Bacillusvelezensis Strains for Protecting Cucumber Plants from Root-Knot Nematode Meloidogyne incognita in a Greenhouse
Abstract
:1. Introduction
2. Results
2.1. Characteristics and Identification of Bacillus strains
2.2. Enzymatic Activity of B. velezensis BZR 86 and B. velezensis BZR 277 Strains
2.3. Cultivation Conditions for B. velezensis BZR 86 and B. velezensis BZR 277 Strains
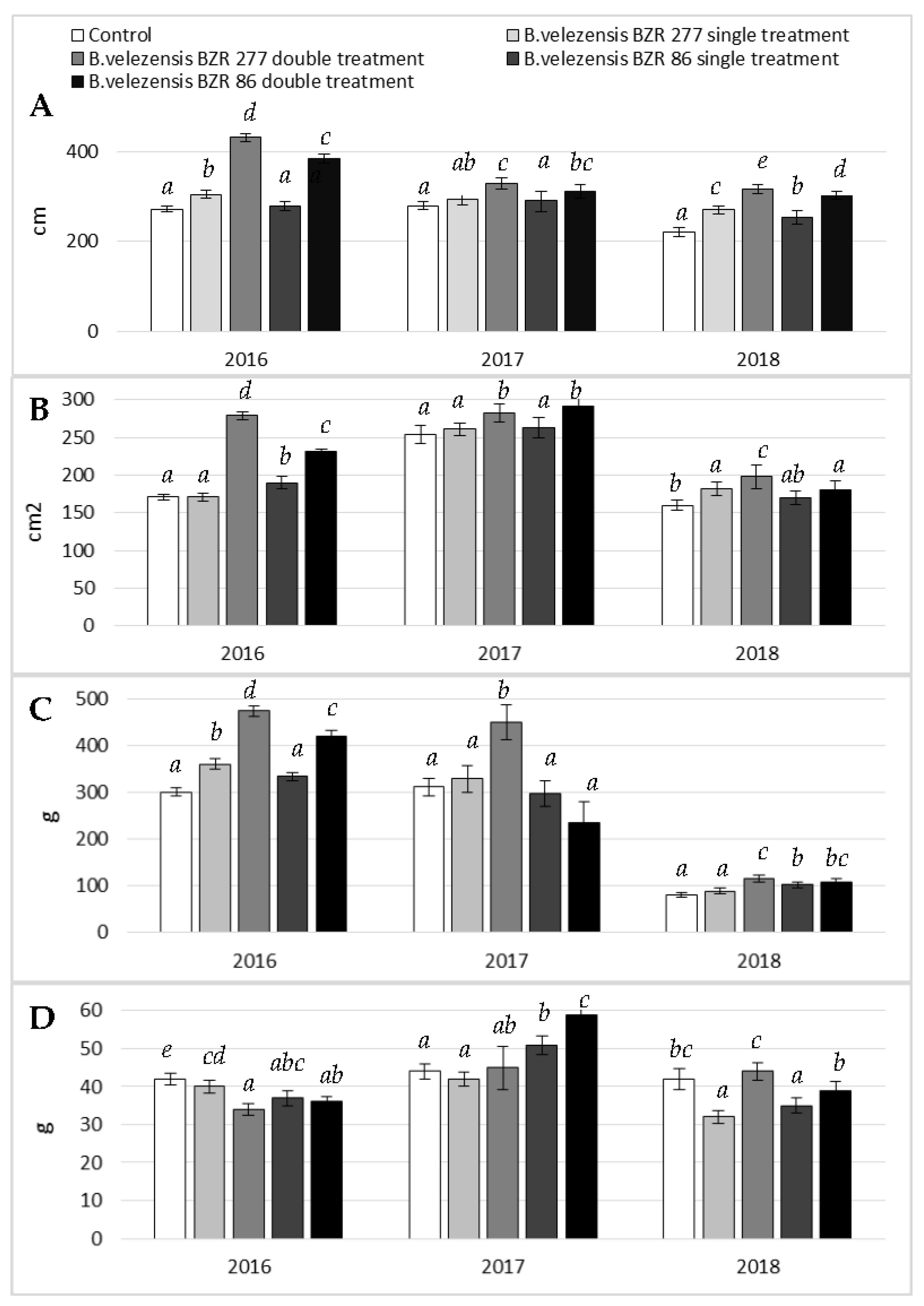
2.4. Study of B. velezensis BZR 86 and B. velezensis BZR 277 Strains under Greenhouse Conditions
3. Discussion
4. Materials and Methods
4.1. Microorganisms
4.2. MALDI-TOF MS Analysis
4.3. Molecular Genetic Identification of Strains
4.4. Cultural and Morphological Characteristics of Bacillus strains
4.5. Enzymatic Activity of Bacillus strains
4.6. Optimal Conditions for the Cultivation of Bacillus strains
4.7. Greenhouse Evaluation of Bacillus strains
- Single treatment by suspension of B. velezensis BZR 86 before planting.
