 c | + | green |
| 808 mg L−1 | 0.53 ± 0.28 a | 1.11 ± 0.33 a | 3.11 ± 0.60 b | 5.56 ± 1.24 ab | − − | green and yellow |
| 909 mg L−1 | 0.51 ± 0.37 a | 1.14 ± 0.38 a | 2.86 ± 1.07 b | 4.86 ± 1.78 ab | − | green and yellow |
| 1010 mg L−1 | 0.49 ± 0.57 a | 1.20 ± 0.42 a | 2.20 ± 0.92 a | 5.10 ± 2.08 ab | − | yellow |
| 1111 mg L−1 | 0.77 ± 0.82 a | 1.17 ± 0.41 a | 2.50 ± 0.55 ab | 6.50 ± 3.27 b | − | yellow |
| 1212 mg L−1 | 0.46 ± 0.61 a | 1.20 ± 0.45 a | 2.20 ± 0.84 a | 3.60 ± 1.34 ab | − | brown |
| 1313 mg L−1 | 0.11 ± 0.04 a | 1.00 ± 0.00 a | 1.43 ± 0.53 a | 2.43 ± 0.53 a | − | brown |
| ZnSO4 × 7H2O Treatment | Plant Height (cm) | Number of Internodes (pcs.) | Number of Leaves (pcs.) | Leaf Blade Length (mm) | Roots (+/−) | Leaf Color |
|---|---|---|---|---|---|---|
| Izumrud | ||||||
| Control 8.6 mg L−1 | 18.90 ± 2.41 c | 6.00 ± 0.67 cd | 6.70 ± 0.67 c | 14.80 ± 2.50 c | + | green |
| 808 mg L−1 | 8.71 ± 4.64 b | 5.86 ± 3.63 c | 11.43 ± 4.89 f | 6.71 ± 2.81 b | − | green and yellow |
| 909 mg L−1 | 5.68 ± 4.58 b | 4.10 ± 1.85 bc | 7.60 ± 3.37 cd | 7.60 ± 3.24 b | − − | green and yellow |
| 1010 mg L−1 | 4.45 ± 4.58 b | 3.25 ± 2.63 b | 4.00 ± 3.16 bcd | 4.50 ± 3.00 ab | − | yellow and green |
| 1111 mg L−1 | 4.35 ± 2.62 b | 4.00 ± 1.41 bc | 8.50 ± 3.54 cf | 6.50 ± 2.12 b | − | yellow and green |
| 1212 mg L−1 | 0.33 ± 0.15 a | 1.00 ± 0.00 a | 2.00 ± 1.00 b | 2.67 ± 0.58 a | − | brown and yellow |
| 1313 mg L−1 | 0.20 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 3.00 ± 0.00 a | − | brown and yellow |
| Izumrud × Inzerskaya | ||||||
| Control 8.6 mg L−1 | 17.61 ± 4.60 b | 5.44 ± 0.73 b | 6.78 ± 1.30 bc | 14.56 ± 4.44 c | + + | green |
| 808 mg L−1 | 11.46 ± 3.85 b | 5.86 ± 0.90 b | 11.43 ± 5.19 c | 8.14 ± 1.57 b | − − | green and yellow |
| 909 mg L−1 | 6.90 ± 6.47 b | 4.20 ± 3.27 b | 7.60 ± 5.32 c | 7.40 ± 3.29 b | − − | green and yellow |
| 1010 mg L−1 | 5.65 ± 6.75 a | 3.50 ± 2.39 ab | 5.88 ± 3.56 abc | 6.75 ± 2.92 b | − − | yellow and green |
| 1111 mg L−1 | 2.98 ± 3.66 a | 2.80 ± 2.05 ab | 4.60 ± 2.41 abc | 8.00 ± 5.05 b | − − | yellow and green |
| 1212 mg L−1 | 0.23 ± 0.06 a | 1.00 ± 0.00 a | 1.67 ± 0.58 ab | 2.00 ± 0.00 a | − − | brown and yellow |
| 1313 mg L−1 | 0.30 ± 0.20 a | 1.00 ± 0.00 a | 1.00 ± 1.00 a | 1.67 ± 1.53 a | − − | brown and yellow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borovaya, S.; Klykov, A.; Barsukova, E.; Chaikina, E. Study of the Effect of Selective Media with High Doses of Zinc on Regeneration Ability and Rutin Accumulation in Common Buckwheat In Vitro. Plants 2022, 11, 264. https://doi.org/10.3390/plants11030264
Borovaya S, Klykov A, Barsukova E, Chaikina E. Study of the Effect of Selective Media with High Doses of Zinc on Regeneration Ability and Rutin Accumulation in Common Buckwheat In Vitro. Plants. 2022; 11(3):264. https://doi.org/10.3390/plants11030264
Chicago/Turabian StyleBorovaya, Svetlana, Alexey Klykov, Elena Barsukova, and Elena Chaikina. 2022. "Study of the Effect of Selective Media with High Doses of Zinc on Regeneration Ability and Rutin Accumulation in Common Buckwheat In Vitro" Plants 11, no. 3: 264. https://doi.org/10.3390/plants11030264
APA StyleBorovaya, S., Klykov, A., Barsukova, E., & Chaikina, E. (2022). Study of the Effect of Selective Media with High Doses of Zinc on Regeneration Ability and Rutin Accumulation in Common Buckwheat In Vitro. Plants, 11(3), 264. https://doi.org/10.3390/plants11030264