- Double treatment by suspension of B. velezensis BZR 86 during planting followed by treatment under the plant root 3 weeks after planting.
- Single treatment by suspension of B. velezensis BZR 277 before planting.
- Double treatment by suspension of B. velezensis BZR 277 during planting followed by treatment under the plant root 3 weeks after planting.
- Plants without treatment were used as control.
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Aydinli, G.; Mennan, S. Identification of root-knot nematodes (Meloidogyne spp.) from greenhouses in the Middle Black Sea Region of Turkey. Turk. J. Zool. 2016, 40, 675–685. [Google Scholar] [CrossRef]
- Briar, S.S.; Wichman, D.; Reddy, G.V. Plant-parasitic nematode problems in organic agriculture. In Organic Farming for Sustainable Agriculture. Sustainable Development and Biodiversity; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2016; Volume 9, pp. 107–122. [Google Scholar] [CrossRef]
- Topalović, O.; Bredenbruch, S.; Schleker, A.S.S.; Heuer, H. Microbes attaching to endoparasitic phytonematodes in soil trigger plant defense upon root penetration by the nematode. Front. Plant Sci. 2020, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Tagiev, M.M. Gall nematodes (Meloidogyne) on the Apsheron Peninsula and their control. Succes. Mod. Sci. Educ. 2015, 5, 22–24. [Google Scholar]
- Singh, M.C.; Yousuf, A.; Singh, J.P. Greenhouse microclimate modeling under cropped conditions: A review. Res. Environ. Life Sci. 2016, 9, 1552–1557. [Google Scholar]
- Abd-Elgawad, M.M.M. Optimizing safe approaches to manage plant-parasitic nematodes. Plants 2021, 10, 1911. [Google Scholar] [CrossRef]
- Peng, D.; Lin, J.; Huang, Q.; Zheng, W.; Liu, G.; Zheng, J.; Zhu, L.; Sun, M. A novel metalloproteinase virulence factor is involved in Bacillus thuringiensis pathogenesis in nematodes and insects. Environ. Microbiol. 2016, 18, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zheng, J.; Zhang, Z.; Peng, D.; Sun, M. Nematicidal spore-forming Bacilli share similar virulence factors and mechanisms. Sci. Rep. 2016, 6, 31341. [Google Scholar] [CrossRef]
- Aballay, E.; Ordenes, P.; Mårtensson, A.M.; Persson, P. Effects of rhizobacteria on parasitism by Meloidogyne ethiopica on grapevines. Eur. J. Plant Pathol. 2012, 135, 137–145. [Google Scholar] [CrossRef]
- Ann, Y.C. Screening for Nematicidal activities of Bacillus species against root knot nematode (Meloidogyne incognita). Am. J. Exp. Agric. 2013, 3, 794–805. [Google Scholar] [CrossRef]
- Mahesha, H.S.; Ravichandra, N.G.; Rao, M.S.; Narasegowda, N.C.; Shreeshail, S.; Shivalingappa, H. Bio-efficacy of different strains of Bacillus spp. against Meloidogyne incognita under in vitro. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2511–2517. [Google Scholar] [CrossRef]
- Abdel-Salam, M.S.; Ameen, H.H.; Soliman, G.M.; Elkelany, U.S.; Asar, A.M. Improving the nematicidal potential of Bacillus velezensis and Lysinibacillus sphaericus against the root-knot nematode Meloidogyne incognita using protoplast fusion technique. Egypt. J. Biol. Pest Control 2018, 28, 31. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Choi, Y.H.; Shin, T.S.; Kim, T.H.; Shin, K.-S.; Park, H.W.; Kim, Y.H.; Kim, H.; Choi, G.J.; Jang, K.S.; et al. Biological control of Meloidogyne incognita by Aspergillus niger F22 producing oxalic acid. PLoS ONE 2016, 11, e0156230. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.T.; Yu, N.H.; Lee, Y.; Hwang, I.M.; Bui, H.X.; Kim, J.-C. Nematicidal activity of cyclopiazonic acid derived from Penicillium commune against root-knot nematodes and optimization of the culture fermentation process. Front. Microbiol. 2021, 12, 726504. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Duraisamy, K.; Jeong, M.-H.; Park, S.-Y.; Kim, S.; Lee, Y.; Nguyen, V.T.; Yu, N.H.; Park, A.R.; Kim, J.-C. Nematicidal activity of chemical intermediates of grammicin biosynthesis pathway in Xylaria grammica EL000614 against Meloidogyne incognita. Molecules 2021, 26, 4675. [Google Scholar] [CrossRef]
- Stoytcheva, M. Pesticides in the Modern World—Pesticides Use and Management; InTech: London, UK, 2011; 532p. [Google Scholar]
- The State Catalog of pesticides and agrochemicals approved for use on the territory of the Russian Federation. Ministry of Agriculture: Moscow, Russia, 2021; pp. 143–144.
- Asaturova, A.M.; Dubyaga, V.M.; Tomashevich, N.S.; Zharnikova, M.D. Selection of promising biological control agents to protect winter wheat against Fusarium pathogens. Electron. Polythem. Sci. J. KubSAU 2012, 75. Available online: http://ej.kubagro.ru/2012/01/pdf/37.pdf (accessed on 18 November 2021).
- Lychagina, S.V.; Migunova, V.D.; Asaturova, A.M. The influence of Bacillus bacteria on the development of cucumber plants affected by the gall nematode. Theor. Pract. Control. Parasit. Dis. 2017, 18, 227–229. [Google Scholar]
- Abd El-Rahman, A.F.; Shaheen, H.A.; Abd El-Aziz, R.M.; Ibrahim, D.S.S. Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt. J. Biol. Pest Control 2019, 29, 41. [Google Scholar] [CrossRef]
- Chahal, P.P.K.; Chahal, V.P.S. Effect of thuricide on the hatching of eggs root-knot nematode, Meloidogyne incognita. Curr. Nematol. 1993, 4, 247. [Google Scholar]
- Dhawan, S.C.; Sarvjeet, K.; Aqbal, S. Effect of Bacillus thuringiensis on the mortality of root-knot nematode, Meloidogyne incognita. Indian J. Nematol. 2004, 34, 98–99. [Google Scholar]
- Vijayalakshmi, S.; Ranjitha, J.; Rajeswari, V.D. Enzyme production ability by Bacillus subtilis and Bacillus licheniformis—A comparative study. Asian J. Pharm. Clin. Res. 2013, 6, 29–32. [Google Scholar]
- Mota, M.S.; Gomes, C.B.; Souza Júnior, I.T.; Moura, A.B. Bacterial selection for biological control of plant disease: Criterion determination and validation. Brazil. J. Microbiol. 2017, 48, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Dihingia, S.; Das, D.; Bora, S. Effect of microbial secretion on inhibitory effect of phytonematode: A review. Int. J. Inform. Res. Rev. 2017, 04, 4275–4280. [Google Scholar]
- Li, X.; Hu, H.J.; Li, J.Y.; Wang, C.; Chen, S.L.; Yan, S.Z. Effects of the endophytic bacteria Bacillus cereus BCM2 on tomato root exudates and Meloidogyne incognita infection. Plant Dis. 2019, 103, 1551–1558. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, D. Nematode Chitin and Application. Adv. Exp. Med. Biol. 2019, 1142, 209–219. [Google Scholar]
- Tran, T.P.H.; Wang, S.-L.; Nguyen, V.B.; Tran, D.M.; Nguyen, D.S.; Nguyen, A.D. Study of novel endophytic bacteria for biocontrol of black pepper root-knot nematodes in the central highlands of Vietnam. Agronomy 2019, 9, 714. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, A.R.; Kiewnick, S.; Sikora, R.A. In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Sci. Technol. 2008, 18, 377–389. [Google Scholar] [CrossRef]
- Geng, C.; Nie, X.; Tang, Z.; Zhang, Y.; Lin, J.; Sun, M.; Peng, D. A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Sci. Rep. 2016, 6, 25012. [Google Scholar] [CrossRef] [Green Version]
- Mekete, T.J.; Kiewnick, S.; Sikora, R. Endophytic bacteria from Ethiopian coffee plants and their potential to antagonize Meloidogyne incognita. Nematology 2009, 11, 117–127. [Google Scholar] [CrossRef]
- Ramezani, M.M.; Mahdikhani, M.E.; Baghaee, R.S. The nematicidal potential of local Bacillus species against the root-knot nematode infecting greenhouse tomatoes. Biocontrol Sci. Technol. 2014, 24, 279–290. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, K.Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. J. Phytopathol. 2016, 164, 29–39. [Google Scholar] [CrossRef]
- Rao, M.S.; Kamalnath, M.; Umamaheswari, R.; Rajinikanth, R.; Prabu, P.; Priti, K.; Grace, G.N.; Chaya, M.K.; Gopalakrishnan, C. Bacillus subtilis IIHR BS-2 enriched vermicompost controls root knot nematode and soft rot disease complex in carrot. Sci. Hortic. 2017, 218, 56–62. [Google Scholar] [CrossRef]
- Basyony, A.G.; Abo-Zaid, G.A. Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab and greenhouse studies. Egypt. J. Biol. Pest Control 2018, 28, 87. [Google Scholar] [CrossRef]
- Hussain, T.; Haris, M.; Shakeel, A.; Ahmad, G.; Khan, A.A.; Khan, M.A. Bio-nematicidal activities by culture filtrate of Bacillus subtilis HussainT-AMU: New promising biosurfactant bioagent for the management of Root Galling caused by Meloidogyne incognita. Vegetos 2020, 33, 229–238. [Google Scholar] [CrossRef]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Cheba, B.A.; Zaghloul, T.I.; El-Mahdy, A.R.; El-Massry, M.H. Effect of nitrogen sources and fermentation conditions on Bacillus sp. R2 chitinase production. Procedia Manuf. 2018, 22, 280–287. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wu, X.Q.; Wang, Y.H. Nematicidal activity of bacteria against Bursaphelenchus xylophilus and its fermentation and culture characteristics. J. Biotechnol. Bull. 2019, 35, 76–82. [Google Scholar]
- Dukariya, G.; Kumar, A. Chitinase production from locally isolated Bacillus cereus GS02 from chitinous waste enriched soil. J. Adv. Biol. Biotechnol. 2020, 23, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Khadka, S.; Adhikari, S.; Thapa, A.; Panday, R.; Adhikari, M.; Sapkota, S.; Regmi, R.S.; Adhikari, N.P.; Proshad, R.; Koirala, N. Screening and optimization of newly isolated thermotolerant Lysinibacillus fusiformis strain SK for protease and antifungal activity. Curr. Microbiol. 2020, 77, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Gauvry, E.; Mathot, A.G.; Couvert, O.; Leguérinel, I.; Coroller, L. Effects of temperature, pH and water activity on the growth and the sporulation abilities of Bacillus subtilis BSB1. Int. J. Food Microbiol. 2020, 337, 108915. [Google Scholar] [CrossRef] [PubMed]
- Pant, G.; Prakash, A.; Pavani, J.V.P.; Bera, S.; Deviram, G.V.N.S.; Kumar, A.; Panchpuri, M.; Prasuna, R.G. Production, optimization and partial purification of protease from Bacillus subtilis. J. Taibah Univ. Sci. 2015, 9, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-K.; Kim, J.; Lee, C.-W.; Song, J.; Seo, S.-I.; Bong, K.-M.; Kim, D.-H.; Kim, P.I. Mass cultivation and characterization of multifunctional Bacillus velezensis GH1-13. Korean J. Org. Agric. 2019, 27, 65–76. [Google Scholar] [CrossRef]
- Usanov, V.S.; Penzin, A.A.; Shishkin, V.V.; Tatarenko, I.Y. Influence of cultivation temperature and active acidity of soybean-corn substrate on the growth dynamics of the Bacillus subtilis bacterium. Far East. Agrar. Bull. 2020, 3, 117–124. [Google Scholar] [CrossRef]
- Mohapatra, S.; Samantaray, D.P.; Samantaray, S.M. Study on polyhydroxyalkanoates production using rhizospheric soil bacterial isolates of sweet potato. Ind. J. Sci. Technol. 2015, 8 (Suppl. 7), 57–62. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.; Llamas, I.; Torres, B.; Toral, L.; Sampedro, I.; Béjar, V. Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. App. Soil Ecol. 2020, 150, 103453. [Google Scholar] [CrossRef]
- Lee, D.R.; Maung, C.E.H.; Choi, T.G.; Kim, K.Y. Large scale cultivation of Bacillus velezensis CE 100 and effect of its culture on control of Citrus Melanose caused by Diaporthe citri. Korean J. Soil Sci. Fertil. 2021, 54, 297–310. [Google Scholar]
- Garcha, S.; Kansal, R.; Gosal, S.K. Molasses growth medium for production of Rhizobium sp. based biofertilizer. Ind. J. Biochem. Biophys. 2019, 56, 378–383. [Google Scholar]
- Gojgic-Cvijovic, G.D.; Jakovljevic, D.M.; Loncarevic, B.D.; Todorovic, N.M.; Pergal, M.V.; Ciric, J.; Loosc, K.; Beskoski, V.P.; Vrvic, M.M. Production of levan by Bacillus licheniformis NS032 in sugar beet molasses-based medium. Int. J. Biol. Macromol. 2019, 121, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Mazeed, T. Optimization of nutrient composition medium and culture condition for mannanase production by Bacillus velezensis nrc-1 using Taguchi method. Al-Azhar J. Pharm. Sci. 2012, 45, 198–207. [Google Scholar] [CrossRef]
- Horak, I.; Engelbrecht, G.; van Rensburg, P.J.J.; Claassens, S. Microbial metabolomics: Essential definitions and the importance of cultivation conditions for utilizing Bacillus species as bionematicides. J. App. Microbiol. 2019, 127, 326–343. [Google Scholar] [CrossRef] [Green Version]
- Pajčin, I.; Vlajkov, V.; Frohme, M.; Grebinyk, S.; Grahovac, M.; Mojićević, M.; Grahovac, J. Pepper Bacterial Spot Control by Bacillus velezensis. Bioproc. Solut. Microorg. 2020, 8, 1463. [Google Scholar] [CrossRef]
- Sahebani, N.; Omranzade, F. Assessment of plant defence induction and biocontrol potential of Bacillus megaterium wr101 against Meloidogyne javanica. Nematology 2020, 22, 1091–1099. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, Y.; Yin, N.; Li, Y.; Ling, J.; Mao, Z.; Yang, Y.; Xie, B. Analysis of the activity and biological control efficacy of the Bacillus subtilis strain Bs-1 against Meloidogyne incognita. Crop Protect. 2019, 122, 125–135. [Google Scholar] [CrossRef]
- Castaneda-Alvarez, C.; Aballay, E. Rhizobacteria with nematicide aptitude: Enzymes and compounds associated. World J. Microbiol. Biotechnol. 2016, 32, 203. [Google Scholar] [CrossRef] [PubMed]
- Marfenina, O.E. Anthropogenic Ecology of Soil Fungi; Medicine for Everyone: Moscow, Russia, 2005; 196p. [Google Scholar]
- Magoc, T.; Salzberg, S. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the genome taxonomy database. Bioinformatics 2020, 36, 1925–1927. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O.; Notes, A. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netrusov, F.I. Workshop on Microbiology; Publishing Center Academy: Moscow, Russia, 2005; pp. 105–107. [Google Scholar]
- Lysak, L.V.; Dobrovol’skaya, D.G.; Skvortsova, I.N. Methods for Assessing Soil Bacterial Diversity and Identifying Soil Bacteria; Max Press: Moscow, Russia, 2003; pp. 56–58. [Google Scholar]
- Guskova, L.A.; Metlitskiy, O.Z.; Danilov, L.G. Guidelines for Conducting State Tests of Nematicides; VNIESKH: Moscow, Russia, 1983; 35p. [Google Scholar]
- Barker, K.R. An advanced treatise on Meloidogyne. In Methodology; North Carolina State University: Raleigh, NC, USA, 1985; Volume II, pp. 19–35. [Google Scholar]
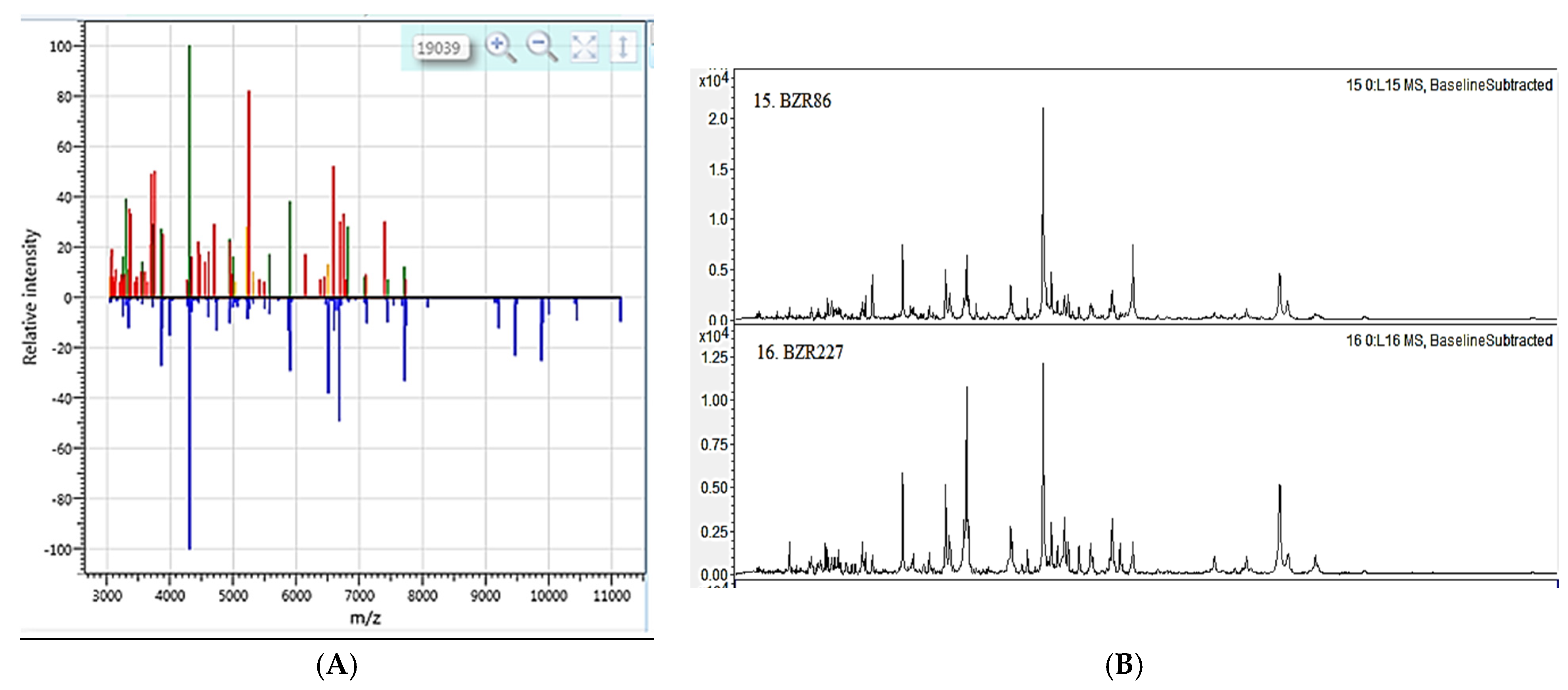



| Strain | Enzymatic Activity | |||
|---|---|---|---|---|
| Lipase | Chitinase | Protease | Gelatinase | |
| B. velezensis BZR 86 | - | + | - | + |
| B. velezensis BZR 277 | +++ | - | +++ | + |
| Parameter | Titer, CFU/ml | |
|---|---|---|
| B. velezensis BZR 86 | B. velezensis BZR 277 | |
| Temperature, °C | ||
| 20.0 | (9.6 ± 0.14) 1 × 10 6 b 2 | (3.4 ± 0.3) × 10 5 b |
| 25.0 | (8.6 ± 0.42) × 10 6 a | (6.2 ± 0.14) × 10 5 a |
| 30.0 | (8.3 ± 0.67) × 10 6 a | (1.4 ± 0.04) × 10 6 c |
| 35.0 | (1 ± 0.05) × 10 7 c | (6.6 ± 0.17) × 10 5 a |
| pH | ||
| 3.0 | (3.2 ± 0.06) × 10 7 c | (4.3 ± 0.2) × 10 6 d |
| 6.0 | (7.6 ± 0.3) × 10 6 a | (1.7 ± 0.3) × 10 6 c |
| 8.0 | (1.1 ± 0.14) × 10 7 b | (1.2 ± 0.02) × 10 6 b |
| 10.0 | (1.1 ± 0.4) × 10 7 b | (1.1 ± 0.05) × 10 6 a |
| Carbon sources | ||
| sucrose | (2.3 ± 0.36) × 10 6 a | (6.2 ± 0.6) × 10 5 a |
| glucose | (3.1 ± 0.22) × 10 6 a | (6.6 ± 0.75) × 10 5 a |
| glycerol | (2.3 ± 0.25) × 10 6 a | (1 ± 0.02) × 10 6 a |
| molasses | (1.6 ± 0.03) × 10 9 b | (5.8 ± 0.39) × 10 8 b |
| Nitrogen sources | ||
| NaNO3 | (3.7 ± 0.4) × 10 6 a | (7 ± 0.66) × 10 7 a |
| peptone | (1.8 ± 0.07) × 10 8 e | (4.7 ± 0.4) × 10 8 c |
| yeast extracts | (4 ± 0.2) × 10 7 b | (5.2 ± 0.5) × 10 7 a |
| corn extracts | (9.4 ± 0.3) × 10 7 c | (1.1 ± 0.05) × 10 8 b |
| Cultivation time, h | ||
| 8 | (2.5 ± 0.15) × 10 6 a | (1.2 ± 0.05) × 10 7 a |
| 16 | (7.4 ± 0.37) × 10 8 b | (4.7 ± 0.35) × 10 8 b |
| 24 | (1.3 ± 0.11) × 10 9 d | (1.7 ± 0.02) × 10 9 d |
| 36 | (1.2 ± 0.06) × 10 9 c | (1.6 ± 0.04) × 10 9 c |
| 48 | (2.3 ± 0.35) × 10 7 a | (4 ± 0.02) × 10 7 a |
| 72 | (1.1 ± 0.2) × 10 7 a | (9.4 ± 0.3) × 10 7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asaturova, A.M.; Bugaeva, L.N.; Homyak, A.I.; Slobodyanyuk, G.A.; Kashutina, E.V.; Yasyuk, L.V.; Sidorov, N.M.; Nadykta, V.D.; Garkovenko, A.V. Bacillusvelezensis Strains for Protecting Cucumber Plants from Root-Knot Nematode Meloidogyne incognita in a Greenhouse. Plants 2022, 11, 275. https://doi.org/10.3390/plants11030275
Asaturova AM, Bugaeva LN, Homyak AI, Slobodyanyuk GA, Kashutina EV, Yasyuk LV, Sidorov NM, Nadykta VD, Garkovenko AV. Bacillusvelezensis Strains for Protecting Cucumber Plants from Root-Knot Nematode Meloidogyne incognita in a Greenhouse. Plants. 2022; 11(3):275. https://doi.org/10.3390/plants11030275
Chicago/Turabian StyleAsaturova, Anzhela M., Ludmila N. Bugaeva, Anna I. Homyak, Galina A. Slobodyanyuk, Evgeninya V. Kashutina, Larisa V. Yasyuk, Nikita M. Sidorov, Vladimir D. Nadykta, and Alexey V. Garkovenko. 2022. "Bacillusvelezensis Strains for Protecting Cucumber Plants from Root-Knot Nematode Meloidogyne incognita in a Greenhouse" Plants 11, no. 3: 275. https://doi.org/10.3390/plants11030275






